Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

hydration/dehydration behaviour is a function of the interlayer cation, with both the
nature of the ‘‘steps’’ and the hysteresis changing as the interlayer cation changes.
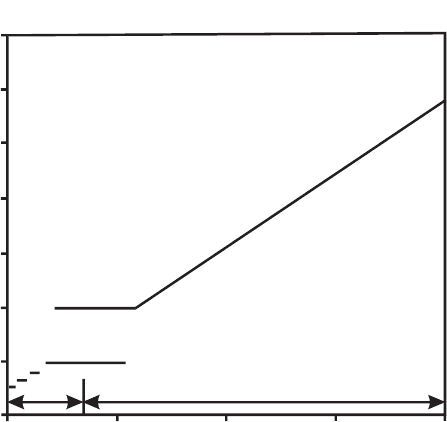
At higher water contents, smectites continue to expand, transforming from a
region where approxim ately integral layers of H
2
O occup y the interlayer space to the
so-called osmotic swelling region (Fig. 13.2.5) (Chapter 5.2). This behaviour stands
in stark contrast to the behaviour exhibited by zeolites, whose structural expa nsion is
limited by their three-dimensional framework structure. The low-layer charge and
weak interlayer bonding in smectites make it possible for the material to delaminate
completely. Both the layer-by-layer expansion and osmotic swelling of smectites are
very important in numerous applications.
The T- or P(H
2
O)-induced structural transitions in zeolites are generally regular
and reversible as a result of the more homogeneous distribution of H
2
O molecule
attraction energies. Although these transitions in zeolites generally do not display
stepwise behaviour, cati on–water interactions and migrations during changes in hy-
dration state can give rise to quantised behaviour. Fortunately, because zeolites are
three-dimensionally ordered, it has been possible to obtain detailed crystal structure
information that is generally not available for smectites. Such structural studies show
that H
2
O molecules in extraframework cavities are bonded to each other, to extra-
framework cations, and/or to the framework, depending on the degree of hydration.
14
12
6
10
2
0
01234
4
8
region 2 (osmotic swelling)
Region 1
g H
2
O / g Na
+
-montmorillonite
interplanar spacing (nm)
Fig. 13.2.5. Interplanar spacing of Na-montmorillonite as a function of water content (mod-
ified from Norrish (1972)).
Chapter 13.2: Parallels and Distinctions Between Clay Minerals and Zeolites1102
T- or P(H
2
O)-induced structural transitions result from a gain or loss of water, but
are also affected by migration of H
2
O molecules (Armbru ster and Gunter, 2001).
These transitions are generally reversible (except at high temperatures where irre-
versible structural changes take place), and hysteretic behaviour is rare.
The structural effects of zeolite hydration and dehydration are easily studied using
powder or single-crystal X-ray diffraction, and the unit-cell parameters often provide
useful insight into the hydration/dehydration process. Such X-ray diffraction data,
combined with thermal analytical data, have shown that the most important variable
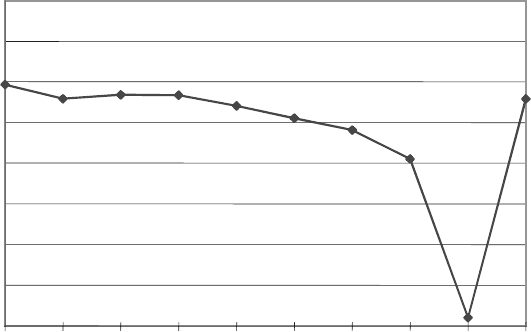
controlling the reaction is the vapour pressure of water. Fig. 13.2.6 illustrates the
behaviour of the clinoptilolite unit-cell volume as a function of RH, going from
water-saturated conditions to 0% RH, and back to 100% RH. These data show the
large volumetric changes occurring simply by changing the vap our pressure of water.
They also demonstrate that the dehydration reaction, eviden ced in the reduction of
unit-cell volume as the RH goes to zero, is reversible as the RH is returned to 100%.
Although seemingly of only academic interest, this P(H
2
O)-induced structural
transition in clinoptilolite is of importance in a variety of applications, including in
the proposed high-level radioactive waste repository at Yucca Mountain, Nevada,
USA. The rocks near the proposed waste repository level consist of both devitrified
volcanic tuff (Topopah Spring Member, primarily alkali feldspar and silica minerals
27 hr. wet
3 hr. wet
75 hr. wet
100%RH
80%RH
60%RH
40%RH
20%RH
0%RH
100%RH
2.120
2.110
2.105
2.100
2.095
2.085
2.090
2.080
2.115
unit cell volume (nm
3
)
conditions
Fig. 13.2.6. Unit-cell volume of Ca-exchanged clinoptilolite from Fish Creek Mountains,
Nevada, USA, as a function of humidity (from Bish and Carey, 2000). ‘‘3 hr wet’’ and ‘‘27 hr
wet’’ samples were wet with liquid water, and the ‘‘75 hr wet’’ contained little, if any, liquid
water.
13.2.3. Crystal Structural Transitions 1103
with minor smectite) and zeolitised volcanic tuff (Calico Hil ls Tuff, primarily
clinoptilolite with lesser amounts of feldspar and silica minerals). Fig. 13.2.7 illus-
trates the axial stress generated by expansion of 2.54 cm diameter cores of these two
rock types. The cores had been previously vacuum dried, without heating, and were
immersed in liquid water for this experiment. The contrast in behaviour of the
devitrified tuff (Topopah Spring) and the zeolite-bearing tuff (Calico Hills) is re-
markable. The Topopah Spring tuff generated a small (o1 MPa) stress, likely due to
hydration of the small amount of smectite in the rock. However, the clinoptilolite-
rich Calico Hills tuff generated an axial stress >10 MPa. One need only consider
that the tensile strength of the Calico Hills Tuff is 5–7 MPa to conclude that de-
hydration-induced tensile cracking is possible (as the hydration-induced volumetric-
expansion stress is greater than the strength of the rocks).
Similar X-ray diffraction experiments on clinoptilolite, mordenite, and erionite,
among others, have shown no evidence of hysteresis in the T- or P(H
2
O)-induced
structural transitions. However, some zeolites show an intriguing mixture of differ-
ent T- and P(H
2
O)-induced structural transitions. Fridriksson et al. (2003) showed
that the zeolite laumontite, Ca
4
[Al
8
Si
16
O
48
]16H
2
O, experiences structural changes
at partial pressures of water below 22 mbar that are similar to those exhibited by
most other zeolites. This behaviour is illustrated in Fig. 13.2.8, where the unit-cell
volumes measured from near 0 to 22 mbar P(H
2
O) are reversible and no hysteresis
is evident. However, Fridriksson et al. (2003a, 2003b) noted an abrupt change in
unit-cell volume between 24 and 29 mbar, followed by gradual and continuous
behaviour above this water vapour pressure. Both the abrupt nature of this change,
and the hysteresis obvious between the hydration and dehydration data are exciting
11
10
9
8
7
6
5
4
3
2
1
0
0 1020 3040506070 8090100
TOPOPAH SPRING TUFF (TSw2)
ZEOLITIZED CALICO HILLS TUFF (CHnz)
stress generated (MPa)
time immersed in water (hours)
Fig. 13.2.7. Axial stress generated against fixed platens (axially confined) in unjacketed cy-
lindrical samples after vacuum drying and immersion in water (Kranz et al., 1989).
Chapter 13.2: Parallels and Distinctions Between Clay Minerals and Zeolites1104
evidence of a first-order phase transition in laumontite. These data illustrate that
cation–H
2
O interactions in zeolite extraframework sites are dynamic, even under
room conditions of temperature and RH.
13.2.4. DEHYDRATION PHENOMENA
For the most part, smectite hydration and dehydration mirror the structural
transitions documented above. Gravimetric experiments are the most common
method of studyi ng hydration and dehydration, and they usually show pronounced
hysteresis between the hydration and dehydration loops. Interestingly, the quantised
behaviour apparent in structural transitions is less pronounced in gravimetric results
(Fig. 13.2.9), but hysteresis is obvious. The gravimetric data in Fig. 13.2.9 show that
the hydration reactions are reversible, and the amount of water taken up by the
smectite depends on the nature of the interlayer cation. Ca
2+
, with its high hydration
energy, takes up the most interlayer water whereas K
+
with its low hydration energy
takes up the least of the three. Note also that at close to 0% RH both K
+
- and Na
+
-
smectites contain a small but finite amount of water whereas the Ca
2+
-smectite
contains 6.5% H
2
O. The fact that all of these smectites retain measurable H
2
O
under low-P(H
2
O) conditions is a reflection of the fact that much of the H
2
O in these
minerals is held with energies higher than in liquid water. Analysis of the data of
Dios Cancela et al. (1997) allows derivation of the partial molar enthalpies of hy-
dration for Na
+
-andCa
2+
-smectites, shown in Fig. 13.2.10. This figure shows the
stepwise hy dration behaviour noted above and demonstrates that H
2
O in smectite is
010203040
1.320
1.340
1.360
1.380
1.400
Hydration
Dehydration
volume (nm
3
)
P
H
2
O
(mbar)
Rietveld refinement
Fig. 13.2.8. Laumontite unit-cell volume as a function of P(H
2
O) at 27.01–29.3 1C. Triangles
represent data from Rietveld refinements, and circles and diamonds represent data from
hydration and dehydration experiments, respectively. Solid lines connect coexisting laumontite
phases during hydration (28–29 mbar) and dehydration (24–25 mbar). From (Fridriksson
et al., 2003).
13.2.4. Dehydration Phenomena 1105
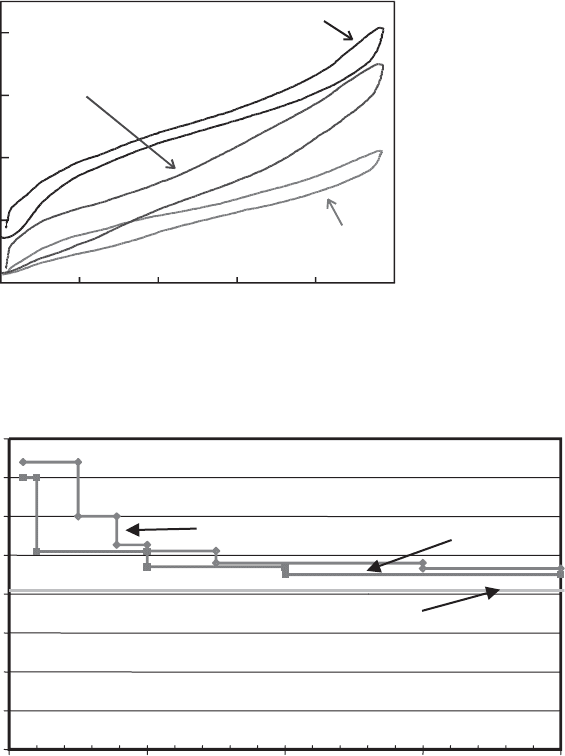
held increasingly strongly as the amount of interlayer H
2
O decreases. Note that
interlayer H
2
O in partially dehydrated smectite has a hydration enthalpy approach-
ing twice that of liquid water. I will discuss the implications of these significant
energetic differences below.
0 20 40 60 80 100
relative humidity (%)
Ca
2+
-exchanged
K
+
-exchanged
Na
+
-exchanged
0.0
0.1
0.2
0.3
0.4
g H
2
O / g dry clay mineral
Fig. 13.2.9. Gravimetric water vapour adsorption isotherms (22 1C) measured for Na
+
-,
Ca
2+
-, and K
+
-exchanged SAz-1 smectite.
-80
-70
-60
-50
-40
-30
-20
-10
0
0 0.05 0.1 0.15 0.2
H
2
O content (g H
2
O/g smectite)
enthalpy of hydration (kJ/mol)
enthalpy of condensation for H
2
O
Na
+
smectite
Ca
2+
smectite
Fig. 13.2.10. Partial molar enthalpies of hydration for Na
+
- and Ca
2+
-smectite, derived
from data of Dios Cancela et al. (1997).
Chapter 13.2: Parallels and Distinctions Between Clay Minerals and Zeolites1106
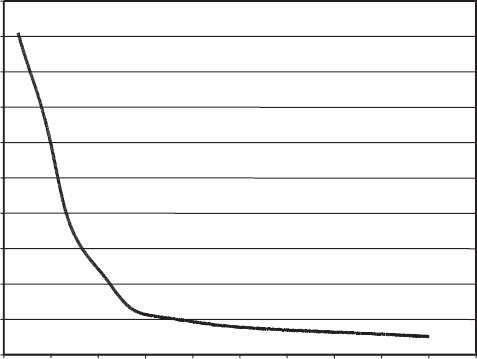
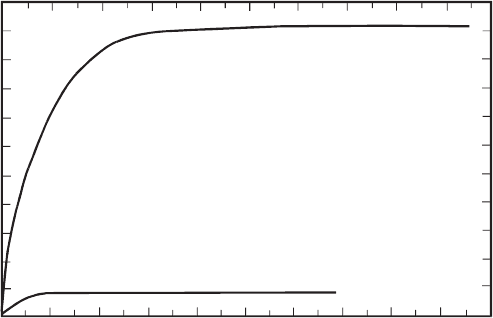
As with smectites, zeolite hydration and dehydration mirror the structural tran-
sitions observed with diffraction methods. Zeolite hydratio n/dehydration reactions
are typically continuous and reversible, usually with no obvious hysteresis (as long as
the zeolite is not heated to temperatures at which it undergoes irreversible phase
transitions). Fig. 13.2.11 shows thermogravimetric analysis data for the zeolite
chabazite, Ca
2
[Al
4
Si
8
O
24
]12H
2
O, illustrating the gradual, multistage loss of weight
that is common for zeolites. The multiple weight-loss events (note inflection points
near 150 1C and 270 1C) are a reflection of the existence of H
2
O in more than one
distinct type of crystallographic site. Also note that chabazite continues to undergo
weight loss at temperatures above 300 1C indicating that H
2
O is held quite strongly.
As a result of the strong H
2
O–cation and H
2
O–framework interactions that occur in
zeolite extraframework sites, this appears to be the rule for many zeolites. It is
possible to quantify the energetics of hydration/dehydration reactions by measuring
the response of a zeolite to changing H
2
O vapour pressure at a given temperature.
These data, known as water vapour adsorption isotherms, measured at a series of
temperatures can provide quantitative information on the nature of the H
2
O mol-
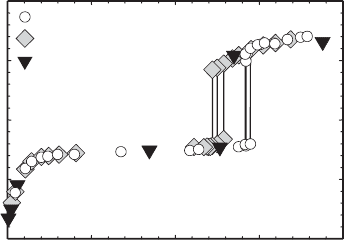
ecule(s) in a zeolite. The data in Fig. 13.2.12 illustrate such adsorption isotherms for
Na
+
-clinoptilolite, measured from 25 to 218 1C. Carey and Bish (1996) used these
data, and similar data for K
+
- and Ca
2+
-clinoptilolite, to determine the partial
molar enthalpies of hydration shown in Fig. 13.2.13. They were able to fit these da ta
assuming a single energetically distinct H
2
O site, but experiments on other zeolites
(e.g., chabazite) show clear evidence for multiple H
2
O sites that must be considered
T (˚C)
101
99
97
95
93
91
89
87
85
83
81
0
100 200 300 400 500 600 700 800 900 1000
wt (%)
Fig. 13.2.11. Thermogravimetric analysis data for chabazite measured at a heating rate of
2 1C/min in a N
2
atmosphere.
13.2.4. Dehydration Phenomena 1107
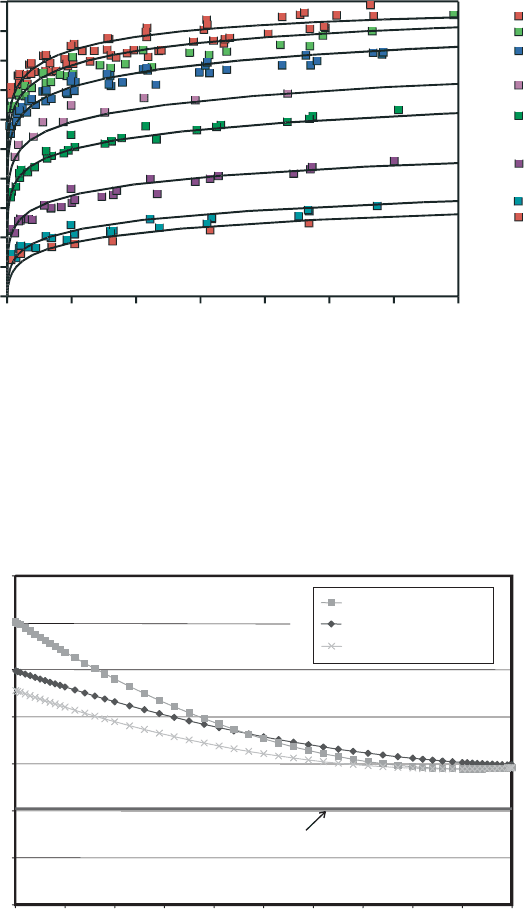
25°C
148°C
218°C
198°C
98°C
48°C
74°C
34°C
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
water vapour pressure (mbar)
0 5 10 15 20 25 30 35
fractional water content Θ
Fig. 13.2.12. Experimental (symbols) and calculated (solid line) H
2
O-vapour adsorption iso-
therms for Na
+
-clinoptilolite measured at the indicated temperatures. y is the fractional water
content, where a y value of 1 indicates full H
2
O occupancy and a y value of 0 indicates
complete dehydration.
-140
-120
-100
-80
-60
-40
-20
0
0 0.1
0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
enthalpy of condensation of H
2
O
2
fractional water content θ
Ca
2+
-clinoptilolite
Na
+
-clinoptilolite
K
+
-clinoptilolite
enthalpy of hydration (kJ/mol)
Fig. 13.2.13. Calculated curves of the partial molar enthalpies of hydration for Na
+
-, K
+
-,
and Ca
2+
-clinoptilolite at 25 1C.
Chapter 13.2: Parallels and Distinctions Between Clay Minerals and Zeolites1108
when fitting experimental isotherm data (Bish et al., 2003b). Fig. 13.2.13 shows that
the energy required to remove H
2
O molecules from clinoptilolite (or the energy
gained on hydration) is significantly greater than the energy required to evaporate
liquid H
2
O, up to almost three times the enthalpy of vapourisation of liquid water in
a largely dehydrated Ca-clinoptilolite. This figure also shows, once again, the large
effect of exchangeable cation on hydration be haviour.
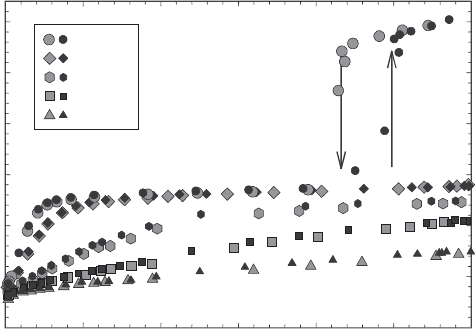
Although zeolite hydration/dehydration is typically continuous and non-hystere-
tic, some zeolites exhibit discontinuous and hyster etic behaviour, notably laumon-
tite. The unit-cell volume data in Fig. 13.2.8 are mirrored well in Fig. 13.2.14,
showing the results of gravimetric H
2
O isotherm measurements on laumontite. Most
interestingly, the discontinuous and hysteretic behaviour shown in Fig. 13.2.8 is
evidenced here only in the 23.4 1C data, and this discontinuous hydration/dehyd ra-
tion reaction does not appear at higher temperatures.
The dehydration and hydration behaviour of smectites and zeolites, and their
associated structural effects, are very important in a wide variety of applications,
both environmental and technological . The importance of these properties possibly
extends beyond Earth, to the surface of Mars. Great excitement was generated with
the recent discovery by the Mars Odyssey spacecraft of heterogeneously distributed
hydrogen at Martian mid-latitudes, suggesting that large areas of the near-equatorial
highlands contain near-surface deposits of ‘‘chemically and/or physically bound H
2
O
and/or OH’’ in amounts up to 8.571.3% water-equivalent hydrogen (Feldman,
Mars conference). Bish et al. (2003a, 2003b) used quantitative data on the hydration
0 5 10 15 20 25 30
3.00
3.25
3.50
3.75
4.00
4.25
4.50
30.1°C
49.5°C
64.5°C
79.3°C
23.4°C
hydration
dehydration
water molecules per 12 framework oxygens
water vapour pressure (mbar)
Fig. 13.2.14. Gravimetric H
2
O adsorption isotherm measurements for laumontite made at
the indicated temperatures. Solid symbols represent data obtained during hydration, and
shaded symbols represent data obtained during dehydration. From Fridriksson et al. (2003).
13.2.4. Dehydration Phenomena 1109
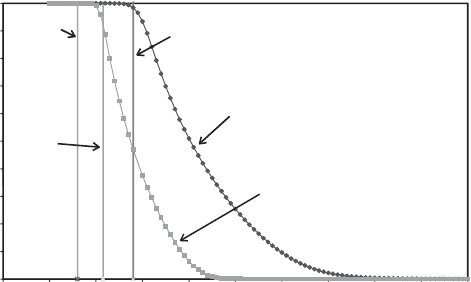
and dehydration of smectites, clinoptilolite, and chabazite, to determine whether or
not these minerals cou ld exist in a hydrated state on the surface of Mars, a cold and
very dry environment (equatorial surface temperatures range from 160 to 280 K,
P(H
2
O) on the surface is 1.5 10
6
bar). Fig. 13.2.15 summarises the calculated
behaviour for Na
+
-clinoptilolite. Not surprisingly, the curve for 0.03 bars, typical of
a humid day on Earth, shows that Na
+
-clinoptilolite is largely hydrated. However,
the curve calculated for Martian P(H
2
O) shows the unexpected result that Na
+
-
clinoptilolite is at least 50% hydrated under Martian surface conditions! The ap-
parent ability of clinoptilolite, and of smectite and other zeolites, to retain H
2
O even
under conditions of very low partial pressure of H
2
O is a direct result of the large
energies of attraction that the minerals have for water molecules.
13.2.5. SUMMARY AND CONCLUSIONS
The data presented here illustrate the important interplay between crystal struc-
ture and hydration/dehydration behaviour for both smectites and zeolites. In spite of
the fact that smectites and zeolites have many similar properties, their very distinct
crystal structures often give rise to very different properties. As a result of their layer
structure, smectite structural transitions are quantised and hysteretic, and they gen-
erally app roximate first-order transitions. This stepwise behaviour is mirrored in the
hydration/dehydration behaviour. Quant itative analysis of H
2
O-vapour adsorption
isotherms shows that the first few layers of H
2
O molecules entering the interlayer
region are held very strongly.
tem
p
erature (K)
Mars day, equator
P = 0.03 bar
P = 1.5 x 10
-6
bar
Mars
subsurface
Mars night,
equator
100 2000 300 400 500 600 700 800 900 1000
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
fractional water content Θ
Fig. 13.2.15. Equilibrium hydration state of Na
+
-clinoptilolite as a function of temperature
at a P(H
2
O) of 1.5 10
6
and 0.03 bar. Mars equatorial night, day, and subsurface temper-
atures are indicated. Data were calculated from the equilibrium isothermal data of Carey and
Bish (1996).
Chapter 13.2: Parallels and Distinctions Between Clay Minerals and Zeolites1110
Zeolites rarely show the hysteretic behaviour that is so common with smectites,
and structural transitions are usually gradual, reversible, and second order. Accurate
crystal structure information has shown that many natural zeolites have multiple
H
2
O sites, and zeolite hydration and dehydration often reflect this, with multiple
weight-loss events often occurri ng up to temperatures >250 1C. How ever, the com-
plex interactions between H
2
O molec ules and exchangeable cations in the zeolite
extraframework sites can give rise to hysteretic stepwise transitions when the cations
and H
2
O molecules migrate during hydration or dehydration. As wi th smectit es,
analysis of adsorption data shows that H
2
O molec ules in zeolite extra framework
sites is held very strongly, up to three times the enthalpies for liquid water. Such high
vapourisation enthalpies have important implications in a variety of areas and mean
that zeolites and smectites can contain H
2
O molecules under very dry conditions.
They are also further reflection of the importance of H
2
O in these minerals; water is
not a passive occupant in zeolite and smectite structures. Perhaps the future will
bring more quantitative information on smectite structures similar to those existing
for zeolites.
REFERENCES
Armbruster, T., Gunter, M.E., 2001. Crystal structures of natural zeolites. In: Bish, D.L.,
Ming, D.W. (Eds.), Natural Zeolites: Occurrence, Properties, Applications. MSA Reviews
in Mineralogy and Geochemistry, vol. 45. Mineralogical Society of America, Washington,
DC, pp. 1–67.
Barrer, R.M., 1978. Cation-exchange equilibria in zeolites and feldspathoids. In: Sand, L.B.,
Mumpton, F.A. (Eds.), Natural Zeolites: Occurrence, Properties, Use. Pergamon Press,
Oxford, England, pp. 385–395.
Bish, D.L., Carey, J.W., 2000. Coupled X-ray powder diffraction and thermogravimetric
analysis of clinoptilolite dehydration behavior. In: Colella, C., Mumpton, F.A. (Eds.),
Natural Zeolites for the Third Millennium. De Frede Editoree, Naples, pp. 249–257.
Bish, D.L., Carey, J.W., 2001. Thermal behavior of natural zeolites. In: Bish, D.L., Ming,
D.W., (Eds.), Natural Zeolites: Occurrence, Properties, Applications. MSA Reviews in
Mineralogy and Geochemistry, vol. 45. Mineralogical Society of America, Washington,
DC, pp. 403–452.
Bish, D.L., Carey, J.W., Vaniman, D.T., Chipera, S.J., 2003a. Stability of hydrous minerals on
the martian surface. Icarus 164, 96–103.
Bish, D.L., Vaniman, D.T., Fialips, C., Carey, J.W., Feldman, W.C., 2003b. Can hydrous
minerals account for the observed Mid-Latitude water on Mars? 6th International Con-
ference on Mars, July 20–25, 2003.
Campbell, B.J., Cheetham, A.K., 2002. Linear framework defects in zeolite mordenite. The
Journal of Physical Chemistry B 106, 57–62.
Carey, J.W., Bish, D.L., 1996. Equilibrium in the clinoptilolite-H
2
O system. American Min-
eralogist 81, 952–962.
Colella, C., 1996. Ion exchange equilibria in zeolite minerals. Mineral Deposits 31, 554–562.
References 1111
