Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.


macrocrystalline form s, notably the micas and vermiculites (see Chapter 2). A similar
concept was advocated by Weaver (1989) who suggested the term ‘physils’ for the
whole family of phyllosilicates (including palygorskite and sepiolite) irrespective of
grain size.
The JNCs have further proposed that non-p hyllosilicate minerals would qualify
as clay minerals if they impart plasticity to clay, and harden on drying or firing.
Table 1.3. Classification of non-planar hydrous phyllosilicates
Modulated
component
Linkage
configuration
Unit layer c sin
b value
Traditional
affiliation
Species
1:1 Minerals with modulated structures
Tetrahedral
sheet
Strips 0.7 nm Serpentine Antigorite, bemenitite
Islands 0.7 nm Serpentine Caryopilite,
ferropyrosmalite,
friedelite, greenalite,
manganpyrosmalite,
mcgillite, nelenite,
pyrosmalite, schallerite
Other None None
2:1 Minerals with modulated structures
Tetrahedral
sheet
Strips 0.95 nm Talc Minnesotaite
1.25 nm Mica Eggletonite,
ganophyllite
Islands 0.96–1.25 nm Mica/
complex
Ferristilpnomelane,
ferrostilpnomelane,
lennilenapeite,
parsettensite,
stilpnomelane,
zussmanite
Other 1.23 nm None Bannisterite
1.4 nm Chlorite Gonyerite
Octahedral sheet Strips 1.27–1.34 nm Pyribole Falcondoite,
loughlinite,
palygorskite, sepiolite,
yofortierite
1:1 Minerals with rolled and spheroidal structures
None Trioctahedral Serpentine Chrysotile, percoraite
Dioctahedral Kaolin Halloysite (nonplanar)
Source: Adapted from Martin et al. (1991).
1.3. Clay Mineral 7
1.4. DISTINCTION BETWEEN CLAY AND CLAY MINERAL
In commenting on the joint report, Moore (1996) has pointed out that ‘clay’ is
used as a mineral term (as well as a size term and a rock term). However, we would
agree with the JNCs that clay should not be used as a mineral term, and that a clear
distinction must be made between clay and clay mineral (Guggenheim and Martin,
1995). The position of the JNCs on using clay as a rock term is still indeterminate.
On the basis of past usage, we would agree with Moore (1996) that the term ‘clay’
can signify a rock, a sedimentary deposit, and the alteration (weathering) products of
primary silicate minerals. It is in one or other of these senses that the terms ‘ball
clay’, ‘fire clay’, ‘bentonite’, ‘bleaching earth’, and ‘fuller’s earth’ have been used in
the literature (Grim, 1962; Weaver and Pollard, 1973, Table 1.1).
The distinction between clay and clay mineral must always be kept in mind, as the
criteria mentioned above are different (Table 1.4). Nevertheless, the literature often
uses the term ‘clay’ for ‘clay mineral’ because the former is shorter and less cum-
bersome. This handbook is primarily concerned with clay minerals rather than clays.
1.5. CLAY MINERAL PROPERTIES
Clay minerals are characterized by certain properties, including
1. a layer structure with one dimension in the nanometer range; the thickness of the
1:1 (TO) layer is about 0.7 nm, and that of the 2:1 (TOT) layer is about 1 nm,
2. the anisotropy of the layers or particles,
3. the existence of several types of surfaces: external basal (planar) and edge surfaces
as well as internal (interlayer) surfaces (e.g., Annabi-Bergaya et al., 1979),
4. the ease with which the external, and often also the internal, surface can be
modified (by adsorption, ion exchange, or grafting),
5. plasticity, and
6. hardening on drying or firing; this applies to most (but not all) clay minerals.
Table 1.4. Distinction between clay and clay mineral
Clay Clay mineral
Natural Natural and synthetic
Fine-grained (o2 mmoro4 mm) No size criterion
Phyllosilicates as principal constituents May include non-phyllosilicates
Plastic
a
Plastic
Hardens on drying or firing Hardens on drying or firing
a
With some exceptions like flint clays.
Chapter 1: Clays, Clay Minerals, and Clay Science8

Many people associate clay minerals with smectites, which have the following
properties:
particles of colloidal size,
high degree of layer stacking disorder,
high specific surface area,
moderate layer charge (Table 1.2),
large cation exchange capacity that is little dependent on ambient pH,
small pH-dependent anion exchange c apacity,
variable interlayer separation, depending on ambient humidity,
propensity for intercalating
2
extraneous substance s, including organic compounds
and macromolecules, and
ability of some members (e.g., Li
+
- and Na
+
-exchanged forms) to show extensive
interlayer swelling in water; under optimum conditions, the layers can completely
dissociate (dela minate).
1.6. ASSOCIATED MINERALS
The clay fraction of soils and sedim ents (having particles of either o2 mmor
o4 mm) often contain s non-phyll osilicate minerals, such as carbonates, feldspars,
and quartz together with the (hydr)oxides of iron and aluminium. Since these min-
erals do not impar t plasticity to clay, they have been referred to as ‘non-clay con-
stituents’ or ‘accessory minerals’ (Greenland and Hayes, 1978; Brown, 1980; Hall,
1987). However, following the recommendation of the JNCs (Guggenheim and
Martin, 1995), we would prefer to call them ‘associated minerals’, a detailed de-
scription of which has been given by Dixon and Schulze (2002). As the name sug-
gests, these minerals are closely associated with the phyllosilicate component of clay,
and hence interfere with its identification. The presence of associated minerals in
natural clay deposits also reduces the commercial value of the resource. For these
reasons, much effort has been expended in purifying raw clays and in synthesizing
‘pure’ clay minerals. Although associated minerals exert a strong influence on the
behaviour of soil and sediment (Dixon and Weed, 198 9 ), a discussion of their nature
and properties lies beyond the scope of the present book.
1.7. ASSOCIATED PHASES
Non-crystalline or X-ray amorphous materials (sometimes also considered as
gels), including organic matter, are referred to by the JNCs as ‘associat ed phases’
whether or not they impart plasticity to clay. Accordingly, allophane would qualify
2
The term ‘intercalation’ denotes both interlayer adsorption and interlayer ion exchange reactions.
1.6. Associated Minerals 9
as an associated phase since this group of hydrated aluminosilicates lacks long-range
order; that is, its structural regularity does not extend much beyond 1nm (Parfitt,
1990). Nevertheless, we propose that allophane be regarded as a clay mineral because
of past usage. The same applies to imogolite, an aluminosilicate with long-range
order in one direction (Wada, 1989). Chapter 2 gives more detail about allophane
and imogolite.
1.8. OTHER SOLIDS WITH SIMILAR PROPERTIES
In this context, we should mention the existence of other types of solids that have
similar properties to the phyllosilicates in terms of layer structure, charge charac-
teristics, and potential for intercalation but are not components of clay. In other
words, these layered materials are neither associated minerals nor associated phases
of the fine-grained fraction of soil and sediment.
Some notable examples are the alkali silicates, crystalline silicic acids, niobates,
phosphates, titanates, and LDH (Lagaly and Beneke, 1991; Schwieger and Lag aly,
2004). Only LDH are included in this handbook (Chapter 13.1). This is partly
because of space limitation, and partly because LDH have been referred to as ‘an-
ionic clays’. Further, LDH have attracted a great deal of attention for their actual
and potential applications in catalysis and environmental protection (Newman and
Jones, 1998; Tichit and Vaccari, 1998; Basile et al., 2001).
According to the definition of ‘clay’, proposed by the JNCs (Guggenheim and
Martin, 1995), LDH should not be considered as clay because of their synthetic
origin. On the other hand, hydrotalcite, the naturally occurring analogue of LDH,
would clearly qualify as clay. The term ‘anionic clays’ may also cause confusion since
the layers of LDH are positively charged, and hence are cationic (the anionic de s-
ignation being a reference to the anion exchange property of LDH). Another name
for LDH, used in the literature, is ‘hydrotalcite- like compounds’ but this term seems
less descriptive and more cumbersome than ‘layered double hydroxides’. Similarly,
the term ‘cationic clays’ to denote the (conventional) phy llosilicates with a negative
layer charge (Tables 1.2 and 1.3) is not advocated. As already mentioned, the term
‘clay mineral’, as defined by the JNCs (Guggenheim and Martin, 1995), may en-
compass non-phyllosilicates (if they impart plasticity to clay). This would open the
way for the inclus ion of natural and synthetic minerals, such as LDH in the clay
mineral family although synthetic LDH are not part of common clay material. This
issue needs to be clarified (and resolved).
Clay minerals are often confused with zeolites because they tend to occur together
and share many attributes (Chapter 13.2). Both groups of materials can be synthe-
sized, modified, and tailored by chemical, physical, and thermal treatments.
Other materials with properties related to phyllosilicates, notably smectites, are
cement and concrete (Chapter 13.3).
Chapter 1: Clays, Clay Minerals, and Clay Science10

1.9. CLAY MINERAL PARTICLES AND AGGREGATES
According the JNCs definition ‘clay minerals’ may be eithe r natural or synthetic,
phyllosilicates or non-phyllosilicates, and have no size connotation. The structures of
phyllosilicates are all based on a tetrahedral (T) and an octahedral (O) sheet that
may condense in eithe r a 1:1 or 2:1 proportion to form an anisotropic TO or TOT
layer. The structure of LDH (‘anionic clay minerals’) is based on an octahedral sheet
(O). The layers or sheets may be negatively charged (as for the majority of clay
minerals), positively charged (as in LDH), or essentially uncharged (as in talc
and pyrophyllite; Table 1.2). The layer charge density and the nature of the co m-
pensating (charge-balancing) cation determine many important surface and colloidal
properties.
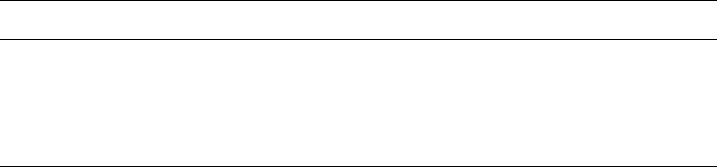
For simplicity sake, we refer to an assembly of layers as a ‘particle’,
3
and an
assembly of particles as an ‘aggregate’. Accordingly we may distinguish between
interlayer, interparticle, and interaggregate pore s (Fig. 1.1). The arrangement of the
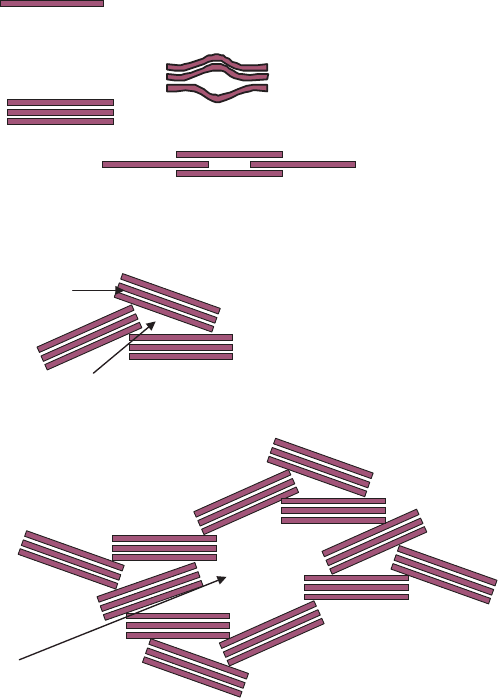
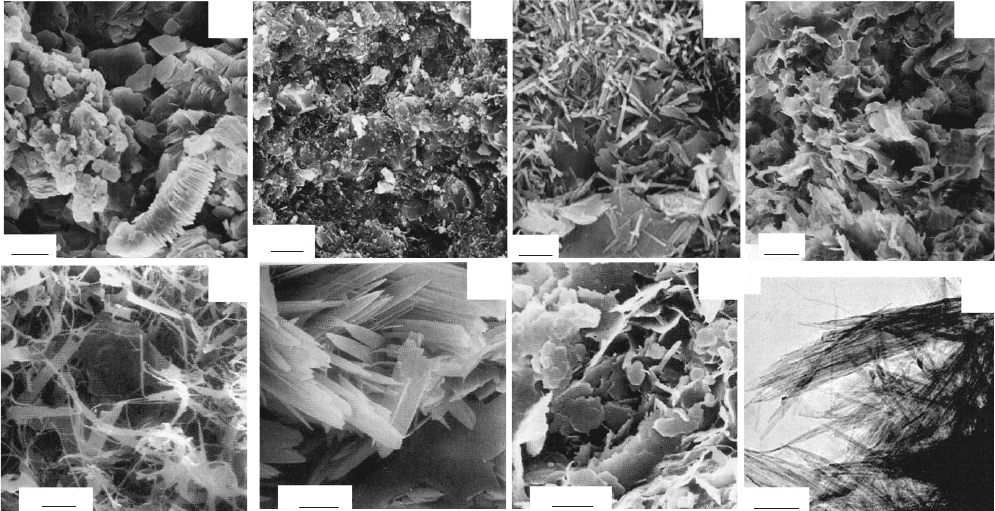
particles or aggregates leads to different morphologies, such as plates, tubules, laths,
and fibres (Fig. 1.2). All phyllosilicates are therefore porous, containing pores of
varied size and shape.
1.10. CLAY MINERALS AND ENVIRONMENT
As clay scientists, we are inter ested not only in the material itself but also in its
interaction with extraneous substances (i.e., in the environment). This interaction is
influenced by many factors, such as the acid–basic character (pH and ionic strength),
and thermodynamic conditions (pressure and temperature) of the surrounding me-
dium. As we need to know the properties of the clay mineral in question, and those
of the interacting compound (water, polymer, iron, organic/inor ganic molecule),
research into the clay–environment interaction requires collaboration between sev-
eral disciplines. Moreover, the interaction between clay minerals and other com-
pounds in the environment can occur at different states of matter (solid, melted solid,
liquid, gas, and plasma). Its study would therefore involve the classical disciplines of
solid/liquid physics and chemistry as well as the developing disciplines of melted- and
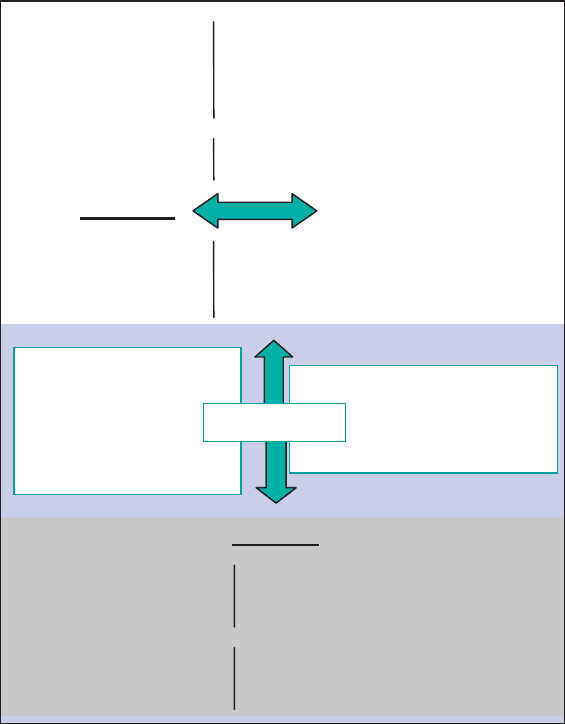
plasma-state science. Fig. 1.3 shows the variety of parameters influencing the clay
mineral–compound interaction, and the importance of relating ‘microscopic’ prop-
erties to ‘macroscopic’ behaviour.
3
In the clay science literature the term ‘particle’, as defined here, is often referred to as ‘crystallite’ (or
even ‘crystal’). For consistency, we have used ‘particle’ throughout the handbook.
1.9. Clay Mineral Particles and Aggregates 11
1.11. ALTERNATIVE CONCEPTS OF CLAY MINERALS
Clay minerals are traditionally classified under ‘silicates’ but since their formulae
(chemical compositions) have more oxygen than Si, Al, or Mg (Table 1.5), these
minerals may arguably be considered as (hydr)oxides of silicon, aluminium, or
magnesium. By the same token, LDH may be regarded as (hydr)oxides of metal ions.
Particle
Interparticle
space
Interlayer
space
Aggregate
Layer
Interaggregate
s
p
ace (
p
ore)
Assembly of Aggregates
A
B
C
D
Fig. 1.1. Diagram showing (A) a clay mineral layer; (B) a particle, made up of stacked layers;
layer translation and deformation can give rise to a lenticular pore; (C) an aggregate, showing
an interlayer space and an interparticle space; and (D) an assembly of aggregates, enclosing an
interaggregate space (pore).
Chapter 1: Clays, Clay Minerals, and Clay Science12
ABCD
E
F
G
H
2 µm
4 µm
1 µm
4 µm
4 µm
10 µm 10 µm 0.2 µm
Fig. 1.2. Transmission electron micrographs of some clay minerals with varied particle morphology: (A) kaolinite from Sasso (Italy)
showing typical books of particles; (B) high-quality flint clay from Gasconade County, Missouri, USA; (C) tubular halloysite particles
alongside kaolinite plates from Sasso, Italy; (D) smectite or illite/smectite from Sasso, Italy; (E) filamentous illite from sandstones in
offshore Netherlands; (F) lath-shaped illite from sandstones in offshore Netherlands; (G) pseudo-hexagonal illite particles from
sandstones in offshore Netherlands; (H) fibrous palygorskite from Southern Georgia (USA). (A), (C), and (D) are taken from
Lombardi et al., (1987); (B) is taken from Keller and Stevens (1983); E, F, and G are taken from Lanson et al., (2002); and (H) is taken
from Krekeler (2004).
1.11. Alternative Concepts of Clay Minerals 13
Thus clay minerals and related layer materials (see Section 1.8) may be referred to as
porous layered (hydr)oxides.
Clay minerals may also be considered as salts of rigid (two-dimensional) poly-
anions with infinite radius and their compensating (exchangeable) cations (Fig. 1.3),
or as inorganic polymers where the continuous octahedral sheet is based on a rep-
etition of an octahedron monomer. The other basic monomer is the silica tetrahe-
dron, which is bound on one or both sides to the octahedral sheet.
(thermodynamic conditions)
temperature, pressure
pH, ionic strength
(acid-base character)
clay minerals adsorb all species
neutral, charged,
inorganic, organic, and polymeric
Clay Mineral and Environment
layer ~ macroanion
layer: 1:1 (TO), 2:1 (TOT)
octahedral sheet: di-or tri-octahedral
nature of cations and anions of the octahedral sheet
distribution of the octahedral cations
substitution in the octahedral (O) and/or
tetrahedral (T) sheet
isolated layers, particles, aggregates
morphology (plates, laths, discs, fibres)
clay mineral
salt =
compensating cation
size and valency
electronegativity
hydration energy and coordination
acidity of water bound to the cations
environment
water, salt solutions,
polar and non-polar organic solvents
inorganic or organic
(in dispersion, in melted state,
or in solid-solid interaction)
liquid phases (or gas)
other solid phases
interaction
rigid polyanion +
compensating cation
Fig. 1.3. Diagram showing the interactions of clay minerals with the environment.
Chapter 1: Clays, Clay Minerals, and Clay Science14

Table 1.5. Layer charge and idealized formulae (chemical compositions) of some represent-
ative 1:1 and 2:1 clay minerals
Charge/
formula unit
Dioctahedral species Trioctahedral species
Serpentine-kaolin group
0 Kaolinite Serpentine
(Si
2
)
IV
(Al
2
)
VI
O
5
(OH)
4
(Si
2
)
IV
(Mg
3
)
VI
O
5
(OH)
4
Talc-pyrophyllite group
0 Pyrophyllite Talc
(Si
4
)
IV
(Al
2
)
VI
O
10
(OH)
2
(Si
4
)
IV
(Mg
3
)
VI
O
10
(OH)
2
Smectite group
0.20.6 Montmorillonite Hectorite
(Si
4
)
IV
(Al
2y
Mg
y
)
VI
O
10
(OH)
2
,
yM
+
.nH
2
O
(Si
4
)
IV
(Mg
3y
Li
y
)
VI
O
10
(OH)
2
,
yM
+
.nH
2
O
Beidellite Saponite
(Si
4x
Al
x
)
IV
(Al
2
)
VI
O
10
(OH)
2
,
xM
+
.nH
2
O
(Si
4x
Al
x
)
IV
(Mg
3
)
VI
O
10
(OH)
2
,
xM
+
.nH
2
O
Vermiculite group
0.60.9 Vermiculite Vermiculite
(Si
4x
Al
x
)
IV
(Al
2y
Mg
y
)
VI
O
10
(OH)
2
,(x+y)M
+
(Si
4x
Al
x
)
IV
(Mg
3y
M
y
3+
)
VI
O
10
(OH)
2
,(xy)/2 Mg
2+
True (flexible) mica group
a
0.91.0 Celadonite Lepidolite
(Si
4x
Al
x
)
IV
(Fe
2y
Mg
y
)
VI
O
10
(OH)
2
,(x+y)K
+
(Si
4x
Al
x
)
IV
(Mg
3y
Li
y
)
VI
O
10
(OH)
2
,(x+y)K
+
Muscovite Phlogopite
(Si
3
Al)
IV
(Al
2
)
VI
O
10
(OH)
2
,K
+
(Si
3
Al)
IV
(Mg
3
)
VI
O
10
(OH)
2
,K
+
Brittle mica group
2.0 Margarite Clintonite
(Si
2
Al
2
)
IV
(Al
2
)
VI
O
10
(OH)
2
,Ca
2+
(Si Al
3
)
IV
(Mg
2
Al)
VI
O
10
(OH)
2
,
Ca
2+
a
Illite is the subject of controversy in the literature. In Table 1.2, illite is classified as a species in the true
mica group. However, illite is also considered to be a separate group of clay-size, dioctahedral mica-type
minerals (Meunier and Velde, 2004). The general formula for illite group may be written as
(Si
4x
Al
x
)
IV
(Al
2y
Mg
y
)
VI
O
10
(OH)
2
,(x+y)K
+
with a charge of 0.9 per O
10
(OH)
2
, although the ex-
istence of Na
+
illite (brammalite) (Bannister, 1943) and NH
4
+
illite (tobellite) (S
ˇ
ucha et al., 1994) has
been documented.
1.11. Alternative Concepts of Clay Minerals 15
These concepts may promote clay science by attracting an increasing number of
chemists and physicists besides the large number of earth and soil scient ists, as is
traditionally the case.
1.12. CLAY SCIENCE
Clay minerals are probably unique in the sense that these materials are studied by,
and used in, many disciplines for fundamental and applied research. The multi-
disciplinary approach is at the frontier between materials science and colloid science,
while the multi-scale approach linking nano-, micro- and macro-scale studies is a
challenge for the futur e of clay science.
Studying the same sample by several techniques simultaneously (Annabi-Bergaya
et al., 1981, 1996) is a method that is seldom followed by researchers. We would
recommend this approach in future investigations as it can provide valuable and
insightful information.
Clay resear ch is being actively pur sued by many people and in many countri es,
and the future of clay science looks bright, exciting, and promising.
1.13. CONCLUDING REMARKS
The aim of this handbook is to attract the attention of clay scientists in academe
and industry as well as in politics (as research needs funding), and to stress the
importance of clay science to society and the quality of life. The economic benefits
seem evident because clays are abundant, widespread, and inexpensive compared
with other raw materials.
The table of contents of this handbook indicates the industrial and environmental
importance of clays and clay minerals. The great variety of physical, chemi cal, and
thermal treatments that may be used to modify clays and clay minerals provide
unlimited scope for future applications, particularly in terms of protecting our en-
vironment.
Because of the multi-disciplinary nature of clay science, its teaching is another
challenging task. By learning about the miner alogical, phy sico-chemical, and indus-
trial aspects of clay science, students would not only gain an appreciation of the
‘scientific method’ and the physical environment but also find suitable employment
and a fulfilling career.
REFERENCES
Annabi-Bergaya, F., Cruz, M.I., Gatineau, L., Fripiat, J.J., 1979. Adsorption of alcohols by
smectites. I. Distinction between internal and external surfaces. Clay Minerals 14, 249–258.
Chapter 1: Clays, Clay Minerals, and Clay Science16
