Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

3 5 7 9 11 13 15
-7.5
-3.5
0.5
4.5
VI
Fe, d Fe-O = 202 pm
IV
Fe, d Fe-O = 195 pmÅ
5%
IV
Fe, 95%
IV
Fe
20%
IV
Fe, 80%
IV
Fe
60%
IV
Fe, 40%
IV
Fe
80%
IV
Fe, 20%
IV
Fe
k
3
(Å
−1
)
6.5
3.5
0.5
-2.5
-5.5
3 5 7 9 11 13
Fe-O, Spokane nontronite
10%
IV
Fe, 90%
VI
Fe
15%
IV
Fe, 85%
VI
Fe
Wavevector k (Å
−1
)
Wavevector k (Å
−1
)
k
3
(k)
Fig. 12.3.9. Application of the FeyO contributions of powder Fe K EXAFS in determining
the tetrahedral Fe content of nontronites. (A) The amplitude and phase changes of the FeyO
wave vector as a function of mixing different amounts of
IV
Fe at d ¼ 195 pm to
VI
Fe at
d ¼ 202 pm. (B) Example least squares fit for the FeyO contribution of Spokane nontronite
assuming d ¼ 195 pm for tetrahedral Fe and d ¼ 202 pm for octahedral Fe. Modified after
Gates et al. (2002).
Chapter 12.3: X-ray Absorption Spectroscopy816
At the opposite extreme, the pre-edge amplitude of the ferruginous smectite SWa-1 is
enhanced, yet the other methods indicated that SWa-1 has nil tetrahedral Fe(III). In
this case, the enhanced amplitude is related to a high degree of distortion of Fe
octahedra caused by the presence of neighbouring Al and Mg octahedra. The dis-
tortion is sufficient to decrease the t
2g
-e
g
splitting, whi ch is better resolved than for
the NG-1, NAu-2 and Spokane nontronites (Fig. 12.3.3B). The South Australian
nontronite, NAu-1, also has an enhanced pre-edg e structure, but this enhancement
seems to be primarily due to its increased Al content compared with the Garfield
sample (Gates et al., 2002). Using other methods, Gates et al. (2002) found that
the Garfield an d NAu-1 nontronites may contain 2–3% of total Fe in tetrahedral
coordination, essentially at the detection limit of pre-edge XAFS spectroscopy
(Manceau and Gates, 1997).
Finally, the presence of different sites in layer structures affects the pre-edge
XAFS spectra. The pre-edge spectra of heat-treated Garfield nontronite (G500) is
shown in Fig. 12.3.3B. The pre-edge amplitude is enhanced, in this case due to the
presence of 30% five-fold coordinated Fe (
V
Fe) in G500. Additionally, the pre-
edge peak of G500 is broadened by as much as 30% of the non-heated samples. Heat
treatment causes dehydroxylation and migration of some M2 cations to M1 sites
(Drits et al., 1998b). The M1 site is slightly larger than the M2 site, so distortion is
introduced in a new average of interatomic angles and distances. The broadening of
G500 is 30% compared with that of the Spokane sample, reiterating just how
uniform the Fe(III) octahedra are in the end member nontronite.
XANES Spectroscopy
Ildefonse et al. (1994, 1998) could estimat e the amount of tetrahedral Al in layer
silicates by decomposition of the Al K XANES spectra (Fig. 12.3.4). By comparison
with a variety of reference minerals, they found that all Al-bearing layered structures
had three amplitude maxima in the near-edge structure related to Al coordination
environment. The first maxima near 1564 eV showed up as an inflection in the rising
edge of references containing no
IV
Al, but became well-resolved and quite pro-
nounced for layer silicates containing appreciable
IV
Al. The two peaks near 1566 and
1569 eV were related to
VI
Al. By decomposing the three peaks in the Al K XANES,
Ildefonse et al. (1994, 1998) estimated accurately the
IV
Al content of several mont-
morillonites and illite–smectite interstratifications. Thus, Al K XANES can be used
to determine tetrahedral Al content in smectites where such techniques as NMR fail
due to the presence of too much Fe (Gates et al., 1996).
The substitution of Ga for Al in synthetic kaolins and smectites was investigated
by Martin et al. (1998), using Ga K XANES, IR, electron microscopy and XRD
analyses. They found that Ga substitution in kaolins was limit ed presumably because
of the size difference between Ga and Al. When the ratio Ga/(Ga+Al) o0.1, Ga
incorporation into the kaolin struc ture was confirmed by an increase in the d(06-33)
spacing. At Ga=ðGa þ AlÞ40:1, kaolin-smectite and kaolin-oxide precipitates oc-
curred, but Ga was predominatly associated with the smectite pha ses as observed by
12.3.2. XAFS Studies on Smectite Structure 817
IR. Ga K XANES spectra of Ga-kaolin and Ga-smectite showed that Ga substituted
for Al in the octahedral sheet of kaolin, but in both octahedral and tetrahedral sites
in smectite, indicating the flexible nature of the 2:1 phyllosilicate structure.
For the Fe-bearing phyllosilicates, the form of the edge crest in the Fe K XANES
spectra pro vides information on oxidation state and degree of crystallinity (Gates
et al., 1997; Dyar et al., 2001). A shoulder within 4 eV above E
0
in the edge crest is
observed in reduced montmorillonite and nontronite, the resolution of which is
improved with increasing levels of Fe(II) (Gates et al., 1997). Similar features were
observed in the Fe K XANES spectra of biotite micas (Dyar et al., 2001) but here the
edge crests are better resolved due to greater crystallinity, and are probably related to
multiple-scattering contributions.
D. Octahedral Cation Distributions and Isomorpho us Substitutions in Smectites
In dioctahedral smectite structures, two types of octahedral cation arrays are
present. Most beidellites, ferruginous smectites, nontronites and illites are composed
of trans-vacant (centrosymmetric) octahedra. Here each (mostly) dival ent octahedral
cation occupies M2 sites, shares its two hydroxyl anions with one adjacent cation in
an M2 site along one edge (2OH edge), and two oxygen anions (2O edge) along edges
with two other M2 cations at 1201 (Fig. 12.3.10). Fe-poor smectites and mont-
morillonites have octahedral cations in both cis (M2) and trans (M1) sites (non-
centrosymmetric structure) (Drits et al., 1998b; Sains-Diaz et al., 2001). In the latter
structure, one M2 site is vacant, and any given octahedral cation (M1, M2) will share
two edges containing both O and OH (O,OH edge) and a 2O edge with adjacent
different (M2, M1) cations. In trioctahedral structures, where all octahedral sites are
filled with divalent cations, each M1 cation shares four O,OH edges and two 2O
edges with M2 cations. Each M2 cation shares one 2OH edge and two 2O edges with
other M2 cations, but two O,OH edges and a 2O edge with M1 cations. Such
distinctions are important as they affect the number of shells, and the number of
atoms within shells that are calculated based on the symmetry of the octahedra sheet
(Manceau et al., 1998; Da
¨
hn et al., 2003; Vantelon et al., 2003).
Table 12.3.3 displays the number of atoms in shells and the distances of the shells
from the absorber (Al in this case) for a hypothetical Ni-saturated and Ni-
substituted Texas montmorillo nite STx-1, as calculated by TKATOMS (Ravel,
1999) in two different symmetries. Note that more oxygen atoms within particular
shells are calculated for c2/m (trans vacant, centrosymmetric) symmetry than for the
correct cis-vacant c2 symmetry. Also, more atoms make up the tetrahedral shells,
which show spli tting due to the makeup of the octahedral sheet. Thus, while an
apparently subtle difference exists between cis- and trans-vacant structures, it can
have a large impact on the parameters used by EXAFS analysis programmes in
calculating scattering paths.
Chapter 12.3: X-ray Absorption Spectroscopy818
Ni Bearing Layer Silicates and Minerals
The occurrence of Ni in various layer silicates attracted considerable attention given
the industrial importance of these minerals as Ni ore sources. Manceau and Calas
(1985, 1986, 1987) and Decarreau et al. (1987) found that Ni associ ated with lateritic
smectite and other layer silicate structures always occurs in Ni-enriched clusters of at
least 2–3 nm diameter. Associated infrared studies (Ge
´
rard and Herbillon, 1983)
indicated a predominance of NiNiMg-OH over NiMgMg-OH absorption stretching
bands, suggesting that Ni substitutes within the octahedral layer near sites initially
having charge deficit.
M1
M1
M1
M2 M2
M2
M2
M2
M2 M1
M1
M1
M2 M2
M2
M2
M2
M2
V
V
V
M2 M2
M2
M2
M2
M2
V
V
V
M2 M2
M2
M2
M2
M2
M1
M1
M1
M2 V
M2
M2
V
V
M1
M1
M1
M2 V
M2
M2
V
V
A
B
C
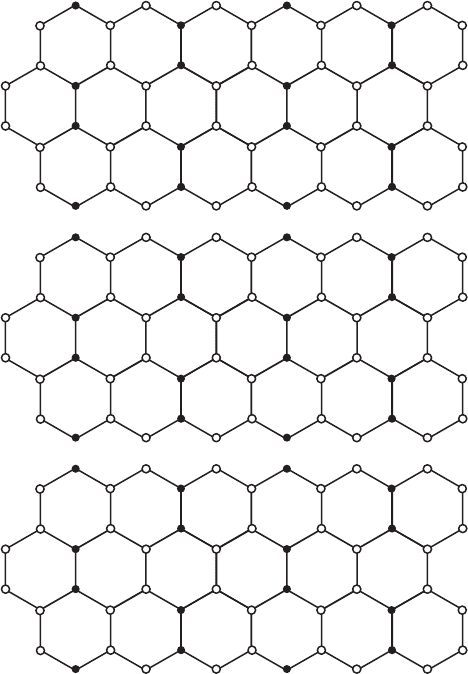
Fig. 12.3.10. Representation of different configurations of the octahedral sheets in diocta-
hedral smectites showing (A) the location of M1 and M2 sites in trioctahedral sheets and the
location of vacancies (V) in (B) trans vacant and (C) cis vacant, dioctahedral sheets. Solid
circles represent hydroxyls and hollow circles represent oxygens.
12.3.2. XAFS Studies on Smectite Structure 819

Ni Substitution in Montmorillonite Using the Hofmann– Klemen Effect
On heating Ni
2+
-saturated Camp Bertaux montmorillonite at 300 1C, Muller et al.
(1997) observed changes in the Ni K EXAFS spectra consistent with the formation
of NiyM(MQFe, Al, Mg) shells at 290 pm from the absorber (Fig. 12.3.11A).
Increases in the b parameter (d(06-33) diffraction peak) indicate that interlayer Ni
2+
migrates into vacant octahedral sites, presumably those closest to sites of Mg
2+
substitution where octahedr al charge is locat ed. The extraordinary amplitude of this
new shell in the EXAFS spectra clearly relates to the close proximity of Fe
3+
to the
substituted Ni
2+
(recall the p-difference in phase-sh ift between heavy and light at-
oms at similar interatomic distances). Muller et al. (1997) interpreted this finding in
terms of the preferential migration of Ni
2+
into vacant M2 (cis) octahedra between
Mg
2+
and pairs of Fe
3+
octahedra. From changes in the OH bending region of the
IR spectrum they furt her deduced that Fe–Mg clustering (Fig. 12.3.11B) occurred
within the octahedral sheet of the montmorillonite.
The IR spectrum of Camp Bertaux montmorillonite showed a band near
800 cm
–1
, assigned by Muller et al. (1997) to OH bending mode of pairs of OH-
sharing octahedral Fe (FeFe-OH). However, Gates (2005) attributed bands at
790–800 cm
–1
in the IR spectra of 32 dioctahedral smectites (including Wyoming
Table 12.3.3. Number of neighbours, N, and interatomic distances calculated for centro-
symmetric and non-centrosymmetric structures of a hypothetical Ni-saturated, Ni-substituted
montmorillonite using chemistry reported by Da
¨
hn et al. (2003) for the montmorillonite STx-1
and unit cell parameters (as determined by XRD) of a ¼ 518 pm, b ¼ 989 pm, and assuming
c* ¼ 967 pm (collapsed interlayer). Chemistry
and symmetry
y
were input into TKATOMS
(Ravel, 1999) enabling scattering paths to be determined by FEFF (Rehr, 2003). Only single
scattering events were taken to determine R
eff
in IFEFFIT (Newville, 2003)
Centrosymmetric (c2/m) Non-centrosymmetric (c2)
NR
eff
(pm) NR
eff
(pm)
NiyO
1
6 201 (2), 204 (2), 213 (2) 6 185 (2), 198 (2), 226 (2)
NiyOct
1
3 307 (2), 330 (1) 3 307 (2), 329 (1)
NiyTet
1
4 324(2), 331 (2) 4 244 (2), 389 (2)
NiyO
2
4 346 (2), 368 (2) 2 325
NiyO
3
6 393 (2), 403 (2), 406 (2) 4 353 (2), 378 (2)
NiyO
4
8 4.21 (2), 457 (2), 462 (2), 463 (2) 6 396 (2), 426 (2), 467 (2)
NiyTet
2
4 445 (2), 467 (2) 4 532 (4)
NiyInt 2 516 2 534
NiyOct
2
2 518 2 518
NiyTet
3
8 534 (2), 557 (2), 569 (2), 578 (2) 4 532
NiyOct
3
4 558 4 558
Al and Mg concentrations were combined for calculations. Ni was assumed to occupy 10% of M2 sites.
y
Al was taken at the origin of an M2 site for both c2 and c2/m symmetry.
Chapter 12.3: X-ray Absorption Spectroscopy820
montmorillonites) containing between 0 and 4
VI
Fe per unit cell, to FeMg-OH in-
dicating that OH sharing between Fe–Mg pairs is common. Since the chemical
composition and IR spectrum of Camp Bertaux montmorillonite are not dissimilar
from those of Wyomi ng montmorillonite, the OH bending band near 800 cm
–1
in the
IR spectr um of Muller et al. (1997) should probably be assigned to FeMg-OH. The
octahedral cation distributions in Camp Bertaux montmorillonite is thus likely to
look like that shown in Fig. 12.3.11C with Fe–Mg pairs as OH-sharing cations. This
model is consistent with the EXAFS analysis of Muller et al. (1997), and similar to
that proposed by Vantelon et al. (2003) for other comparable smectites.
The pioneering work by Muller et al. (1997) made available to clay science a
valuable new approach to quantifying octahedral occupancies in smectites. Although
limited to montmorillonites and those smectites where the layer charge is dominantly
located in the octahedral sheet, this method serves as a model for future researchers
who wish to improve their understanding of the distribution of octahedral cations in
dioctahedral smectites.
Octahedral Cation Distribution in Fe-rich Layer Silicates
The P-EXAFS study by Manceau et al. (1990a) on the distribution of Fe and Mg in
the octahedral sheet of biotite indicated that Fe–Mg clustering is also common
among biotites, in agreement with NMR data (Sanz and Stone, 1983). However,
P-EXAFS can provide additional information that is not attainable by NMR. The
amplitudes and phases of the FeyM
1
shell contribution in the reverse FT of Fe K
EXAFS spectra are nearly identical for biotite minerals with widely differing oc-
tahedral Fe/Mg ratios. This would indicate that in biotites the local environment
around Fe is the same regardless of chemical composition. In other words, Fe and
Mg are not randomly distributed (Manceau, 1990; Dyar et al., 2001).
Using powder XRD, Manceau et al. (2000a) showed that the octahedral sheets
of nontronite were trans-vacant (centrosymmetric) within 5% error. By assuming
complete centrosymmetric layer structures, the calculated (DVLS–FEFF) partial P-
EXAFS spectra were in excellent agreement with those extracted from experimental
spectra. A recent EXAFS study also indicated that a centrosymmetric layer structure
for the ferruginous smectite, SWa-1, was appropriate (Vantelon et al., 2003).
P-EXAFS spectra indicated that the distribution of octahed ral cations in SWa-1
was random with respect to FeyFe and Fey(Al,Mg) cation proxim ities (Manceau
et al., 2000a). This contrasted with probability determ inations by Madejova
´
et al.
(1994) using IR spectroscopy. They indicated that FeyFe and AlyMg pairings
were more likely at the expense of FeyAl and FeyMg pairings than would be
allowed by simple random distribution. In a centrosymme tric structure, the OH
stretching and bending bands (in the IR spectra) preferentially probe octahedral
cation pairing along the b cell dimension, whereas all directions within the octahedral
plane are probed by EXAFS. The EXAFS data ( Manceau et al. 2000a) can be
reconciled with IR data (Madejova
´
et al., 1994) by arranging the cations in such a
12.3.2. XAFS Studies on Smectite Structure 821

way that the octahedral chemical composition, IR absorbances and EXAFS data are
satisfied (Fig. 12.3.12).
Octahedral Cation Distributions in Fe-poor Smectites
Vantelon et al. (2001, 2003) applied IR an d powder Fe K EXAFS to the study of
octahedral occupancy in smectites with Fe content s ranging from 0.1 to 0.6
atoms per O
2
(OH)
4
. In order to acceptably fit their EXAFS data, they used cis-
vacant (no n-centrosymmetric) octahedral sheets for all Fe-poor smectite samples
(Table 12.3.3).
The results (Vantelon et al., 2003) would suggest a strong departure from random
distribution of cation ordering within the octahedral sheet. On the basis of their
EXAFS spectra the smectites may be classified into four distinct groups in agreement
with IR (Vantelon et al., 2001) and a host of previous analyses (e.g., Gu
¨
ven, 1988).
For Wyoming montmorillonites (SWy-1 and SWy-2) the octahedral cation distri-
bution indicated Fe–Fe avoidance. For the other smectites, the distribution indicated
varying degrees of Fe clustering, but not necessarily as OH-sharing pairs (as inferred
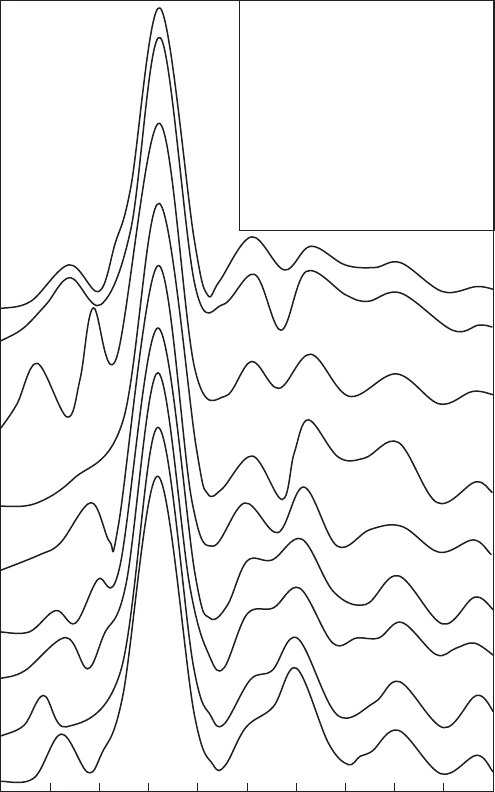
from IR). The FTs of the k
3
weighted Fe K EXAFS spectra revealed a splitting
of the second peak between 220 and 350 pm (Fig. 12.3.13 ). The FEFF-modelled
EXAFS spectra ( Vantelon et al., 2003) showed that splitting of the first cation shell
was due to the presence of ocahedral Al. Thus, splitting is strongly suppressed for
octahedral arrays where each Fe
3+
has as neighbours on average 2 other Fe
3+
and 1
Al
3+
(or Mg
2+
) cations, as for the ferruginous smectite SWa-1. With increasing
Al
3+
and Mg
2+
content the splitting increases, with a shoulder growing on the
shorter R* side of the FeyM
1
shell (Fig. 12.3.14). This splitting is a result of
interference from the p-phase difference between light (Mg, Al) and heavy (Fe)
backscattering atoms (Fig. 12.3.5 and Section 12.3.1E).
The models proposed by Vantelon et al. (2003) for the distribution of cations in
the octahedral sheets of Fe-poor smectites, are in general agreement with previous
studies indicating clustering of certain cations in smectites (Decarreau et al., 1987;
Muller et al., 1997). However, since FeMg-OH bands occur in the IR spectra of
many smectites, including Wyoming (SWy-2) (Madejova
´
et al., 1994) and Arizona
(SAz-1) (Gates, 2005) montmorillonites, the models of Vantelon et al. (2003) appear
to neglect OH-sharing Fe–Mg octahedra. In Fig. 12.3.15, the octahedral cation
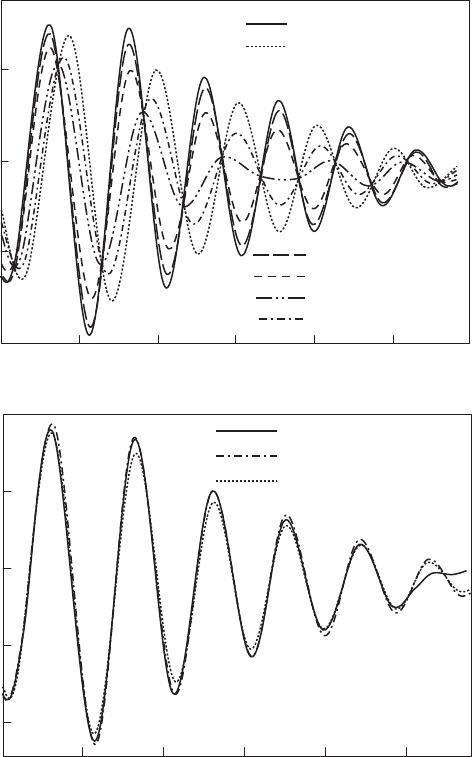
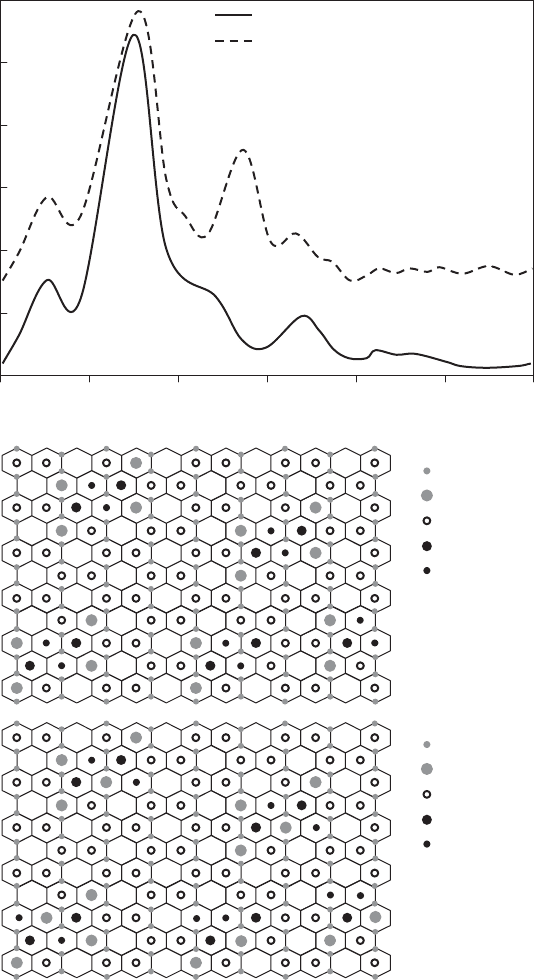
Fig. 12.3.11. (A) Powder Ni K EXAFS spectra of Ni-saturated Camp Bertaux montmorillo-
nite before and after heating to 250 1C, redrawn from Muller et al. (1997). Labels for atomic
shells from interlayer (unheated) or octahedral (heated) Ni derived from TkATOMS and
FEFF scattering calculations of montmorillonite with chemistry reported by Muller et al.
(1997). (B) Two-dimensional cation distribution in the octahedral sheet of Camp Bertaux
montmorillonite based on Muller et al. (1997). (C) Rearrangement of the octahedral cation
array based on re-assignment of the IR band near 800 cm
1
as FeMg-OH bending (Gates,
2004).
Chapter 12.3: X-ray Absorption Spectroscopy822
OH
Mg
Al
Ni
Fe
B
Ni
2+
-montmorillonite, unheated
Ni
2+
-montmorillonite, heated to 250 °C
Normalized Intensity
01
Distance (Å)
Ni-O
Ni-Fe,Ni
Ni-Si
Ni-O
Ni-O(water)
Ni-Si
Ni-Fe,
Si,Al
Ni-O
A
OH
Mg
Al
Ni
Fe
C
23456
12.3.2. XAFS Studies on Smectite Structure 823
distributions, deduced from EXAFS analysis (Vantelon et al., 2003), were modified
by taking the IR data into account (Gates, 2004).
Insightful studies such as those by Vantelon et al. (2003) have the potential to
provide important information on the variety of structures associated with smectites
that may be used to advantage in nanotechnology or other industrial or environ-
mental applications. An obvious and rich area of study is to extend such analyses
(incorportating P-EXAFS techniques) to smectites with compositions intermediate
between SWy-2 (5% Fe
2
O
3
) and SWa-1 (25% Fe
2
O
3
), and to correlate octahe-
dral compositions with geochemical information on formation and weathering of
smectites.
12.3.3. ORIENTATION OF INTERCALATED ORGANIC MOLECULES
Only limited use was made of synchrotron-based techniques to study the orien-
tation of intercalated organic species in layer silicates, and all analyses were carried
out with micas or related minerals (Ha
¨
hner et al. 1996a, 1996b; Lin et al., 1997;
Fischer et al., 1998; Brovelli et al., 1999; Fenter and Sturchio, 1999). In majority of
the cases, the alkyl and aromatic groups of organic molecules are oriented at high
angles with respect to the interlayer surface (Bedzyk and Cheng, 2002; Fenter, 2002).
This is partly due to the high (negative) layer charge, and partly to co-sorption of the
organic cation–inorganic anion (in the case of alkylammonium halides), giving rise
to a high packing density (Slade and Gates, 2004a).
Brovelli et al. (1999) used polarised C K NEXA FS spectroscopy to determine the
orientation of monolayers of octadecyltrimethylammonium (ODTMA) and di-
octadecyldimethylammonium (DODMA) cations on the external surface of mica
crystals (the layer charge, X, was unspecified but assumed to be 2e
–
per O
20
(OH)
4
).
For DODMA, a well-ordered self-assembled monolayer was observed with the alkyl
OH
Mg
Al
Fe
SWa-1
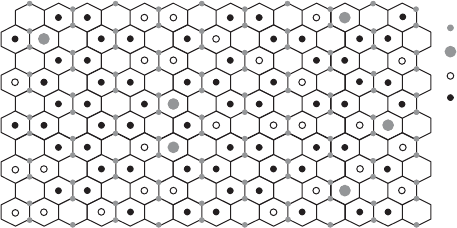
Fig. 12.3.12. Model for the two-dimensional cation distribution in the octahedral sheet of
SWa-1, based on Manceau et al. (2000a), but modified using updated chemical and IR data
(Gates et al., 2002; Gates, 2005).
Chapter 12.3: X-ray Absorption Spectroscopy824
1054
Bavaria
SWy-1
Milos
SWy-2
Georgie
North
Africa
SAz-1
STx-1
k
3
(k)
Fe per
Sample N
FeFe
SWy-1 0.47 0.0
SWy-2 0.45 0.0
China 0.10 0.4
STx-1 0.14 0.5
Georgie 0.36 0.5
Milos 0.46 0.7
SAz-1 0.17 0.9
North Africa 0.28 1.0
Bavaria 0.57 1.3
O
20
(OH)
4
China
32
R* (Å)
Fig. 12.3.13. Fourier transforms of k
3
weighted Fe K EXAFS spectra of Fe-bearing dioc-
tahedral smectites. Inset: the number of Fe atoms per O
20
(OH)
4
reported by Vantelon et al.
(2003) for each sample and the number of Fe neighbours in the FeyM
1
shell (N
FeFe
) de-
termined by least squares FEFF analysis. Modified and redrawn from Vantelon et al. (2003).
12.3.3. Orientation of Intercalated Organic Molecules 825
