Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

chain standing at an average angle of 521 with respect to the layer plane. In the case
of ODTMA, the invariance in NEXAF S amplitude with respect to measurement
angle was interpreted in terms of complete disorder of the alkyl chain, although the
opposite possibility of perfect or der with a tilt angle at 54.71 could not be ruled out.
The difference in orientation between the di- and mono-alkyl ammonium ions was
probably a result of the packing order and density of alkyl chains on the external
mica surface with DODMA having twice the chain density per formula unit than
ODTMA. Given that the layer charge of mica can be nearly twice that of low-charge
vermiculites, these results are not surprising. In the case of intercalated hexadecyltri-
methylammonium (HDTMA) in vermiculite (X ¼ 1:23 and 1.5e
–
) the alkyl chains on
243
R* (Å)
k
3
(k)
3N
Fe
0N
Al
2N
Fe
1N
Al
1N
Fe
2N
Al
0N
Fe
3N
Al
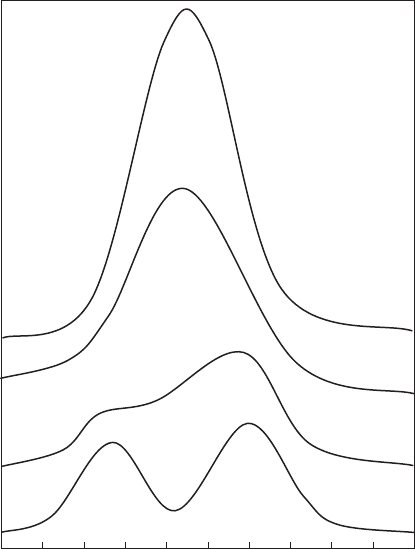
Fig. 12.3.14. Fourier transforms calculated from FEFF showing the FeyM
1
shell of
smectites in which the first cation shell is varied between Fe and Al. Non-centrosymmetric
structure assumed for N
Fe
¼ 0, N
Al
¼ 3 and N
Fe
¼ 1, N
Al
¼ 2 shells; centrosymmetric struc-
ture assumed for N
Fe
¼ 2, N
Al
¼ 1 and N
Fe
¼ 3, N
Al
¼ 0 shells. FeyFe and FeyAl inter-
atomic distances were held constant at d ¼ 307 pm and d ¼ 301 pm, respectively.
Chapter 12.3: X-ray Absorption Spectroscopy826
opposing layers will protrude into the inter layer space, doubling the chain packing
density as compared with a single surface (Slade and Gates, 2004b ). Using scaled
models of intercalated HDTMA-vermiculites based on powder and single-crystal
X-ray transmission diffraction data, Slade and Gates (2004b) deduced an angle of
repose of about 501. By analogy the octadecyl chains (of ODTMA) on the external
surfaces of mica (Brovelli et al., 1999) might have a highly close-packed two-
dimensional order with the chains making an angle of 54.71 to the surface. Slade and
Gates (2004b) also found that at loadings satisfying the vermiculite layer charge,
HDTMA assembled into a 3 a b super cell commensurate with the dimens ions of
the silicate surface. When uptake of HDTMA-Br was in excess of the layer charge,
the alkyl tails of the HDTMA-Br ion pairs caused closest packing and rotation ,
destroying the commensurate structure, although transmis sion diffraction still in-
dicated a highly ordered organic interlayer structure. Obviously, this is a potenitally
fruitful area of research with implications for nanotechnology and self-assembly, and
for which synchrotron-based techniques will be very useful.
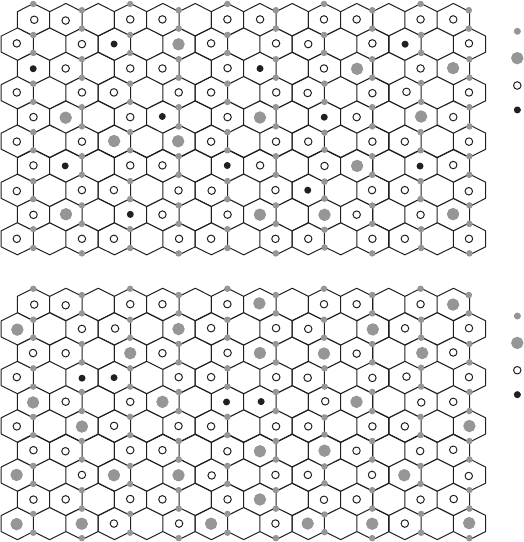
OH
Mg
Al
Fe
SAz-1
OH
Mg
Al
Fe
SWy-2
Fig. 12.3.15. Models for the two-dimensional distribution of cations in the octahedral sheet
of montmorillonites SWy-2 and SAz-1 based on the XAFS analyses of Vantelon et al. (2003),
but modified using chemical and IR data (Gates et al., 2002; Gates, 2004).
12.3.3. Orientation of Intercalated Organic Molecules 827
Lin et al. (1997) studied the location of Br
counterions sorbed during the self-
assembly of organic mono- and multi-layers on Mo/Si surfaces, using X-ray standing
wave techniques. The standing wave coherence indicated a narrow distribution
of positions for the Br
–
anions, located about 1 nm above the surface. These inner
Br
–
positions remained unchanged when multilayers of organic compounds were
sorbed, and the distances from the surface of additional layers of Br
–
were found to
be consistent with the distances between positively charged nitro groups of the or-
ganic molecules (bromopropyl trichlorosilane derivatives). These results strongly
suggest that self-assembly produces ordered superlattice structures, as Slade and
Gates (2004b) observed for HDTMA-vermiculites. Sorption of the halide anion
along with the organic cati on within the structures of organo-clays clearly influence
many of their practical applications, as shown by the thermal stability of organo-clay
complexes (Davis et al., 2004).
Finally, the studies by Fisher et al. (1998) and Ha
¨
hner et al. (1996b) provided
information on the attachment of dye molecules to mineral surfaces. Using C and N
K NEXAFS Ha
¨
hner et al. (1996b) showed that the fused aromatic ring plane of
methylene blue resides at steep angles (651–701) with respect to the mica surface.
Fischer et al. (1998) observed similarly for crystal violet and malachite green. These
results indicate that the specific surface areas of smectites, determined by sorption of
methylene blue, are overestimated if the dye is assumed to lie with its aromatic rings
flat to the silicate surface. The steep angle of inclination may be due to the high
packing density of methylene blue on high-charge micas. Visible spectroscopic stud-
ies ( Bujda
´
k and Komadel, 1997; Bujda
´
k et al., 2001) showed that the inter action of
methylene blue molecules with reduced-charge smectites (with a layer charge o0.25
that of micas) is more complex. When the layer charge is very low, methylene blue is
intercalated as oligomers by van der Waals interactions with a residence time pro-
portional to layer charge. Eventually these oligomers are protonated and degrade to
isolated monomers at the surface. A steeply inclined orientation of the oligomers,
with the aromatic rings lying parallel to one another, would favour dye–dye electron
transfer.
12.3.4. SPATIAL RESOLUTION WITH MICROPROBE XAFS
Micro-XAFS (m-XAFS, or specific applications such as m-XRF, m-EXAFS,
m-XANES or m-XRD) can now achieve spatial resolution of o1 mm
2
with respect to
chemical interactions between metal sorbates on minerals (Bajt et al., 1993, 1994,
1995; Hunter and Bertsch, 1998; Manceau et al., 2002a), plant material (Schulze et
al., 1995a, 1995b; Punshon et al., 2003) and animal material (Hunter et al., 1997).
Spatially resolved XRF studies showed that the distribution of trace metals at
mineral surfaces influences their incorporation during crystallisat ion (Hunter and
Bertsch, 1998; Bertsch and Hunter, 2001; Manceau et al., 2002a). Trace metals
also influence mineral formation during pedogenesis (Manceau et al., 2002b;
Chapter 12.3: X-ray Absorption Spectroscopy828
Strawn et al., 2002). The reviews by Schulze et al. (1999), Bertsch and Hunter (2001),
Fenter et al. (2002) and Hirshchmugl (2002) provide excellent background on the
various applications, as well as the pros and cons, of synchrotron-based microprobe
XAFS, X-ray and infrared spectromicroscopy.
12.3.5. XAFS STUDIES OF REACTIVITY OF CLAYS AND CLAY
MINERALS
Synchrotron-based XAFS made an important contribution to environmental sci-
ence in the past 20 years because of its ability to assess structural, compositional and
reaction mechanisms under in situ conditions. Such applications include the study of
phosphates (Hest erberg et al., 1999) or biogenic silica (Gehlen et al., 2002). Because
the EXAFS phenomenon occurs at a much faster time-scale than many processes
such as diffusion, exchange, sorption, precipitation and even electron transfer,
EXAFS can provide nearly instantaneous ‘snapshots’ of these reactions, especially
those involving chemically unstable intermediate minerals. Recent applications in-
clude assessing the structure of hydrated mineral surfaces (Eng et al., 2000), and that
of the diffuse electric double layer (Sturchio et al., 1997). Studies of the sorption of
heavy metals by phyllosilicates are summarised in Table 12.3.4. The interested reader
is referred to Hayes and Katz (1991), Schulze et al. (1999) and Fenter et al. (2002)
for comprehensive reviews on the application of various synchrotron-based tech-
niques (fluorescence, reflectivity, etc.) to study the reactivity of minerals and mineral
surfaces.
Table 12.3.4 shows that the application of P-EXAFS to the sorption of various
metals by smectites provided an insight into the types of complexes formed. The
examples described below illustrate that unambiguous interpretation of XAFS ex-
periments is very difficult. They also show the importance of a solid understanding of
the mineral phase involved.
A. Exchange/Sorption Reactions at the Smectite– Water Interface
The terms ‘outer-sphere’ (OS) and ‘inner-sphere’ (IS) are often used in the non-clay
mineral literature to describe two distinctly different sorption mechanisms. OS im-
plies an indirect electrostatic bonding where a coordination sphere surrounds the
sorbed species that is not directly bound to the surface other than by hydrogen
bonding or electrostatic attr action. Hydrated exchangeable cations occupying in-
terlayer sites in smectites are examples of OS sorption complexes. Although the
exchangeable cations have more affinity for the surface than the diffuse swarm of
ions in the electric double layer, both are considered OS sorption complexes because
the interaction is electrostatic. An IS sorption complex is one where direct coor-
dination bonding exists between the sorbed species and the surface, usually but not
necessarily through a surface ligand. The sorption of phosphate or citrate at the
12.3.5. XAFS Studies of Reactivity of Clays and Clay Minerals 829

(continued on next page)
Table 12.3.4. Summary of metals and metaloid ion sorption complexes at the phyllosilicate/solution interface studied by XAFS
spectroscopy. Individual references should be consulted for exact conditions of pH, ionic strength, metal concentration, surface
coverage and other experimental variables. See Brown and Sturchio (2002) for a more complete listing of sorption mechanisms
involving related minerals
Metal Layer silicate Complex reported Reference
Co(II) Kaolinite IS edge sites Charlet and Manceau (1994)
IS multinuclear, bidentate O’Day et al. (1994a, 1994b)
Chen and Hayes (1999)
OS low pH charge sites Chen and Hayes (1999),
Thompson et al. (1999, 2000)Co(II)Al LDH precipitate
Illite OS low pH charge sites Chen and Hayes (1999)
Co(II)Al LDH precipitate
Hectorite OS low pH charge sites Papelis and Hayes (1996) , Chen
and Hayes (1999)
Co(II)Al LDH precipitate Chen and Hayes (1999)
IS edge sites Schlegel et al. (1998, 1999a,
1999b)
Montmorillonite OS low pH, charge sites Papelis and Hayes (1996)
IS polynuclear (high pH)
Co(II)Al LDH precipitate
Sepiolite IS exchange site Fukushima and Okomoto (1987)
IS edge site Manceau and Decarreau (1988),
Charlet and Manceau ( 1994)
Ni(II) Kaolinite Ni(II)Al LDH precipitate Eick and Fendorf (1998)
Pyrophyllite Ni(II)Al LDH precipitate Scheidegger et al. (1996b, 1997,
1998), Elzinga and Sparks
(1999), Ford et al. (1999b),
Scheinost and Sparks (2000),
Scheckel et al. (2000)
Chapter 12.3: X-ray Absorption Spectroscopy830
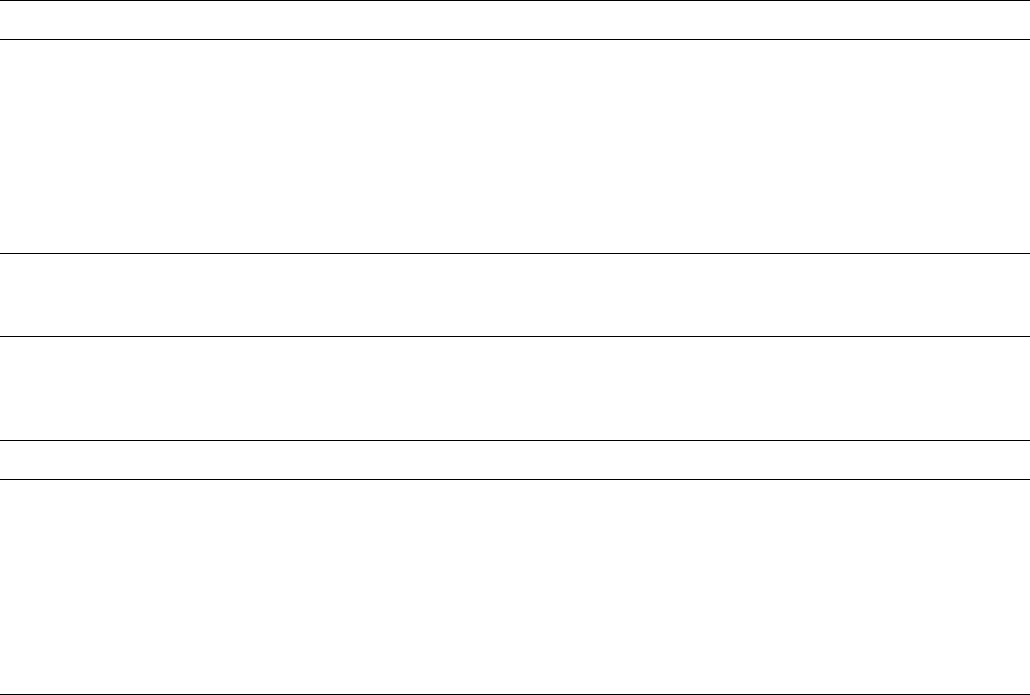
Table 12.3.4. (Continued )
Metal Layer silicate Complex reported Reference
Montmorillonite Ni(II)Al LDH precipitate Scheidegger et al. (1996, 1997,
1998)
IS edge sites Elzinga and Sparks (1999) ,
Da
¨
hn et al. (2001, 2002b, 2003)
Pyrophyllite Smectite mix Ni(II)Al LDH precipitate & IS
edge sites
Elzinga and Sparks (1999)
Talc a-Ni(OH)
2
precipitate Scheinost and Sparks (2000),
Scheckel et al. (2000)
Soil clay Ni(II)Al LDH precipitate Roberts et al. (1999)
Cu(II) Muscovite IS at defects Farquhar et al. (1996, 1997)
Biotite OS and IS Farquhar et al. (1997)
Montmorillonite OS & IS edge sites monodentate Morton et al. (2001)
Zn(II) Pyrophyllite Zn(II)Al LDH precipitate Ford and Sparks (2000)
Hectorite IS edge sites Manceau et al. (2000c) , Schlegel
et al. (2001a, 2001b)OS exchange sites
Schlegel et al. (2001b)
As(III) Kaolinite Oxidation to As(V) Foster et al. (1998b)
Sr(II) Kaolinite OS/IS edge sites (low
concentration)
Chen et al. (1998), Sahai et al.
(2000), O’Day et al. (2000b),
Parkman et al. (1998)
Illite OS Chen et al. (1998), Chen and
Hayes (1999)
Hectorite OS Chen et al. (1998), Chen and
Hayes (1999)
Montmorillonite OS Chen et al. (1998), Chen and
Hayes (1999)
(continued on next page)
12.3.5. XAFS Studies of Reactivity of Clays and Clay Minerals 831

Table 12.3.4. (Continued )
Metal Layer silicate Complex reported Reference
Cd(II) Muscovite OS Farquhar et al. (1997)
Biotite OS, IS Farquhar et al. (1997)
I(-I) Biotite IS (IO
3
sorbed) Fuhrmann et al. (1998)
Cs(I) Montmorillonite OS hydrated, interlayer Kemner et al. (1997)
Illite OS dehydrated, interlayer Kemner et al. (1997)
Phlogopite OS dehydrated, interlayer Kemner et al. (1997)
Pb(II) Montmorillonite OS low I, pHo6.5 Strawn and Sparks (1999)
OS/IS high I, pH>6.5
Th(IV) Montmorillonite IS bidentate (low concentration) Da
¨
hn et al. (2002a)
Th(OH)
2
precipitate (high
concentration)
U(VI) Kaolinite IS multiple sites Thompson et al. (1998)]
Montmorillonite OS interlayer (low pH) Sylwester et al. (2000a, 2000b)
IS neutral pH
Hydrobiotite OS interlayer, IS when dry Hudson et al. (1999)
Vermiculite OS interlayer, IS when dry Hudson et al. (1999)
Muscovite U(VI) hydroxide precipitate Moyes et al. (2000)
Chapter 12.3: X-ray Absorption Spectroscopy832
broken edges of smectit e particles would be an example of an IS sorption complex
since the anion forms a coordination bond with one or more oxygen atoms exposed
at the octahedral and tetrahedral edge site. IS sorption complexes can occur as
isolated units (mononuclear) or in clusters (multinuclear) as with polymers. IS com-
plexes can be mono-, bi-, tri-, tetra- or multidentate, implying the formation of 1, 2,
3, 4 or more bonds, respect ively, connecting the sorbed species with the surface.
Two other variants of OS and IS exist (i) ‘fixed’ cations in the (collapsed) in-
terlayers of illit es, micas, and some smectites are considered to be OS as the forces
holding the cation are electrostatic and physical in nature, and not covalent , even
though the fixed cation lost its waters of hydration; and (ii) layer substituted cations
which enter the structure either by exchange, diffusion or coprecipitation. Layer
substitution is considered different from simple IS sorption as it generally involves
multidentate bonding.
Table 12.3.4 illustrates that sorption complexes formed by transition metals at
smectite surfaces are highly dependent on experimental conditions. Generalisations
to conditions differing strongly from those reported should be made with care, but
trends can be discerned. For example, monovalent (e.g., Cs
+
) and certain divalent
cations (Co
2+
,Cd
2+
,Sr
2+
) form OS complexes in the interlayers under low pH or
low ionic strength (Papelis and Hayes, 1996; Farquhar et al., 1997; Kemner et al.,
1997; Chen and Hayes, 1999; Strawn and Sparks, 1999). The same divalent cations
may form IS sorption complexes at clay mineral edges under high pH or ionic
strength conditions (Papelis and Hayes, 1996; Farquhar et al., 1997; Kemner et al.,
1997; Schlegel et al., 1999a, 1999b; Strawn and Sparks, 1999). Other divalent cations,
such as Cu
2+
and Zn
2+
appear to form mostly IS sorption complex es, but were
shown to sorb as OS complexes for a short time (Farquhar et al., 1997; Morton et al.,
2001; Schlegel et al., 2001a, 2001b). Still other divalent cations such as Ni
2+
only
appear to form IS sorption complexes, regardless of pH and ionic strength. Indeed,
these metal ions also react strongly with layer silicates that have no permanent (pH-
independent) charge (Scheidegger et al., 1996a, 1996b, 1997, 1998). Finally, Th
2+
was shown to be IS at low electrolyte concentrations, but OS at high electrolyte
concentrations (Da
¨
hn et al., 2002b).
Interestingly, these same metals and metaloids behave differently with respect to
other mineral surfaces from what was described above for smectites. Thus, the fol-
lowing divalent metals generally form isolated IS complexes at low concentra tions,
but multinuclear IS complexes at higher sorption densities: Co(II) (Chisholm-Brause
et al., 1989a, 1990a, 1991; Towle et al., 1995, 1999a, 1999b), Pb(II) (Chisholm-Brause
et al., 1989b, 1990b; Roe et al., 1991; Trainor et al., 2002; Bargar et al., 2004), Cu(II)
(Weesner and Bleam, 1997; Xia et al., 1997; Cheah et al., 1998, 1999, 2000; Bochatay
et al., 1997) and Zn(II) (Trainor et al., 1999; Trivedi et al. 2001a, 2001b; Roberts et
al., 2003). The reasons for these differences in sorption complexes are probably as
varied as the number of studies. However, it appears that the greater the tendency
for a metal or metaloid to undergo hydrolysis, the greater its tendency to form IS
sorption complexes. Metal hydrolysis reactions are strongly de pendent on pH, thus
12.3.5. XAFS Studies of Reactivity of Clays and Clay Minerals 833
the formation of sorption complexes is sensitive to pH. The following two examples
of XAFS studi es of hydrolysing and non-hydrolysing cations at the surfaces of
smectite further illustrate the complexity of OS or IS sorption.
In an EXAFS study of Cs–Ca competition at exchange sites on montmorillonite
(SWy-1), illite (Silver Hill) and phlogopite mica, Kemner et al. (1997) found that at
Cs loadings of 100% of the cation exchange capacity (CEC), Cs L EXAFS spectra
showed limited long-range order. On the other hand, at low Cs loading s (e.g., 10%
CEC), the Cs environment showed strong long-range order up to 600 pm. The 10%
CEC loadings gave similar FT amplitudes of the atomic shells for illite and mica, but
3–4 times less intense for montmorillonite. The Cs L EXAFS FT for montmorillo-
nite with 10% CEC Cs also showed a shift of the FT peaks to higher R*. These
observations were interpreted in terms of Cs occupying interlayer sites for which the
extent of interlayer collapse increases in the sequence montmorillonite>illite>mica.
Although the authors did not venture beyond that explanation, it seems reasonable
to suggest that Cs occupies hydrated OS coordination environments in the inter-
layers of hydrated montmorillonite, but dehydrated OS (or fixed interlayer) sites in
illite and mica. (Note that Brown and Sturchio (2002) preferred to label fixed in-
terlayer sorption as IS, but in reality, the attraction is electrostatic and the immo-
bilisation of the cation between the smectitic layers is due primarily to steric
hindrance.)
What else might the Cs L EXAFS data tell us? Recall that both illite and mica have
a higher total layer charge as well as considerable Al
3+
for Si
4+
substitution in the
tetrahedral sheets as compared with montmorillonite. Thus, the enhanced structural
order of Cs at low CEC coverage, observed by Cs L EXAFS, is a function of the
probability of exchangeable Cs occurring near sites of tetrahedral substitution, where
a local site specificity may exist. Comparison of the layer charges (montmorillonite
XE0.75 about 25% of which is in the tetrahedral sheet; illite XE1.5 of which 67% is
tetrahedral; mica XE2 of which 67% is tetrahedral) indicates that tetrahedral-
to-total charge ratios agree well with the reported amplitude differences in the Cs L
EXAFS spectra (Kemner et al., 1997). The greater long-range order of Cs in illite and
mica are due to Cs residing between two adjacent layers within a distinct site, prob-
ably above the ditrigonal cavity near sites of Al
3+
substitution in the tetrahedral
sheet. With an anhydrous radius of 196 pm, Cs can partially sit within the ditrigonal
cavities of dioctahedral smectites (Gu
¨
ven, 1988). The high layer charge density, and
hence the high field strength at the basal surfaces, of illite and mica cause dehydration
of most monovalent cations occupying any interlayer exchange sites, and ultimately
interlayer collapse. Thus, the Cs L EXAFS results of Kemner et al. (1997) clearly
differentiate between a hydrated (in montmorillonite) and a dehydrated (in illite and
mica) exchangeable cation at low CEC coverage. This interpretation is in agreement
with previous NMR interpretations (Weiss et al., 1990). A worthwhile extension
would be to study the interlayer structures of La
3+
-orBa
2+
-smectites, the structures
of which, at least for vermiculites, were well characterised by conventional powder
X-ray transmission techniques (Telleria et al., 1977; Slade et al., 1998).
Chapter 12.3: X-ray Absorption Spectroscopy834
As a secon d example, Da
¨
hn et al. (2002a) studied the sorption of Th by mont-
morillonite at under- (10
–6
–10
–4
M, pH 2–3) and super-saturated (10
–5
–10
–4
M,
pH 5) conditions (with respect to ThO
2
). They precluded the formation of OS
(exchange) complexes by includ ing 0.1 M NaClO
4
. The Th K EXAFS of the su-
persaturated Th -mon tmorillonite most resemble d poorly crystalline Th(OH)
4
,but
at under-satur ated condi tion s, their spectral analysis was consistent with the for-
mation of IS edge-sharing bi dentate complexes. The analysis clearly s howed that (i)
IS complexes of Th formed at low electrolyte concentrations with Si making up the
neighbouring cation shell at R*E380–390 pm, and (ii) the Th–Si interatomic dis-
tance was consistent with edge-shar ing polyhedra. The results were also consistent
with those reported by O
¨
stols et al. (1997) for the sorption of Th by poorly crys-
talline Si. The lack of pleochroism, however, implied that Th did not form IS
complexes at the smec tite surface, and they thus prop osed that Th sorption com-
plexes at Si/Al edges were disordered with respect to the montmorillonite surface.
They discounted the possibili ty of Th sorbing to a poorly crystalline SiO
2
phas e
beca use the measured Si release rates wer e no t affected by the addi tion of Th.
However, they did report that solubl e Si increased continually throu ghout the ex-
periment , whereas soluble Al remained consta nt. This was taken as an indi cation
that silica precipi tation was unlikely under the conditi ons of their s tudy. One sourc e
of Si considered by Da
¨
hn et al. (2002a) was poorly crystalline Si O
2
, but it escaped
detection by XRD in the bulk montmorillonite (STx-1) used. Therefore, Da
¨
hn et al.
(2002a) preferred the interpretation that Th for med bidentate, edge-shari ng com -
plexes with Si and Al at the edges of montmorillonite that unde rwent incongruent
dissolution. Obviously, fur ther wo rk is required to decipher the a mbiguities of Th
sorption by mont morillonite.
Of the oxyanions (e.g., As(V), As(III), Se(VI), Se(IV) and U(VI)), only U(VI)
sorption by smectite (Chisholm-Brause et al., 1992; Dent et al., 1992; Chisholm -
Brause et al., 1994; Peterson et al., 1996; Giaquinta et al., 1997a, 1997b; Sylwester
et al., 2000; Ritherdon et al., 2003), hydrob iotite and vermiculite (Hudson et al.,
1999), muscovite mica (Moyes et al., 2000) and kaolin ( Thompson et al., 1998) was
studied. This is because swelling uranyl–clay complexes are potent photo-oxidisers
(Suib and Carrado, 1985; Duff et al., 1999). The aqueous uranyl oxyanion (UO
2
2+
)
forms OS complexes (exchangeable cation) at low pH. At pH 5–6, uranyl-hydroxide
species begin to form (Toth and Begun, 1981). The hydrolysis of UO
2
2+
at higher pH
results in the formation of IS bidentate complexes in the presence of competing ions
(Sylwester et al., 2000). At low pH the U L EXAFS spectra of U(VI)-montmorillo-
nite resemble those of aqueous uranyl (Chisholm -Brause et al., 1994; Peterson et al.,
1996; Giaquinta et al., 1997a, 1997b; Sylwester et al. 2000), but at higher pH (6.4) the
spectra more closely resemble U(VI) sorption complexes on silica or alumina (IS
bidentate complex) (Reich et al., 1998; Allard et al., 1999; Sylwester et al., 2000). The
composite evidence indicates that U(VI)-surface complexes are commonly formed
with montmorillonite and most minerals (Thom pson et al., 1997), although Duff
et al. (2002) showed that U(VI) sorption by goethite also involved layer substitution
12.3.5. XAFS Studies of Reactivity of Clays and Clay Minerals 835
