Basdevant J.-L., Rich J., Spiro M. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
Подождите немного. Документ загружается.

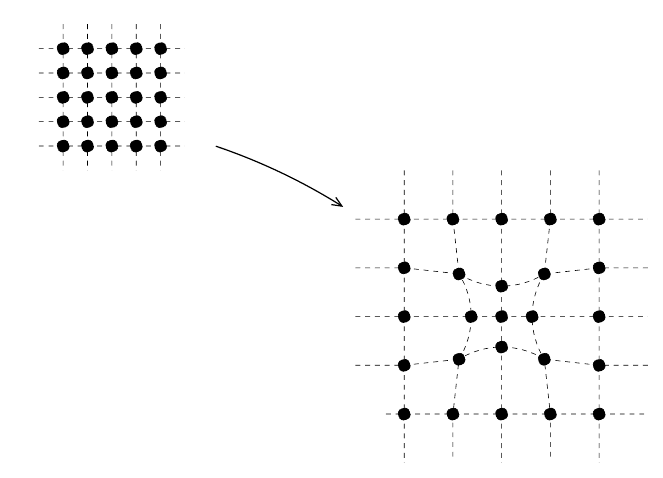
9.3 Gravitation and the Friedmann equation 413
We also note that the equations of general relativity for a homogeneous
Universe will be simple because the coordinate grid can be described by one
function a(t). In an inhomogeneous Universe, the situation is much more
complicated, as illustrated in Fig. 9.5. In the figure we have supposed that
there is an excess of mass in the center of the figure. The gravitational at-
traction of matter to this excess will cause the test particles to expand in
a non-uniform manner. Even if the coordinate system is simple at the be-
ginning, it will evolve into a complicated system with distances between test
particles defined by three metric functions
dS
2
==g
xx
(t, x, y)dx
2
+ g
xy
(t, x, y)dxdy + g
yy
(t, x, y)dy
2
. (9.46)
We now have 3 functions of position and time to describe the coordinate
system. The three functions g
xx
, g
xy
and g
yy
define the (spatial) metric of
the coordinate system. The equations that govern the metric can be quite
complicated.
dS
2
=dx +dy
22
dS
2
=g
xx
dx
22
dy
yy
+gdxdy
xy
+2g
Fig. 9.5. Same as Fig. 9.4 but with a mass excess near the center of the region.
The excess gravity causes the test particles to separate less rapidly near the center
than in the homogeneous case. The resulting formula for dS is more complicated.
Fortunately for us, the metric of a homogeneous Universe is described by
only one function a(t) and we can expect the Einstein equation to be simple.
It turns out to be

414 9. Nuclear Cosmology
¨a
a
=
−4πG
3c
2
(ρ +3p) , (9.47)
where ρ is the energy density and p is the “pressure.” As expected, a positive
energy density ρ>0 works to decelerate the Universe. In fact, the part of the
r.h.s. that is proportional to ρ can be guessed through a simple Newtonian
argument, as shown in Exercise 9.4. On the other hand, the appearance of
a correction proportional to the pressure p is surprising, especially since a
positive pressure also works to decelerate the Universe, out of line with our
intuition of thermal pressure encouraging the expansion of a gas. This is
however not the role of the pressure in the gravitational context where it acts
as a source of gravitation that is normally ignored in Newtonian problems.
To see why it can often be ignored, we note that for a collection of particles
(number density n), the pressure can be defined to be proportional to the
mean value of |p|
2
c
2
/3E:
p ≡ n|p|
2
c
2
/3E . (9.48)
For instance, for a thermal collection of nonrelativistic particles of mass m,
the energy density is ρ = mc
2
n and the pressure is given by
p = n(|p|c)
2
/3E = nkT , (9.49)
wherewehaveused|p|
2
/2m =(3/2)kT. This value of the pressure is just
that of the ideal gas law. Because kT mc
2
for a non-relativistic gas, this
shows that the relativistic correction is small. On the other hand, for a rela-
tivistic gas (|p|c ∼ E)wehave
p = n(|p|c)
2
/3E = ρ/3 . (9.50)
An example of such a gas is the cosmic (photon) background radiation. For
such a gas, the relativistic correction proportional to the pressure is large and
doubles the deceleration rate.
In an adiabatic expansion, the volume and energy of a gas change accord-
ingtothelawdE = −pdV . This suggests that in a cosmological context the
pressure can be defined by the way that the energy density changes as the
Universe expands:
p = −
dρa
3
da
3
. (9.51)
Using the laws (9.39) and (9.40) for the evolution of the energy density, we
see that (9.51) implies that, as expected, p = 0 for a non-relativistic gas and
p = ρ/3 for a relativistic gas. On the other hand, the fact that the vacuum
energy density is constant (9.41) implies that for vacuum energy we have
p = −ρ vacuum . (9.52)
A negative pressure is perhaps counter intuitive but we should not forget that
our intuition for pressure is based on the pressure of collections of particles
whereas the vacuum is the absence of particles.

9.3 Gravitation and the Friedmann equation 415
The solution ˙a(t) of (9.47)with the pressure (9.51) is
˙a
2
=
8πGρ
3c
2
a
2
+const.
To evaluate the constant we use the present values ˙a(t
0
)=H
0
a
0
and
8πGρ(t
0
)/3c
2
= H
2
0
Ω
T
to find
˙a
2
=
8πGρa
2
3c
2
+ H
2
0
a
2
0
(1 − Ω
T
) . (9.53)
Dividing (9.53) by a
2
we find the “Friedmann equation”:
˙a
a
2
=
8πGρ
3c
2
+ H
2
0
(1 − Ω
T
)
a
0
a
2
. (9.54)
We note that the measurement previously discussed of the Cosmic Back-
ground Radiation anisotropies indicate Ω
T
∼ 1 so that the second term on
the r.h.s. nearly vanishes. At any rate, it can be ignored in the primordial
Universe since it diverges as a
−2
whereas the radiation density diverges as
a
−4
.
We will be mostly interested in the expansion rate in the primordial Uni-
verse when the energy density and pressure were dominated by relativistic
radiation given by the Stefan–Boltzmann law:
ρ(T )=g(T )
π
2
30
(kT)
4
(¯hc)
3
, (9.55)
where g(T ) is the effective number of relativistic spin degrees of freedom in
thermal equilibrium at the temperature T (g = 2 for a blackbody photon
gas). At T = 1 MeV, photons, three neutrino species and electron–positron
pairs are in equilibrium corresponding to g(kT =1MeV)∼ 10.75. (Note
that a fermion degree of freedom counts for only ∆g =7/8 because the Pauli
principles restricts the number of fermions.) When nucleosynthesis occurs at
kT ∼ 50 keV, only photons and neutrinos [with T
ν
∼ (4/11)
1/3
T
γ
] are present
giving g(kT =50keV)∼ 3.36.
We then have
˙a
a
=
8πG
3
g(T )
π
2
30
(kT)
4
(¯hc)
3
1/2
∼ 0.65 s
−1
kT
1MeV
2
g(T )
10
1/2
. (9.56)
The Hubble time, i.e. the time for significant changes in the temperature is
the inverse of ˙a/a. It is about 1 sec for T ∼ 1 MeV and about 100 sec for
kT ∼ 100 keV when nucleosynthesis begins.
416 9. Nuclear Cosmology
9.4 High-redshift supernovae and the vacuum energy
The general relativistic equation (9.47) combined with the surprising nega-
tive pressure for a positive vacuum energy (9.52) means that the expansion
accelerates in a Universe dominated by vacuum energy:
¨a
a
=+
8πG
3c
2
ρ
V
if ρ
tot
∼ ρ
V
. (9.57)
The observational evidence for acceleration involves the observed photon flux
from high redshift supernovae.
Understanding the relation between the measured flux of a supernova and
its redshift requires the use of general relativity. However, we can understand
the relationship qualitatively through the following argument. The flux is
determined by the present distance R to the supernova
φ ∝
1
R
2
∼
1
c
2
(t
0
− t
1
)
2
, (9.58)
where in the second form we replace the distance by the flight time c(t
0
−t
1
)
where t
1
and t
0
are the explosion and detection times.
On the other hand, the redshift, z, is defined by the emitted (λ
0
)and
observed (λ
1
) wavelengths of photons
1+z ≡
λ
1
λ
0
. (9.59)
For nearby objects, this redshift is most simply interpreted as a Doppler shift
(9.27). However, equation (9.35) tells us that more generally the redshift is
the universal expansion factor between the time of the explosion and the time
of the observation
1+z =
a(t
0
)
a(t
1
)
. (9.60)
If the expansion is accelerating (decelerating), the expansion rate in the
past was relatively slower (faster) implying a longer (shorter) time between
the explosion and observation. Equation (9.58) then implies that in an ac-
celerating (decelerating) Universe, a fixed redshift therefore corresponds to a
relatively small (large) photon flux.
Two teams [95, 96] have observed that the fluxes of supernova at z ∼ 0.5
are about 40% smaller than that expected for a decelerated Universe of mass
density equal to the critical density. The effect can be explained by a vacuum
energy Ω
Λ
∼ 0.7.
9.5 Reaction rates in the early Universe
These days, not much goes on in intergalactic space. There are no nuclear
reactions occurring and photons and neutrinos very rarely scatter on matter.

9.5 Reaction rates in the early Universe 417
More quantitatively, the number of reactions per particle per unit time, λ,
is much less than one reaction per Hubble time. For example the rate of
Compton scattering per photon is
λ
γe→γe
= n
e
σ
T
c ∼ 1.4 × 10
−3
H
0
, (9.61)
where the free electron density is set equal to the baryon density from Table
9.1 and σ
T
is the Thomson cross-section. The numerical value in the second
form means that only one photon out of 700 will scatter in the next Hubble
time. As long as the Universe continues to expand, reactions will become
rarer and rarer as the density decreases. In fact, we will see that the typical
photon will never scatter again.
Things were quite different in the early Universe. Just before electrons
and nuclei recombined to form atoms, the temperature was 1000 times its
present value so the density of electrons was 10
9
times the present den-
sity. The expansion rate, given by the Friedmann equation (9.54), was only
√
Ω
M
1000
3/2
∼ 2 × 10
4
times the present rate:
λ
γe→γe
(t
rec
) ∼ n
e
(t
rec
) σ
T
c ∼ 80 H(t
rec
) . (9.62)
At this epoch, a typical photon suffered 80 collisions per Hubble time.
The thermal spectrum of photons resulted from the high reaction rate in
the early Universe. Elastic scattering, e.g.
γ e
−
↔ γ e
−
, (9.63)
caused energy exchanges between particles and generated “kinetic” equilib-
rium, i.e. a thermal momentum distribution. Inelastic collisions changed the
number of particles and generated “chemical” equilibrium where the parti-
cle densities have thermal values. For example, bremsstrahlung and photon
absorption
e
−
p ↔ e
−
pγ , (9.64)
creates and destroys photons, generating a thermal number density
n
γ
=
2.4
π
2
(kT)
3
(¯hc)
3
(9.65)
The elementary reactions
γγ ↔ e
+
e
−
↔ ν
¯
ν (9.66)
generated thermal (blackbody) densities of electron–positron pairs and neu-
trinos:
n
e
+
= n
e
−
= n
ν
i
+ n
¯
ν
i
=(3/4)n
γ
kT m
e
c
2
. (9.67)
The factor (3/4) comes from the fact that fermions must respect the Pauli
principle and, as such, have smaller numbers in thermal equilibrium. Also
important are the neutron–proton transitions:
ν
e
n ↔ e
−
p
¯
ν
e
p ↔ e
+
n . (9.68)

418 9. Nuclear Cosmology
These reactions established chemical equilibrium between protons and neu-
trons (4.35):
n
n
n
p
=exp
−(m
n
− m
p
)c
2
kT
kT > 1MeV . (9.69)
This determines the number of neutrons available for nucleosynthesis.
The minimal requirement for the establishment of thermal equilibrium is
that the reaction rate per particle be greater than the expansion rate:
λ
˙a
a
⇒ thermal equilibrium . (9.70)
The expansion rate is the relevant parameter because its inverse, the Hubble
time t
H
, gives the characteristic time for temperature and density changes
due to the universal expansion. Collisions can therefore perform the necessary
readjustments of momentum and chemical distributions only if each particle
reacts at least once per Hubble time.
Because of the expansion, the collision epoch was bound to end when the
reaction rate became less than the expansion rate, λ ˙a/a.Whathappens
to the thermal distributions once the collisions cease depends on the type of
equilibrium. For purely kinetic equilibrium, i.e. the momentum spectrum of
particles, the thermal character of the spectrum may be maintained by the
expansion. This is the case for the CBR photons.
On the other hand, chemical thermal equilibrium is maintained only by
reactions and the equilibrium is lost once the reactions cease. At the present
low temperature, chemical equilibrium would imply that nucleons would tend
to be in their most bound states near
56
Fe.Thisisnotthecasebecausethe
nuclear reactions necessary to reach this state ceased when the temperature
was much higher, kT ∼ 30 keV. Most nucleons were thus “stranded” in hydro-
gen and helium. We say that the nuclear reactions “froze” at a temperature
T
f
∼ 30, keV. The “freeze-out” left the Universe with a “relic” density of
hydrogen and helium nuclei that is far from the equilibrium density.
The nuclear freeze-out left the Universe with a reserve of free energy,
i.e. energy that can now be degraded by entropy producing (exothermic)
nuclear fusion reactions. In particular, hydrogen can be converted to helium
and helium to heavier elements once matter is gravitationally confined in
stars. Fusion reactions in stellar interiors transform mass into kinetic energy
of the reaction products which is then degraded to thermal energy including
a multitude of thermal photons. It is this increase in the number of photons
which is primarily responsible for the entropy increase.
3
After the photons escape from a star, entropy production can continue
if the photons are intercepted by a cold planetary surface. On Earth, solar
photons (T ∼ 6000 K) are multiplied into ∼ 20 thermal photons (T ∼ 300 K).
The accompanying entropy increase more than compensates for the entropy
decrease associated with the organization of life induced by photosynthesis.
3
Entropy is always approximately proportional to the number of particles.

9.5 Reaction rates in the early Universe 419
Without the thermal gradient between the Sun and Earth photosynthesis
would not be possible because of the second law of thermodynamics. We see
that the loss of thermal nuclear equilibrium in the early Universe provides the
free energy necessary for life on Earth. Without this energy source, life would
depend on the photons produced during the contraction phases of stars (Fig.
8.3).
The explanation for the current thermal disequilibrium is one of the great-
est triumphs of modern cosmology. Nineteenth century physicists were puz-
zled by the disequilibrium because they knew that all isolated systems tend
toward thermal equilibrium. They also worried about the future “heat death”
of the Universe when equilibrium will be reached, terminating all intelligent
activity. Modern cosmology appears to have inverted the sequence of events
since the state of thermal equilibrium occurred in the past rather than the
future.
It should be noted, however, that the early Universe can be said to have
been in thermal equilibrium only if we ignore the possibility of gravitational
collapse of inhomogeneities. Gravitational collapse results in the radiation of
photons (Sect. 8.1.1) and this process generates entropy. The initial homo-
geneous (and therefore low entropy) conditions of the Universe are therefore
“special” and require an explanation as to how they were established.
In order to establish the formalism for the study of reactions in an ex-
panding Universe, we consider a general two-body reaction
ij → kl (9.71)
In Sect. 3.1.4 we saw that the reaction rate per particle i is proportional to
the number of j particles present and to the cross-section times velocity:
λ
ij→kl
≡ n
j
σ
ij→kl
v . (9.72)
We note that λ
ij→kl
= λ
ji→kl
. This is simply because the reaction rate per
particle i is proportional to the number of particles j and vice versa.
The Boltzmann equation governing the time dependence of n
i
was derived
in Chap. 3 for a time-independent volume (3.32). For an expanding Universe,
the equation is modified to include the effect of the expansion:
dn
i
dt
= −3
˙a
a
n
i
− n
i
λ
ij→kl
+ n
k
λ
kl→ij
. (9.73)
The three terms in this equation describe the three effects that change n
i
:
the expansion of the Universe, destruction of i particles, and creation of i
particles.
While there is no analytic solution to the Boltzmann equation, there are
two very simple limits with approximate solutions. The first is if the expansion
rate is much greater than the destruction rate, ˙a/a λ
ij→kl
, and production
rate ˙a/a (n
k
/n
i
)λ
kl→ij
. In this case the first term dominates
dn
i
dt
= −3
˙a
a
n
i
⇒ n
i
∝ a
−3
. (9.74)

420 9. Nuclear Cosmology
As expected, the number density of i particles just dilutes with the expansion
of the Universe.
The second case occurs if the reaction rates are much greater than the
expansion rates, the Boltzmann equation is simply that for a gas of particles
in a fixed volume
dn
i
dt
= − n
i
λ
ij→kl
+ n
k
λ
kl→ij
⇒ n
i
∼ n
i
(T ) . (9.75)
The solution in this case is the thermal equilibrium solution (principle of
detailed balance). This comes about since if n
i
is greater than (less than)
the equilibrium value, the first term (second term) dominates and the time
derivative of n
i
is negative (positive). The equation then pushes n
i
to the
equilibrium value where the two terms cancel. The slow time dependence
due to the temperature decrease with the expansion is then enforced by the
first term of (9.73).
We can therefore get a qualitative understanding of the solution of the
Boltzmann equation by simply comparing expansion and reaction rates. De-
pending on which is greater, we will either have free dilution or thermal
equilibrium. This will be our strategy in the following sections.
9.6 Electrons, positrons and neutrinos
As a first application of the Boltzmann equation, we will treat the case of
electrons and positrons. These particles are created and destroyed principally
by the reaction
e
+
e
−
↔ γγ . (9.76)
The diagram is shown in Fig. 9.6. For high center-of-mass energies, E
cm
m
e
, the annihilation cross-section is
σ
e
+
e
−
→γγ
=(¯hc)
2
2πα
2
E
2
cm
[2ln(E
cm
/m
e
) − 1] E
cm
m
e
. (9.77)
For low energy, v c, the cross-section is proportional to 1/v as is expected
for barrier-free exothermic reactions:
σ
e
+
e
−
→γγ
v
c
=(¯hc)
2
πα
2
m
2
e
c
4
v c. (9.78)
To determine if the electrons and positrons are in chemical equilibrium
with the photons, we only need to calculate the thermal equilibrium value
of the annihilation rate and compare it with the expansion rate given by the
Friedmann equation (9.56). The expansion and annihilation rates are shown
in Fig. 9.7. For the annihilation rate, there are two simple limits, kT m
e
c
2
and kT m
e
c
2
.
For kT m
e
c
2
,wesimplyreplaceE
cm
in (9.77) with its mean value ∼ kT
so σv∝T
−2
(ignoring the logarithmic factor). The densities of electrons
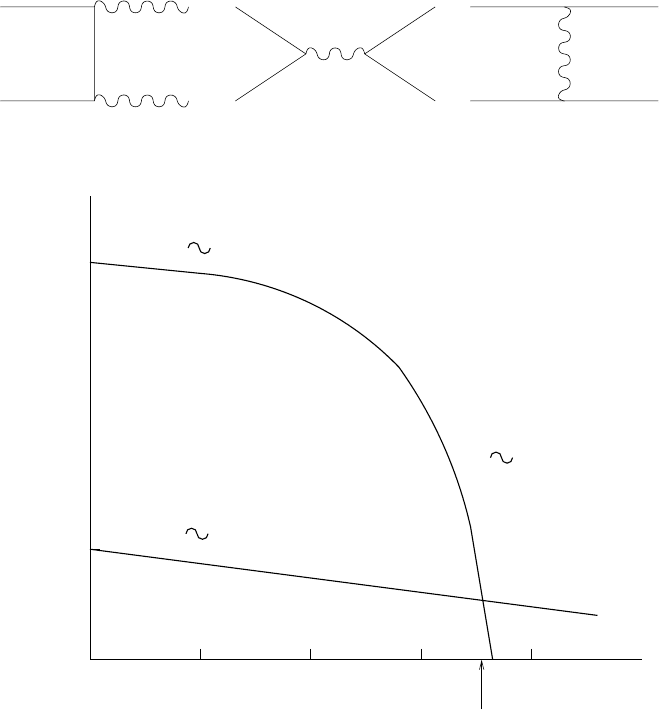
9.6 Electrons, positrons and neutrinos 421
W
e
ν
ν
γ
e
e
γ
e
e
e
e
ν
ν
Z
Fig. 9.6. The diagrams for the reactions e
+
e
−
↔ γγ and ν
¯
ν ↔ e
+
e
−
.
Γ
α
2
T
H
T
2
Γ
e
−m
e
f
c
2
c
2
/kT
10
−2
10
−1
Γlog (T)
110100
T
log H
kT / m
e
Fig. 9.7. The annihilation rate λ(e
+
e
−
→ γγ) and the expansion rate H =˙a/a
as a function of temperature under conditions of thermal equilibrium and with
n
e
−
= n
e
+
= n
e
.ForT>T
f
, λ> ˙a/a and n
e
will take its equilibrium value. For
T<T
f
, λ< ˙a/a and the reactions are “frozen.” After the freeze-out, the number
of electrons and positrons is constant so n
e
decreases as 1/a
3
.

422 9. Nuclear Cosmology
and positrons are proportional to T
3
and the annihilation rate is therefore
proportional to the temperature:
λ
e
+
e
−
→γγ
∼ ¯h
−1
α
2
kT ∼ 10
18
s
−1
kT
1MeV
kT m
e
c
2
, (9.79)
where we have suppressed the numerical prefactors. Comparing (9.56) and
(9.79), we see that λ>˙a/a for kT < 10
14
GeV. For example, at kT ∼ m
e
c
2
,
λ ∼ 10
18
˙a/a, i.e. 10
18
reactions per Hubble time. We can conclude that the
electrons and positrons were in chemical equilibrium with the photons for
m
e
<T <10
14
GeV.
The equilibrium is inevitably lost for T m
e
because, for a Universe
with equal numbers of electrons and positrons, the equilibrium density falls
rapidly with an exponential Boltzmann factor (Exercise 9.6):
n
e
−
= n
e
+
∝ exp(−m
e
c
2
/kT ) m
e
c
2
kT . (9.80)
The annihilation rate drops accordingly and one finds numerically that it falls
below the expansion rate when kT ∼ m
e
c
2
/40 when the electron–photon ratio
is ∼ 2 × 10
−16
. The temperature at which this occurs is called the “freeze-
out” temperature T
f
because after this temperature is reached the number of
electrons and positrons is frozen.
Figure 9.8a shows the ratio between the number of electrons and the
number of photons as a function of temperature calculated by numerically
integrating the Boltzmann equation. For T T
f
the electron–photon ratio
is fixed at about ∼ 2 × 10
−16
, confirming our qualitative argument.
The evolution of n
e
−
and n
e
+
for a universe like our own with an excess
of electrons over positrons is shown in Fig. 9.8b. The electron excess coupled
with charge conservation leads to a small number of electrons surviving the
primordial epoch.
The evolution of the neutrino density is governed by the same principles
as that of the electron–positron density. The three neutrino species can be
produced and destroyed at kT ∼ MeV by the reaction (Fig. 9.6)
ν
¯
ν ↔ e
+
e
−
. (9.81)
Since this reaction is due to the weak interactions, the cross-section for all
species is of order
σ ∼
G
2
F
E
2
ν
(¯hc)
4
m
e
c
2
E
ν
m
W
c
2
. (9.82)
The annihilation rate is therefore
λ
ν
¯
ν→e
+
e
−
= n
ν
σv∼
G
2
F
(kT)
5
¯h(¯hc)
6
∼ 1s
−1
kT
1MeV
5
m
e
c
2
kT , (9.83)
