Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

disordered illite–smectite minerals. One of these has about 50% smectite layers; the
other has 20% smectite and is less abundant. Both types co-exist in the same ho-
rizon, with beidellite and montmorillonite as the expanding layers. Smaller amounts
of illite, kaolinite, and vermiculite are also present, but rarely chlorite. Irrespective of
climate (continental) and parent material, a convergence in mineralogy towards
mixed-layer phases seems to occur. That is, there is a tendency towards the same
chemical and mineralogical equilibrium, be it metastable or not. Nevertheless, the
clay minerals along a soil profile often show great diversity because of the complex
transformation and reaction processes that operate with increasing depth.
In the weathering environment, most clays are formed by incongruent dissolution
of unstable silicates. Reaction of the unstable mineral with less soluble compounds
can produce new clay minerals. Direct precipitation from solution can also give rise
to neoformed clay minerals with compositions that are very different from those of
the (dissolved) parent minerals. Eventually, a clay mineral can become unstable
because of intense drainage, forming oxides and liberating most ions.
Hydrolysis generally involves the exchan ge of protons from the solution for sol-
uble mono- and divalent cations in the minerals. Thus, the flow rate and acidity of
water play a key role in the formation of clay minerals. In a soil profile developed
under high rainfall, kaolinite is found in the upper part, while montmorillonite
commonly occurs in the lower part of the weathering profile, and closer to the rock
where the accumulation of leached ions can produce more chemically complex clay
minerals. Leaching is stronger when the topography is smooth or plain, and kaolinite
can predominate in soils of slight slope, while smectites are dominant in soils formed
in depressions (Kantor and Schwertmann, 1974).
In the classic example of granite weathering, a large part of the soils consists of
metastable muscovite, biotite, and chlorite. These minerals alter progressively into
clay minerals. Muscovite is degraded into illite, and both minerals can transform into
regular or random illite–smectite (I/S) mixed-layer minerals. Biotite and chlorite
transform into smectite through the formation of a regular mixed-layer phase
containing biotite and smectite, or chlori te and smectite. Corrensite, a 1:1 chlo-
rite–saponite mixed-layer mineral, is very common in soils. In many cases all these
primary minerals (muscovite, biotite, chlorite) can be transform ed into vermiculite.
When rainfall is high, muscovite and biotite eventually transform into kaolinite in
the upper part of the profile. Like the micas, plagioclase and orthoclase are also
destabilized and transform into kaolinite and some illite. Kaolinite decreases to-
wards the lower part of the alteration profile, while illite, I/S mixed-layer minerals,
vermiculite, and neoformed clay minerals (smectites) tend to increase with depth.
This evolution pathway also typifies the weathering of gneisses, and other types of
aluminous pellitic rocks.
In basic rocks, smectites (Fe-beidellite, nontronite) and vermiculite can form from
Ca-plagioclases, amphiboles, and pyroxenes. Depending on the Al content, minor
amounts of kaolinite may be formed. On ultrabasic rocks, saponite, serpentine,
chlorite, and talc are the most frequent phy llosilicates derived from pyroxenes and
14.1. Geological Environments for Clay Formation 1133
olivine. Nevertheless, under a tropical climate, kaolinite can be the major clay min-
eral formed from basalt (Siefferman and Millot, 1969), anorthosites (Gomes et al.,
1994), or gabbros (Gala
´
n et al., 1996) by hydrothermal or weathering alteration.
The weathering of quartz–chlorite schists can produce a complex sequence of clay
minerals starting from chlorite and finishing with kaolinite. In the host rock of
uranium ore deposit at Koongarra, Australia, Murakami et al. (1996) observed the
following weathering sequence: (i) chlorite; (ii) chlorite–vermiculite intergrade; (iii)
interstratified chlorite and vermiculite; (iv) vermiculite; and (v) kaolinite.
A specia l case of weathering takes place when eruptive rocks react with sea water
in a deep ocean environment. Basalt, the most frequent rocks found in ocean floors,
are hydrolyzed to form clays on the floor surface. These clays contain much Fe, and
become progressively enriched in alkaline elements at lower temperatures. Clay
mineral formation begins below 30 1C, with saponite forming at high, and Fe-rich
beidellite and celadonites at intermediate temperatures.
The genesis of the various clay minerals found in specific weathering profiles
remains to be resolved in detail. The multitude of factors involved in this environ-
ment are responsible for many variations in weathering sequence an d the crystallo-
chemical properties of the intermediate and end member products. However, some
generalization is possible.
Illite and other micaceous minerals are inherited from parent rocks or other
materials, where they form under different P–T conditions from those existing at the
earth’s surface (Fanning et al., 1989). The formation of illite by a pedogenic process
is possible but it is very rare (Wilson, 1999). The formation of illite from micaceous
minerals involves cleavage of the relatively thick, micro-size mica particles with a full
content of K into very thin particles depleted in K (illite) (Roberts et al., 1991;
Romero et al., 1992). The majority of micaceous soil clays are dioctahedral and
aluminous, although some rare trioctahedral illites, derived from biotite, do exist.
Smectite formation in soils takes place when the following factors come together:
base-rich parent rocks, poor drainage, low-lying topography, high pH , high silica
activity, and abundance of basic cations (Table 14.1)(Keller, 1970; Borchardt, 1989).
These conditions can occur under very different climates (temperate, cold, or even
tropical) when leaching is limited. Soil smectites consist principally of dioctahedral
montmorillonite and beidellite that are somewhat enriched in Fe as compared with
their ideal compositions (Wilson, 1987a). They are also somewhat different from the
smectites in ben tonite deposit s. This might be because the chemical variability is
higher in the weathering environment than during bentonization since the smectites
in most soils can be inh erited, transformed, and ne oformed (Table 14.2). Smectites of
different composition may crystallize locally in microenvironments from different
parent materials (Aoudjit et al., 1995). The illite-to-smectite transformation seems to
be a simple degradation pr ocess but illitization of smectite (‘aggradation’) in wea-
thering environments is not fully understood.
Vermiculite is very common in soils and weathering profiles developed on acidic
and basic rocks. Vermiculite seems to be formed by the weathering of micas, mainly
Chapter 14: Genesis of Clay Minerals1134

Table 14.1. Geological environment of primary-stage montmorillonitization (Keller, 1970)
A. Retention of Mg
2+
,Ca
2+
,Fe
2+
,Na
+
a.
Evaporation exceeds precipitation, as semi-arid climate
b.
Ineffective leaching
Stagnant water and water logging
Ash in lakes and marine basins
Low effective permeability of rocks
c.
Alkalinity
d.
Fe
2+
not combined with O
2–
or S
2–
e.
Silicates characterized by
High specific surface, as for volcanic ash
High susceptibility to hydrolysis
B. Retention of silica
a.
Flocculated by Ca
2+
,Mg
2+
, and other cations
Rock rich in Ca
2+
,Mg
2+
, and Fe
2+
b.
Ineffective leaching
c.
Clay-size cristobalite
C. Retention of parent texture and mineralogy
a.
Shards or flow
b.
Igneous-type minerals
Table 14.2. Abundance of smectites in soils as assessed from the literature (Wilson, 1999)
Soils Inheritance Neoformation Transformation
Entisols +++ +++ +
Aridisols +++ +++
Inceptisols +++ +++ +++
Vertisols +++ +++ +
Mollisols + ++ ++
Alfisols + +++ ++
Spodosols + +++
Ultisols +++ +
Oxisols
+++ major importance, ++ moderate importance, + minor importance, no importance.
14.1. Geological Environments for Clay Formation 1135
from biotite (trioctahedral vermiculite), involving the release of interlayer K
+
, and
the oxidation of structural Fe
2+
(Vicente-Herna
´
ndez et al., 1983). Soil vermiculite
may also derive from muscovite (dioctahedral vermiculite) but this transformation
was not well understood until the application of high-resolution transmission elec-
tron microscopy (HRTEM). Aoudjit et al. (1996) presented HTREM images of the
vermiculitization of muscovite in an acidic soil, showing particles with a muscovite
core and a vermiculite rim consisting of about 15 layers. Very fine-grained muscovite
particles of high specific surface area and reactivity seem necessary for transforma-
tion into vermiculite in soil. The degradation of chlorite by decomposition of the
interlayer hydroxide sheet is another means of obtaining vermiculite (Makumbi and
Herbillon, 1972; Ross and Kodama, 1976).
Chlorite is an inherited clay mineral in soils and weathering crusts. This layer
silicate is easily weatherable and transformable into regularly interstratified ch lo-
rite–vermiculite and vermiculite (Proust, 1982; Buurman et al., 1988). Chlorite can
also be formed pedogenically by the intercalation of Al (hydr)oxides into pre-existing
smectite, vermiculite, or interstratified expansible minerals. The source of the inter-
layer Al is the degradation of both the tetrahedral and octahedral sheets of layer
silicates, weathering of Al-minerals (i.e., feldspars), or decomposition of organic
matter containing adsorbed Al. Like smectite and illite, chlorite is very sensitive to
acidification. In soils affected by ‘acid rain’ or acidified surface water, trioctahedral
chlorite is destroyed in the E horizons, illite transforms into vermiculite via regularly
interstratified illite–vermiculite, interlayer hydroxy-Al sheets are liberated (particu-
larly in B horizons), and poorly ordered material (probably allophane) is preci-
pitated in B horizons (Wilson, 1987b). Gala
´
n et al. (1999) shown that acid mine
drainage is responsible for the progres sive degradation of chlorite in the basin of the
Tinto River (Spain).
Kaolinite can be a neoformed, transformed , or inherited mineral in soils. As
already menti oned, high rainfall and a temperate climate can transform muscovite
and biotite into kaolinite together with some illite. Al-(hydr)oxides liberated by
extensive leaching can react with silica to produce kaolinite under slightly acidic
conditions (pH E5), and when silica activity is moderate and the concentration of
basic cations is low. Keller (1970) summarize d the general conditions for the weath-
ering formation of kaolinite (Table 14.3). Like kaolinite that derives from weath-
ering, soil kaolinite is usually highly disordered and may contain Fe
3+
from
isomorphous substitution. In some cases, it is also interstratified with smectites
(Linares and Huertas, 1971; La Iglesia and Van Oosterwyck-Gastuche, 1978;
Herbillon et al., 1981 ; Dixon, 1989).
Halloysite occurs frequently as a neoformed mineral in weathering crusts devel-
oped on acidic volcanic rocks (Siefferman and Millot, 1969). Under supergene con-
ditions halloysite can transform into kaolinite according to the sequence: disordered
halloysite-ordered halloysite-metahalloy site-disordered kaolinite-ordered ka-
olinite (Ponder and Keller, 1960; Chem, 1969). As La Iglesia and Gala
´
n (1975)
shown, this process is one of recrystallization, requiring the presence of organic
Chapter 14: Genesis of Clay Minerals1136

acids. Kaolinite can also transform into halloysite (Singh and Mackinnon, 1996;
Bobos et al., 2001).
Palygorskite and sepiolite, the fibrous clay minerals in soils and paleosols, are
generally neoformed (Singer, 1979). The instability of these minerals in wet climates
favours their presence in soils under dry or semi-dry climates. The occurrence of
palygorskite in soils is associated with one of the following conditions: (i) in modern
soils that, at present or in the past, were affected by rising ground water of pH 7–8
and abundant salinity; (ii) in soils with distinct and sharp textural transitions because
these minerals accumulate in the coarse fraction (this group includes many paleo-
sols); and (iii) in calcretes, caliches (Singer, 1984). In all these cases, palygors-
kite (and very rarely sepiolite, when Al is absent or immobilized (Jones and Weir,
1983)) is precipitated by evaporation of the vadose water. Cemented soils (duri-
crusts) can be caused by palygorskite (palycretes), or by silica (silcretes) and calcite
(calcretes) (Rodas et al., 1994). The transformation of smectite into palygorskite was
described in terms of a dissolution–precipitation process (Jones and Gala
´
n, 1988), or
Table 14.3. Geological environment for primary kaolinitization (Keller, 1970)
A. Removal of Ca
2+
,Mg
2+
,Fe
2+
,Na
+
,K
+
a.
Precipitation exceeds evaporation
b.
Permeable rocks
c.
Percolating water
d.
Oxidation of Fe
2+
to Fe
2
O
3
or FeS
2
B. Addition of H
+
a.
Fresh water
b.
Acids
Sulphur compounds
Carbonic, air and soil atmosphere
Organic, living and dead organisms
C. High Al:Si ratio
a.
Removal of silica
Na and K silicates
Organic complexes
b.
High concentration of Al
Al
3+
in acid solution
Al–OH polymers
14.1. Geological Environments for Clay Formation 1137
transformation through kerolite–smectite mixed-layer minerals (Eberl et al., 1982).
The transformation of smectite and illite to palygorskite was verified by Suarez et al.
(1995), Lo
´
pez Galindo et al. (1996), and Sa
´
nchez and Gala
´
n (1995). Palygorskite is
more easily formed in weathering environments than sepiolite, or else sepiolite is less
stable under supergene conditions, and hence is rarer. Traces of palygorskite found
in some arid soils are considered as inherited (Shadfan and Dixon, 1 984; Mackenzie
et al., 1984).
In summary, we can say that under weathering conditions most common rock-
forming silicates (except quartz) are destroyed, and practically all clay minerals can
form. Illite derives from micaceous minerals and feldspars, chlorite can be inherited
or formed from biotite. Kaolinite can be neoformed, or derived from muscovite and
feldspars, and more rarely from smectite or vermiculite. Vermiculite comes from
muscovite/biotite and chlorite through interstratified structures. Smectite can form
directly by alteration of basic silicates (pyroxenes, amphiboles), or from illite and/or
vermiculite. Palygorskite can be directly precipitated under certain climatic condi-
tions, or formed from smectite and illite. Sepiolite seldom forms in soils. The com-
plexity of the systems is very large and not all the possibilities were yet demonstrated.
Some of the most frequent pathways are illustrated in Fig. 14.2.
14.1.2. Sedimentation
Ancient sedimentary rocks are composed of about 70% mudstones (containing
about 50% clay-size particles) and shales (Blatt et al., 1980). Presently, sedimentary
environments that contain muds cover about 60% of marine continental shelves and
40% of deep ocean basins (Hillier, 1995). Continental aquatic environments such as
lakes, rivers, estuaries, and deltas also contain high proportions of clays. The
amounts and varieties of clay minerals found in sediments and sedimentary rocks are
a direct result of source area differences, changes occurring during transport and
deposition, and changes taking place after sedimentation (diagenesis).
The erosion of soils is governed by the profile structure, the humidity, the clay
content, the slope, and the climatic conditions. A well-developed soil is more sen-
sitive to erosion, because it shows a thick upper zone where clays predominate and
which can be easily removed. The finest particles of the profile (mostly clay minerals)
are most easily transportable. On the other hand, clays can contain significant
amounts of adsorbed water, providing protection against ero sion by sudden abun-
dant rainfall, which is the principal erosion factor.
Clay transportation mainly occurs by water flow (stream, river), but the timespan
of this transport is very short and has practically no influence on clay alteration. The
deposition zone spreads from the mouth of rivers to lagoons, lakes, seas, and oceans,
producing a particle-size fractionation from the mouth to the interior, together with
different mineral settings. According to Parham (1966), the order of the first ap-
pearance of major clay mineral groups from shoreward to basinward areas is as
follows: kaolinite, illite, chlorite, palygorskite, and sepiolite. Montmorillonite and
Chapter 14: Genesis of Clay Minerals1138
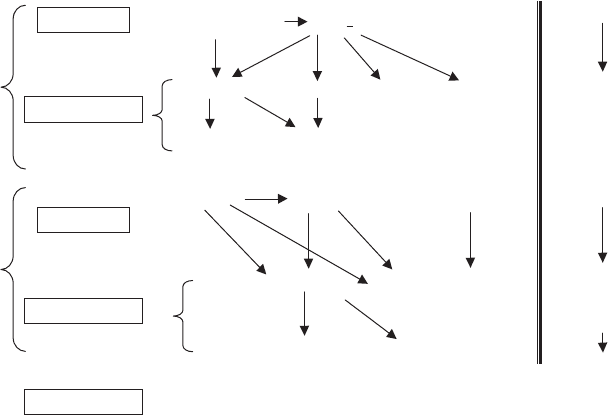
I/S mixed layered minerals are present over the entire range. This approach con-
tinues to be true in the most cases, except for the fibrous clay minerals (specially
sepiolite). On the other hand, salinity changes can also produce coagulation proc-
esses affecting some clay minerals. A typical clay mineral distribution found from the
coastline to the open sea is: kaolinite-illite- smectite.
After deposition the inter stitial water solution in the sedim ent has a higher ionic
concentration than that in the weathering profile, leading to a change in chemical
equilibrium. The chemical potential of the elements in solution is different from that
for clay formation in the continent, and clays react with the environment solution.
The time of connection between the sediment pore water and water reservoir can be
very long or very short depending on sediment accumulation rates. Typical reactions
are clay–clay, and clay-(hydr)oxide transformations with chemical contribution from
the sedimentary solution. The new phases formed depend on the redox potenti al of
the ambient solution which is partly controlled by organic matter, and partly by
living organ isms at the water/sediment interface. For example, microbial action can
change the oxidation state of Fe, or stabilize sulphides, and hence control the
Transformation
Vermiculite
Smectite
Palygorskite
Smectite
(Kaolinite)
V-S
B-V
Inheritance
Chlorite (**) Smectite (***)
Ch-V
Ch-S
Ke-S
B-S
Neoformation Kaolinite Smectite
Transformation
Vermiculite Smectite
Smectite
Kaolinite
(Palygorskite)
AlV-S
Inheritance
I-S
I-V
Acidic
Rocks
Basic
Rocks
(Kaolinite)
Biotite
Palygorskite
(Sepiolite)
(Kaolinite)
Smectite/
Chlorite/
Vermiculite
Talc
Ca-Plag/Amp/Py
Illite + Kaolinite
Feldspars
Muscovite (Biotite)
Illite*
Fig. 14.2. Pathways for the formation of clay minerals in weathering environment from
different starting materials. Minerals in bold type indicate the most probable end products. In
brackets are minerals that do not form easily according to that pathway, or are rare. Clay
minerals derived from other rock-forming silicates could be formed by transformation and/or
neoformation. (
) Illite can be considered inherited in soils, and as the first degradation
product of muscovite, (
) Chlorite may be inherited or transformed from biotite, (
)
Smectite may be inherited in soils. Also some palygorskite may be inherited in soils.
14.1. Geological Environments for Clay Formation 1139
availability of certain elements that clays can fix. This early post-deposition changes
are usually termed ‘early diagenesis’.
In general, clay minerals of sedimentary sequences (in areas not subject to tectonic
activity) mainly reflects the climate, relief, and lithology of source areas. However, in
zones of intense tectonic activity (flysch) the clay mineral assemblages in sediments
can reflect the composition of the parent rocks because tect onic activity prevents the
formation of soils in the continent. The rapid transport of mate rial by turbidity
currents with high solid concentrations does not produce well-sorted sediments;
rather, the sediments usually have a grain-size distribution similar to that of the
parent suspensions (Ruiz Cruz, 1999). The mineralogy of recent sediments in coastal
shelves and estuaries is generally consistent with terrigenous origin, that is, from soils
and weathered rocks of the hinterland (Chamley, 1989 ). Sometimes a particular
mineral can be an indicator of continental origin. For example, sediments from the
Galician Coast of Spain contain significant amounts of gibbsite, a common con-
stituent of soils found in weathering profiles of this region (Belzunce-Segarra et al.,
2002).
In continental-shelf and deep-sea sediments the bulk of the clay fraction is detrital
(Kastner, 1981; Chamley, 1993) but some of the smectites, I/S mixed-layer minerals,
glauconite, and berthierine are neoformed by alteration of volcanic material, and by
low-temperature interaction between X-ray amorphous hydr(oxides) and biogenic
silica.
Certain clay minerals formed in marine environments can serve as paleoenviron-
mental indicators. For example, both glauconite and berthierine are typical marine
clay minerals formed from pre-existing clays (kaolinite, smectite, and iron oxides, at
temperature around 4 1C) by diffusion of ions in the solid state and then recrys-
tallization. Berthierine does not incorporate K
+
as does glauconite, but takes up
large amounts of Fe
2+
and Fe
3+
. The geological environment of both minerals
seems to be different. Glauconite occurs in a calmer ambient situation (shallow
shores of oceanic platforms) than berthierine.
Glauconization is analogous to illitization. It involves a gradual alteration of
neoformed Fe-rich smectite into Fe-illite, similar to celadonite, through intermediate
mixed-layer structures. Glauconitization is complete when the layers no longer ex-
pand and the K
2
O content increases to 9%, which requires about 1 Ma (Odin, 1988).
Transformations of detrital clay minerals at the bottom of seas and oceans include
the formation of Al-smectites, showing that these minerals can be stable in this
environment. In addition, devitrification of volcanic materials in the deep ocean
environment yields new crystalline phases, such as smectites and zeolites.
Palygorskite of detrital origin can also be found in oceans, having come from the
continent, as in the case of deep-sea Atlantic palygorskite close to Morocco, which
was transported by SW winds from near-shore shallow African basins into the ocean
(Pletsch et al., 1996). Palygorskite can also be derived from smectite by diagenetic
transformation in the marine environment (Lo
´
pez Galindo, 1986), sometimes
associated with marine phosphorites (Chahi et al., 1999). Sepiolite can also be of
Chapter 14: Genesis of Clay Minerals1140
diagenetic origin through the transformation of magnesite at pH 10.5–11.5 in silica-
rich lake waters (Ece, 1998). However, most of the palygorskite and sepiolite in the
world are neoformed (precipitated) in lacustrine and peri-marine environments. Al-
though these minerals can be derived from other clay minerals, the most common
mechanism is crystallization from solution, promoted by the presence of amorphous
silica. Experiments by Birsoy (2002) indicated that low aqueous Al-activities favour
sepiolite and kerolite relative to palygorskite and saponite.
In some shallow restricted basins with evaporitic conditions fibrous clay minerals
and Mg
2+
-rich smectites (saponite, stevensite, kerolite) can form authigenic min-
erals, usually together with sulphate and carbonate minerals. In this environment
smectite can also be transformed into palygorskite (Sa
´
nchez and Gala
´
n, 1995). In a
typical playa-lake sequence, from the basin edge to the centre, detrital miner als
(illite, ka olinite, smectite) dominate at the lake edge, palygorskite with some
Al
3+
-smectite near the shore, while sepiolite and other Mg
2+
-clay minerals abound
towards the centre.
In summary, we can say that sedimentary basins are repositories of clay miner als,
forming part of the clay cycle (weathering-deposit ion-diagenesis/metamor-
phism-magmagenesis). The clay minerals can be formed by neoformation (i.e.,
direct precipitation from solution or through ageing of amorphous materials, such as
glassy volcanic ash), inheritance (i.e., transported from the source to the basin with
little modification), and transformation (i.e., when neoformed and inherited clays in
the basin react by diagenesis and low-grade metamorphism). Basins formed at active
plate tectonic margi ns tend to have a greater diversity of clay origins than at passive
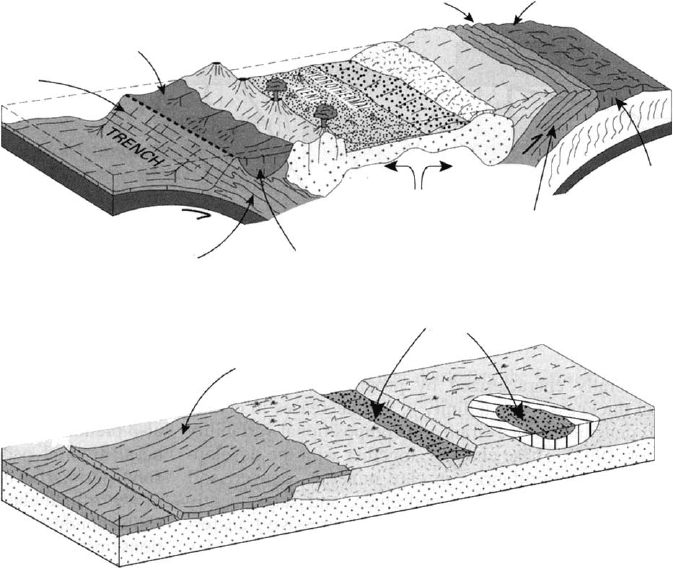
margins or within-plate basins (Fig. 14.3).
14.1.3. Burial Diagenesis and Low-Grade Metamorphism
When sedimentary clays are buried, free or weakly bonded water is eliminated. As a
result, the porosity decreases from 80% to about 20% at the first kilometer of burial.
As depth increases, sediments tend to reach a global chemical equilibrium and new
phases can form under the new environmental conditi ons. The unstable phases tend
to disappear and the number of species diminishes. The reactions are mostly of the
clay–clay and clay–solution types with time and temperature being the principal
factors governing the transformations. The new minerals are more highly ordered
and of large size than their original minerals.
After the disappearance of most inherited soil clay minerals, new clay minerals are
formed: I/S- and chlorite–smectite mixed-layer minerals, chlorite, kaolinite together
with zeolite, some quartz, and feldspars. In general, illite is the most common dia-
genetic product, and I/S mixed-layer minerals are the main indicator of diagenetic
evolution of a sedimentary basin (Dudek and S
´
rodon
´
, 2003).
The last stage of diagenesis is the beginning of metamorphism (anchimetamor-
phism) when most of the interstratified clay minerals and kaolinite are destroyed,
resulting in an illite–chlorite facies with pyrophyllite, biotite, and paragonite.
14.1. Geological Environments for Clay Formation 1141
Typical low-grade metamorphic rocks derived from the clay sediments are pelitic
rocks (slates), mainly composed of mica (or illite), chlorite, alkali feldspar and
quartz, with some pyrophyllite, and/or paragonite. Shales are gradually transformed
into phyllites, and with increasing metamorphism, into schists. Phyllosilicate par-
ticles become too large to be considered clay minerals, although they can be similar
crystallochemically.
Perhaps the most significant mineral change on burial diagenesis is the gradual
loss of expandable miner als, mainly the transformation of Al
3+
-smectite into illite
through I/S mixed-layered structures. The change occurs step-by-step. The first one
is the random interstratification of smectite with illite (Reichweite, R ¼ 0), and
composition ranging from pure to 50% smectite. The 50% I/S mixed-layer structure
is a regular interstratification of good stability. The last stage is the formation of illite
Transformed
Clays
Fore Arc Basin
Transformed
Clays
Inherited
Clays
Neoformed (volcanogenic)
and inherited clays
Inherited
Clays
Forelan
d
Basin
Fold-and-thrust
Belt
Remnant
Arc
Back-Arc
Basin
Active
Arc
Accretionary
Complex
Neoformed
Clays
Inherited + Neoformed
clays
Neoformed + Inherited
clays
Mid-ocean ridge
and ab
y
ssal
p
lain
Passive
Margin
Continental
Rift Basin
Continental
Sag Basin
Fig. 14.3. Characteristic modes of origin of clays in sedimentary basins at an active plate
margin (above), and a passive margin and within-plate setting (below). From Merriman
(2003).
Chapter 14: Genesis of Clay Minerals1142
