Bhadeshia H.K.D.H., Honeycombe R. Steels: Microstructure and Properties
Подождите немного. Документ загружается.

118 CHAPTER 5 FORMATION OF MARTENSITE
Table 5.3 Values of difference in enthalpy of element X in austenite (γ) and in marten-
site (α
′
)
X CNMnNiCuCrWMoVTi
H
α
′
→γ
X
(kJ mol
−1
) −33.9 −22.4 −10.7 −8.4 −5.4 −5.0 +5.7 +5.7 +11.8 +37.7
rewritten, expressing the driving force of the reaction G
α
′
→γ
as:
G
α
′
→γ
= (1 − x)G
α→γ
Fe
+ xH
α
′
→γ
X
. (5.13)
After determining H
α
′
→γ
X
, the free energy change for the martensite reaction
G
α
′
→γ
can be calculated. Values for T
0
, the temperature at which γ and α
′
have the same free energies, can be calculated by putting G
α
′
→γ
equal to zero.
Alloying elements either expand the γ-loop, i.e. stabilize γ, or contract the
loop and encourage α-formation, and this will have different effects on H
α
′
→γ
X
(Section 4.1). Elements which expand the γ-loop will make this term negative
and lower T
0
, while elements which favour α-formation will make the term
positive and raise T
0
.
It is interesting to look at the values of H
α
′
→γ
X
which are available in the
literaturefor anumber ofcommon alloying elements(Table 5.3).There aresome
anomalies, e.g. chromium, which contracts the γ-loop, has a negative H value,
suggesting that H has been computed from data at too low a temperature.
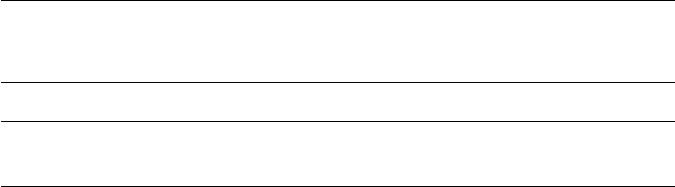
Cohen and co-workers have provided detailed data for iron–carbon alloys
between 0 and 1.1 wt% carbon and in Fig. 5.19 the temperature dependence
of G
α
′
→γ
is plotted for several carbon levels. The intersection of the curves
with the H
α
′
→γ
=0 axis provides values of T
0
for the various compositions. It
was found that the driving force G
α
′
→γ
at the M
s
temperatures of the alloys
was practically constant, approximately 1250 J mol
−1
, independent of carbon
content. However, work on iron–nickel alloys has shown that the driving force
increases with increasing nickel content, i.e. as the M
s
is depressed.
5.6.3 The effect of deformation
The effect of uniaxial stress on the martensitic transformation is normally to
raise the M
s
temperature. The superimposed stress field from the plastic, or
elastic, deformation reinforces that caused by the nucleation of a martensite
plate, and in one sense the subsequent shape change is a further plastic deform-
ation process. We can define a temperature M
d
, greater than M
s
, above which
deformation of the parent phase does not form any martensite. However, it is
likelythat deformation ofthe austenite aboveM
d
willalter the M
s
onsubsequent
cooling through the martensitic range. Usually in these circumstances, the M
s
is
5.6 KINETICS OF TRANSFORMATION TO MARTENSITE 119
Fig. 5.19 Free energy change for the austenite–martensite reaction as a function of
temperature and carbon content (Kaufmann and Cohen, Progress in Metal Physics 7, 165, 1958).
lowered, and the resultant increased stability of the austenite is referred to as
mechanical stabilization.
5.6.4 Stabilization
Stabilization means a reduction in the amount of transformation of austenite
to martensite, as a result of processes which interfere with the nucleation and
growth of the plates. Plastic deformation above the M
d
temperature can achieve
this. However, the term stabilization is normally applied when the cooling of a

120 CHAPTER 5 FORMATION OF MARTENSITE
steel is arrested in the M
s
−M
f
range. The transformation, when resumed by
lowering the temperature, does not result in as complete a transformation to
martensite as would have been the case if no isothermal pause had occurred.
At the chosen delay temperature, the degree of stabilization increases to a
maximum with time, and as the temperature approaches M
f
, the extent of sta-
bilization increases. It appears that stabilization is at a minimum when only a
small amount of martensite is present in the matrix.
The explanation of these complex effects lies in the fact that the formation
of martensite plates leads to accommodating plastic deformation in the sur-
rounding matrix, which can result in high concentrations of dislocations in the
austenite. Interaction of some of these dislocations with the glissile dislocations
in the martensite plate boundary will then cause it to be no longer mobile, so
that the plate cannot grow further. Any phenomena which help to encourage
this process will achieve stabilization. Resting at an intermediate temperature
gives time for plastic relaxation, i.e. movement of dislocations, as well as the
locking of interfacial dislocations by carbon atoms.
5.7 THE STRENGTH OF MARTENSITE
The high hardness and brittleness of rapidly quenched steels is the result of the
formation of martensite, yet many shear transformations in non-ferrous alloy
systems do not produce this dramatic hardening. Indeed, if carbon is eliminated
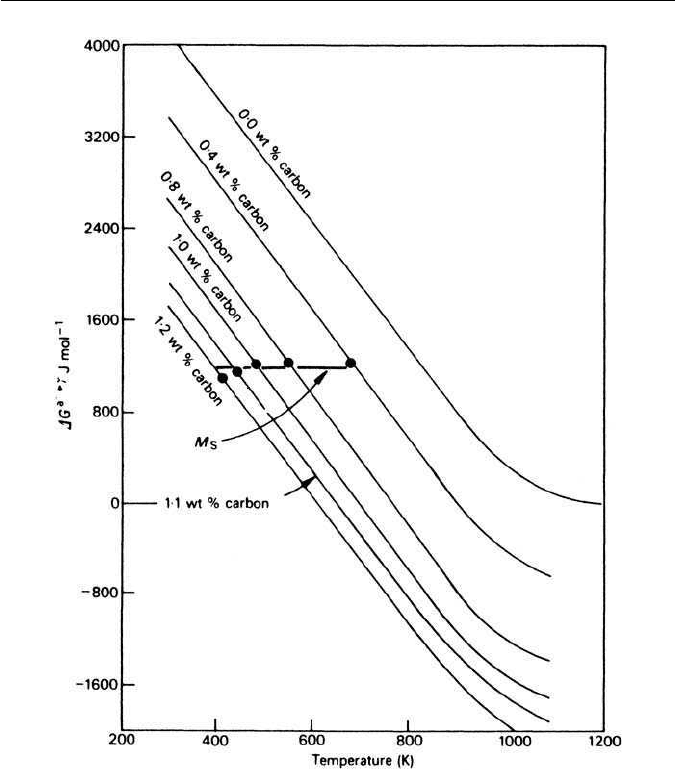
from the steel the resulting hardness is very much lower. Figure 5.20 shows the
large effect of carbon content on the hardness of martensite compared with the
relatively small effect of carbon on the strength of austenite, retained to room
temperature by the addition of nickel.
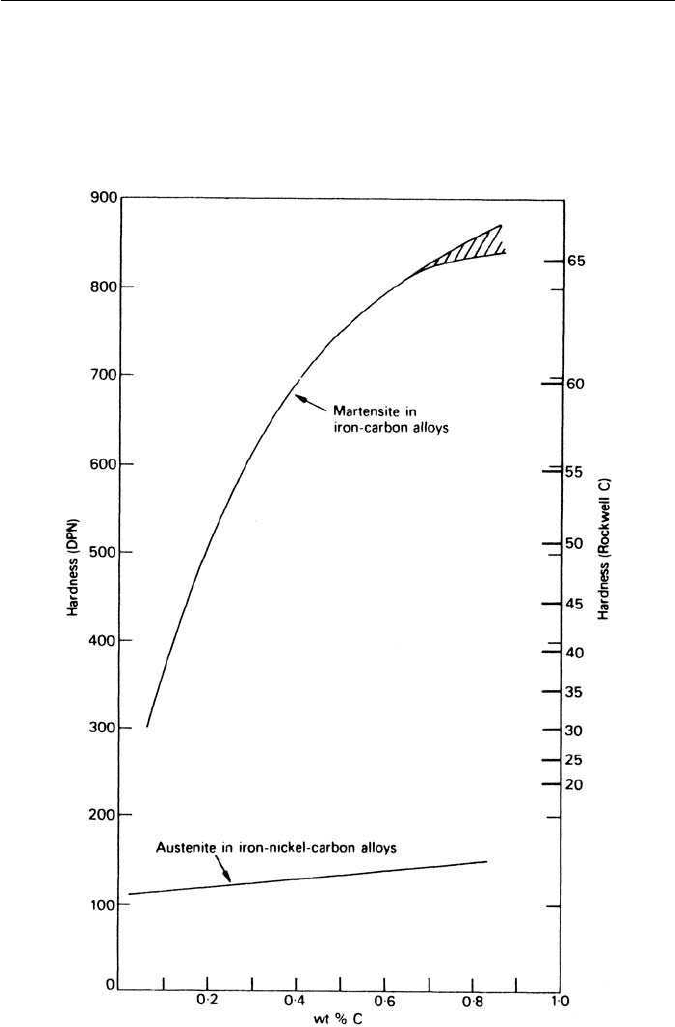
The strength levels reached depend also on the detailed structure of the
martensite, e.g. whether it has remained stable during quenching and testing at
room temperature. By addition of nickel to iron carbon alloys, Winchell and
Cohen depressed the M
s
temperature to −35
◦
C,so that martensite formed only
at low temperatures and auto-tempering was eliminated (Chapter 8). In addi-
tion, the samples were deformed at 0
◦
C, with the results shown in Figure 5.21,
indicating that the flow stress of martensite increases with carbon content up
to about 0.5 wt% C. Allowing the martensite to rest for 3 h at 0
◦
C, resulted in
the upper curve (Fig. 5.21), demonstrating that martensite can age harden at
ambient temperature or below.
The question of the origin of the high strength of martensite is a difficult one,
compounded by the complexity of the structure, a tetragonal lattice with inter-
stitial carbon in solid solution, formed by shear which leads to high densities of
dislocations and fine twins. There are, as a result, several possible strengthening
mechanisms:
(a) substitutional and interstitial solid solution;
(b) dislocation strengthening, i.e. work hardening;
5.7 THE STRENGTH OF MARTENSITE 121
(c) fine twins;
(d) grain size;
(e) segregation of carbon atoms;
(f) precipitation of iron carbides.
Fig. 5.20 The effect of carbon on the hardness of martensite and austenite (Winchell and
Cohen,Transactions of the Metallurgical Society of AIME 224, 638, 1962).
122 CHAPTER 5 FORMATION OF MARTENSITE
Fig. 5.21 Ageing of martensite at 0
◦
C in Fe–Ni–C alloys (Winchell and Cohen, Transactions
of the Metallurgical Society of AIME 224, 638, 1962).
The interstitial solid solution of carbon which results in the tetragonality of
martensite is a prime candidate for the role of major strengthening factor. The
work of Winchell and Cohen enabled the determination of the yield stress as a
function of carboncontent under conditionswhen thecarbon atoms wereunable
to diffuse to form atmospheres and precipitates. The flow stress was shown to
vary as c
1/3
, where c =carbon and concentration, but later it was found that the
strength could be shown equally well to vary as c
1/2
.
5.7 THE STRENGTH OF MARTENSITE 123
Fleischer examined the situation theoretically with a model of a dislocation
bending away from interstitial solute atoms with short range interactions, and
using a parameter ε, the difference in longitudinal and transverse lattice strain
caused by an interstitial carbon atom in martensite (ε ≃0.38). He found the
following expression for the flow stress τ:
τ = τ
0
+
2Gεc
1/2
3
, (5.14)
predicting that the flow stress is proportional to c
1/2
. The curve has a slope of
G/15 to G/20. Other experiments on martensites with low M
s
temperatures sup-
port the c
1/2
relationship, with slight differences in slope depending on whether
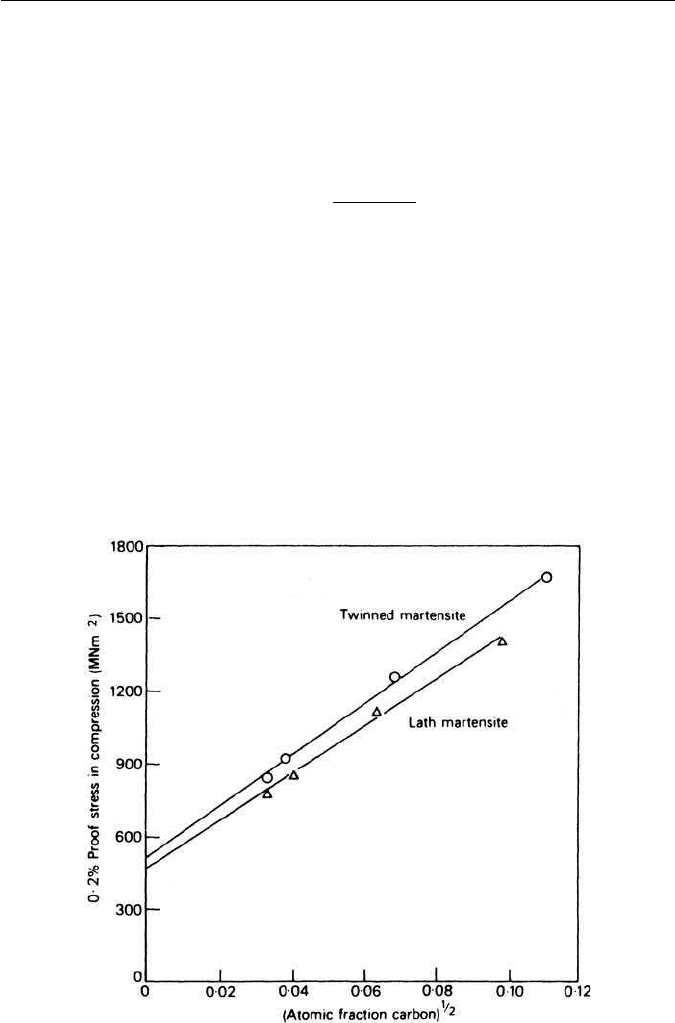
the martensite is of lath type or twinned (Fig. 5.22).
The proposal that the fine twins characteristic of higher carbon marten-
sites make a major contribution to strength has not received wide acceptance.
Certainly, a large increase in strength is not found when the transition from
dislocated martensite to twinned martensite takes place. However, the high
dislocation densities of twin-free martensite must make some contribution to
strength, estimated to be not greater than 300 MN m
−2
, and there is reason to
believe that the fine twinning makes a similar, but not additive, contribution.
Fig. 5.22 Effect of carbon on the strength of martensite (Chilton and Kelly, Acta Metallurgica
16, 637, 1968).
124 CHAPTER 5 FORMATION OF MARTENSITE
The austenitic grain size determines the maximum size of a martensitic plate,
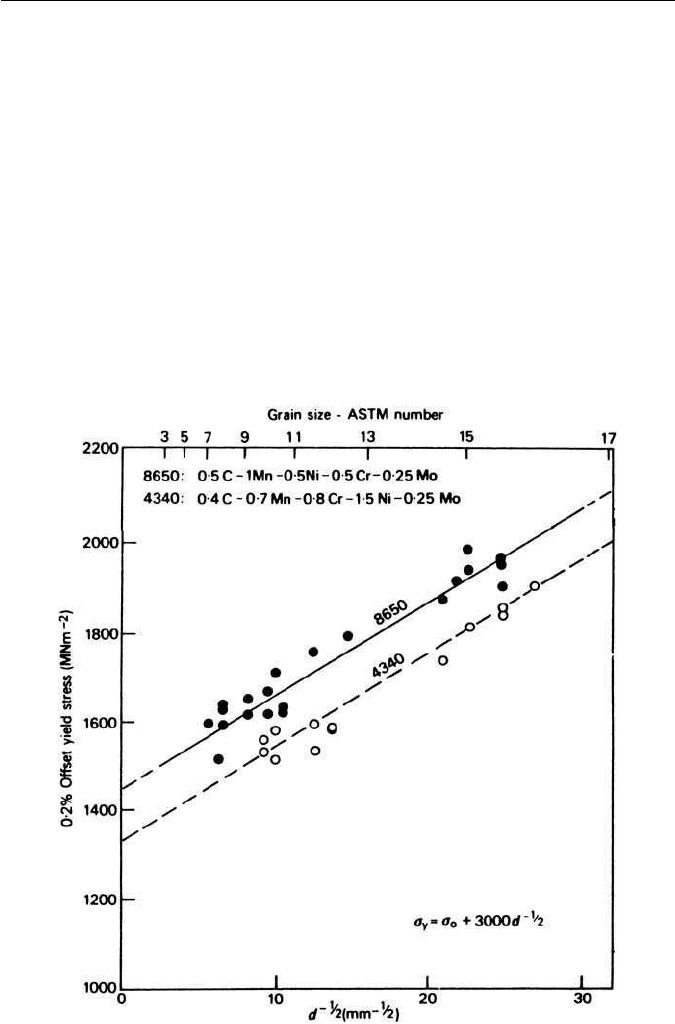
so somedependence of strengthon grain size might be expected. In fact,a Petch-
type plot has been found for several alloy steels of different austenitic grain
sizes tested in the martensitic condition (Fig. 5.23). However, when the fine
structure of martensite is examined other possible grain sizes much finer than
the austenitic grain size can be considered as contributors to strength. Firstly,
there is the packet size in lath martensite, or the individual plate in lenticular
martensite, and beyond these there is the lath substructure which is usually well
below 1 µm in thickness. While many of these boundaries are really low angle
sub-boundaries, they do present obstacles to dislocation movement and must,
therefore, be considered to make some contribution to the overall strength.
It is also to be expected that carbon atoms segregate to the high disloca-
tion populations typical of martensite, bearing in mind the strong interactions
found in the case of ferrite. Internal friction measurements by Kurdjumov and
Fig. 5.23 The effect of prior austenite grain size on the strength of martensite (Grange,
Transactions of the American Society for Materials 59, 26, 1966).
5.7 THE STRENGTH OF MARTENSITE 125
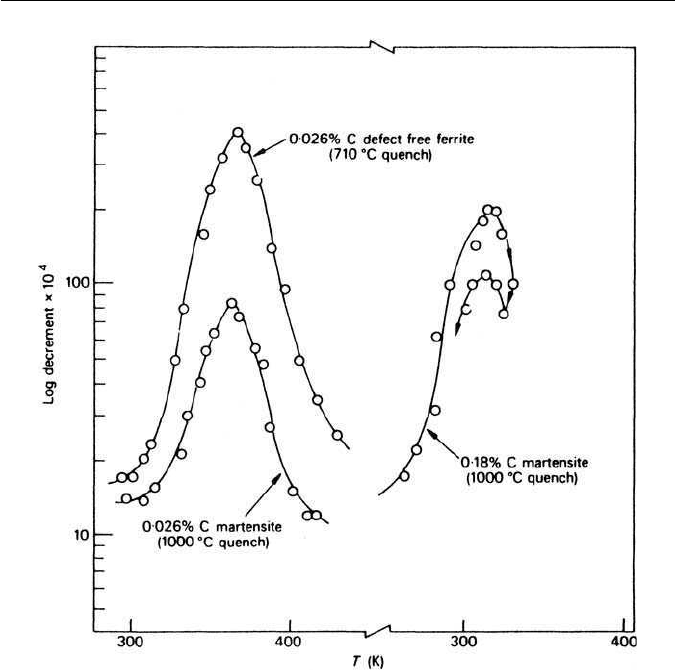
Fig. 5.24 Comparison of the internal friction behaviour of low carbon martensites with that
of ferrite (Speich,Transactions of the Metallurgical Society of AIME 245, 2553, 1969).
co-workers have revealed the well-defined temperature-dependent peak, the
Snoek peak (p. 6), which occurs as a result of the stress-induced movement of
carbon atoms in ferrite and martensite. Figure 5.24 shows that the Snoek peak is
much lower in a 0.026 wt% C martensite than in ferrite of the same composition.
This is a direct result of the reduction in free carbon atoms in the martensite
structure due to pinning by the high concentration of dislocations. These pinned
carbon atoms cannot contribute directly to the Snoek peak, the height of which
is proportional to the concentration of free carbon atoms in the lattice. In con-
trast, ferrite has a very low dislocation density and exhibits a much higher Snoek
peak (Fig. 5.24), because a greater concentration of carbon atoms is available
to move interstitially between the octahedral sites.
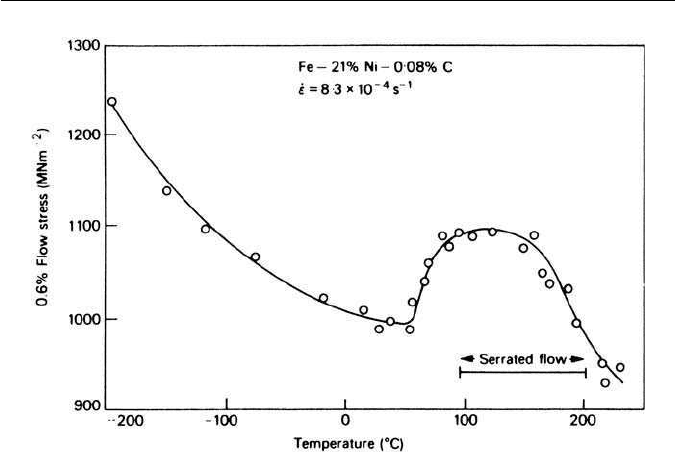
Work on the temperature dependence of the flow stress of martensite in
Fe–Ni–C alloys has shown a strong temperature dependence, together with a
126 CHAPTER 5 FORMATION OF MARTENSITE
Fig. 5.25 Temperature dependence of the flow stress of Fe–Ni–C martensite (Owen and
Roberts, International Conference on Strength of Metals and Alloys,Tokyo, 1968).
peak in the curve associated with serrated flow in the stress–strain curve (Fig.
5.25). Like the development of the yield point in α-iron, this has been attributed
to the Cottrell–Bilby interaction of carbon with dislocations.
However, this phenomenon leads to precipitation of iron carbide on the
dislocations which is responsible for the increase in strength shown by marten-
site aged at room temperature or just above. Also martensites with relatively
high M
s
temperatures will form cementite dispersions during the quench
(auto-tempering) which will also make some contribution to the observed
strength.
The yield strength of martensite, like that of ferrite, is markedly temperature
dependent, but this dependence is little affected by the presence or absence of
precipitate or by the amount of carbon in solution. It is, therefore, likely that
the temperature dependence arises from the basic resistance of the lattice to
dislocation movement, i.e. it is a result of the temperature dependence of the
Peierls–Nabarro force.
5.8 SHAPE MEMORY EFFECT
The shape deformation accompanying martensitic transformation can be
reversedby transforming backtothe parent phase. Suppose a crystalofaustenite
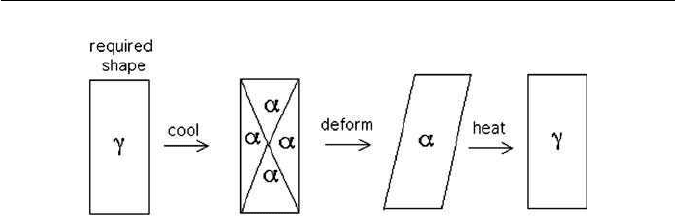
FURTHER READING 127
Fig. 5.26 Shape memory effect.
is cooled to form many variants of martensite, in such a way that they accommo-
date and hence the overall shape is unaffected by transformation. When a stress
is applied, the favoured variant of martensite grows, leading to a shape change
(Fig. 5.26). On heating the shape change is reversed, thus regaining the original
shape. This is the basis of the shape memory effect. The memory can be lost by
introducing defects during transformation, e.g. by repeated cycling. Excessive
deformation, beyond that required to produce a single martensite variant, will
lead to irreversible strain and a loss of memory.
The most successful shape-memory alloys are based on nickel containing
titanium and aluminium. A large variety of iron-based shape-memory alloys
exists but their recoverable strains are smaller and less reversible. They do
have cost advantages and find engineering applications such as pipe-couplings,
where the memory effect need operate only once to make an integral joint.
Some of these alloys exploit the γ →α
′
transformation whereas others rely on
γ →ǫ martensite. There are even alloys in which the austenite transforms into
face-centred tetragonal martensite.
FURTHER READING
Bhadeshia, H. K. D. H., Geometry of Crystals, 2nd edition, Institute of Materials, London,
2001. www.msm.cam.ac.uk/phase–trans/2001/crystal.html
Bhattacharya, K., Microstructure of Martensite, Oxford University Press, Oxford, UK, 2003.
Christian, J. W.,Theory of Transformations in Metals and Alloys, 3rd edition, Pergamon Press,
Oxford, 2003.
Kajiwara, S., Shape memory effect and transformation behaviour in iron-based alloys,
Materials Science and Engineering A273–A275, 67, 1999.
Krauss, G., Steels: Heat Treatment and Processing Principles. ASM International, Ohio, USA,
1990.
Krauss,G.,Martensite in steel:strength and microstructure, Materials Science and Engineering
A273–A275, 40, 1999.
Maki, T., Recent developments in iron-based shape memory alloys, 1st Japan International
SAMPE Symposium, 225, 1989.
Martensite (ATribute to Morris Cohen) (eds Olson,G. B. and Owen,W. S.),ASM International,
Ohio, USA, 1992.
