Baggott J. The Quantum Story: A History in 40 Moments
Подождите немного. Документ загружается.

the quantum story
30
fi nish it in a hurry, and to do that I have taken off a couple of days from
the laboratory (this is also a secret).
The model was still nevertheless rife with contradictions. He summa-
rized his work in a manuscript he submitted to Rutherford on 6 July,
but which was never published. He left Manchester and returned to
Copenhagen a couple of weeks later, carrying the problems back with
him in his briefcase. On 1 August he and Margrethe were married. They
honeymooned in England, Bohr returning to the Cambridge and Man-
chester laboratories for brief visits before setting off for a holiday in
Scotland and then back to Copenhagen, where an academic position
now awaited him.
Bohr continued to work on the problem of atomic structure through
the rest of 1912 and into early 1913. For his hypothesis concerning the sta-
ble orbits to have any value, he needed to use it to explain the results of
recent experiments or predict the results of experiments yet to be done.
His next breakthrough came in February 1913, when he was given a clue
that would unlock the entire mystery. Hans Hansen, a young professor
of physics who had done some experimental work on atomic spectros-
copy at the University of Göttingen in Germany, drew Bohr’s attention
to something called the Balmer formula.
Spectroscopy is the study of the absorption and emission of electromag-
netic radiation by atoms and molecules. The simplest atoms exhibit the
simplest spectra and the hydrogen atom, consisting of a nucleus and just
one electron, has the simplest spectrum of all. Whilst it might have been
anticipated from classical physics that atoms such as hydrogen should
absorb and emit energy continuously, favouring no particular radiation fre-
quencies, the opposite is actually found. The hydrogen emission spectrum
is a ‘line’ spectrum of discrete, narrowly defi ned frequencies.
In 1885, the Swiss mathematician Johann Jakob Balmer had studied the
measurements of one series of hydrogen emission lines and found them
to follow a relatively simple pattern. He found that the frequencies of the
lines are proportional to the differences between the inverse squares of
pairs of integer numbers. In other words, the frequencies depend on the
numbers m and n where, for the hydrogen series that Balmer had investi-
gated, m = 2 and n takes the values 3, 4, 5, and so on.
a little bit of reality
31
Balmer’s formula was generalized in 1888 by Swedish physicist
Johannes Rydberg. He found that other spectral series follow a similar
relation, with the value of m taking different integer values. In themselves
the Balmer and Rydberg formulas were purely empirical and their origin
in terms of the underlying physics of the atom was quite obscure. Bohr,
however, immediately understood where the integer numbers had come
from.
He realized that an electron moving from an outer, high-energy orbit
to a lower-energy inner orbit causes the release of energy as emitted
radiation. If, as he had hypothesized, the orbits are fi xed, with energies
that depend on integer numbers that can be counted outwards from the
nucleus in a linear sequence, then the energy differences between the
orbits are also therefore fi xed.
For example, in the case where an electron circling the nucleus in
orbits characterized by the integer numbers n = 3, 4, 5, . . . drops down
into a lower-energy orbit characterized by m = 2 the result is the series
of emission lines studied by Balmer, which had become known as the
Balmer series. Putting m = 3 and n = 4, 5, 6, . . . gives another series that
had been observed in 1908 by German physicist Friedrich Paschen. Bohr
predicted the existence of a further series in the ultraviolet with m = 1,
and series in the infrared characterized by m = 4 and 5.
Bohr was further able to show that the constant of proportionality that
appeared in Rydberg’s formula (known as the Rydberg constant) can be
calculated directly by combining a number of fundamental physical con-
stants, including Planck’s constant, the electron charge, and the electron
mass. The Rydberg constant was well known at the time from spectro-
scopic measurements. Bohr’s calculation was within six per cent of the
experimental value, a margin well within the experimental uncertainty
of the values he had used for the fundamental constants.
A further series of emission lines named for American astronomer
and physicist Edward Charles Pickering was thought by experimental-
ists also to belong to the hydrogen atom. However, at the time, the Pick-
ering series was characterized by half-integer numbers which are not
admissible in Bohr’s theory. Instead, Bohr proposed that the formula
be rewritten in terms of integer numbers, suggesting that the Pickering
series belongs not to hydrogen atoms but to ionized helium atoms. An
the quantum story
32
awkward mismatch was later resolved by Bohr himself. The correction
gave a Rydberg constant for ionized helium some 4.00163 times greater
than that for hydrogen. The experimentalists had found this ratio to be
4.0016. This kind of agreement between theory and experiment was
simply unprecedented.
Bohr’s idea of stable electron orbits had a further consequence. The
electrons had to possess a fi xed angular momentum—a fi xed momen-
tum associated with their ‘rotation’ around the central nucleus—in units
of h divided by 2p. Transitions between the orbits had to occur in instan-
taneous ‘jumps’, because if the electron were to move gradually from one
orbit to another, it would again be expected to radiate energy continu-
ously during the process. Transitions between inherently non-classical
stable orbits must themselves involve non-classical discontinuous jumps.
Bohr wrote that:
. . . the dynamical equilibrium of the systems in the [stable orbits] can be
discussed by help of the ordinary mechanics, while the passing of the sys-
tems between the different [stable orbits] cannot be treated on that basis.
The integer numbers that characterize the electron orbits would come
to be known as quantum numbers, and the transitions between different
orbits would be called quantum jumps.
Bohr wrote to Rutherford on 6 March 1913, enclosing with his letter a
manuscript with the title: ‘On the Constitution of Atoms and Molecules’.
In his reply Rutherford reacted favourably but raised some diffi cult ques-
tions. He was particularly puzzled by the fact that, in Bohr’s model, an
electron in a high-energy orbit would somehow need to ‘know’ before-
hand the energy of the fi nal, destination, orbit in order to emit radia-
tion of just the right frequency. Rutherford was already ringing alarm
bells about the implications of the new quantum theory for our under-
standing of cause-and-effect, bells that would continue to peal loudly for
another century.
He also warned that Bohr’s manuscript was rather long: ‘I do not know if
you appreciate the fact that long papers have a way of frightening readers,’
he wrote. Bohr was nonplussed. The day before he received Rutherford’s
letter he had sent a revised draft of his manuscript. It was even longer.
a little bit of reality
33
Bohr resolved to go at once to Manchester and discuss the paper directly
with Rutherford. He wrote back on 26 March and declared his intention
to visit Rutherford in the fi rst days of the following week. Rutherford was
patient. After discussions through several long evenings, during which
he declared he had never thought Bohr should prove to be so obstinate,
he agreed to leave all the detail in the fi nal paper and communicate it to
the journal Philosophical Magazine on Bohr’s behalf.
The paper was published in July 1913, and was followed by two further
papers published in the same journal in September and November that
year.
Bohr’s model of atomic structure was a triumph. But, much like Planck’s
achievement in 1900, it was also rather mysterious. There were many
unanswered questions. The most pressing of these concerned the quan-
tum numbers. What did they mean? From where, exactly, had they
come?

34
Experiment forced the pace on the new quantum theory of the atom. It turned out that
the spectrum of the hydrogen atom was not quite so simple, after all. Some of the lines in
the spectrum had already been found some years before actually to be two, closely spaced
lines. Furthermore, lines in the spectrum were found to be split in low-intensity elec-
tric and magnetic fi elds. In addition to Bohr’s quantum number n, German physicist
Arnold Sommerfeld introduced two additional quantum numbers, k and m, to explain
the experimental results. These new quantum numbers were thought to be related in some
way to the quantization of the geometry of the stable electron orbits.
As the various lines in the spectrum were identifi ed with different quantum jumps
between different orbits, it was soon discovered that not all the possible jumps were
appearing. Some lines were missing. For some reason certain jumps were forbidden. An
elaborate scheme of ‘selection rules’ was established by Bohr and Sommerfeld to account
for those jumps that were allowed and those that were forbidden.
In the meantime, evidence for Einstein’s light-quantum hypothesis was also accu-
mulating. Einstein had used his hypothesis to make predictions about the photoelectric
effect, predictions that were duly borne out in experiments by American physicist Rob-
ert Millikan in 1915.
1
The status of light ‘particles’ was further enhanced when, in 1923,
4
la Comédie Française
Paris, September 1923
1
Einstein was awarded the 1921 Nobel Prize for physics for his work on the photoelectric
effect (not relativity). Bohr was awarded the 1922 Nobel Prize for his work on atomic structure.
Planck had been awarded the Prize in 1918 for his discovery of the quantum.
la comédie française
35
American physicist Arthur Compton and Dutch theorist Pieter Debye showed that light
quanta could be ‘bounced’ off electrons, with a consequent (and predictable) change in
their frequencies. These experiments appeared to demonstrate that light consists of parti-
cles with directed momenta, like small projectiles.
Despite this evidence, many physicists, including Planck and Bohr, dismissed the
light-quantum. They preferred to think of quantization as having its origin in atomic
structure, retaining Maxwell’s classical wave description for electromagnetic radiation.
Whatever theory was going to replace classical physics had to confront the diffi cult task
of somehow reconciling the wave-like and particle-like aspects of light in a single theory.
And, somehow, this theory also had to account for the inner structure of the atom. It had
to account for the stable electron orbits characterized by their quantum numbers.
An important clue would come from Einstein’s special theory of relativity.
Einstein had introduced his special theory in the fourth paper he pub-
lished in 1905. He had struggled, and failed, to fi nd a way to reconcile one
of the most disturbing observations in late nineteenth-century physics
with the body of accepted classical theory.
When in 1887 Michelsen and Morley could fi nd no evidence for
relative motion between the earth and the hypothetical, all-pervading
ether they concluded that the speed of light is constant and independent
of the motion of the light source. This was one of the most important
‘negative’ experiments ever performed, and led to the award of the 1907
Nobel Prize for physics to Michelsen.
The absence of an ether and an apparently universal speed of light
could not be accommodated in any kind of Newtonian conception of
absolute space and time. As he had done some months earlier for the
light-quantum, now Einstein decided to build a new theory out of the
bare minimum of assumptions in which the observations made by
Michelson and Morley would result. He found that he needed only two.
He assumed that the laws of physics should be identical for all observ-
ers. In particular, they should not depend in any way on how an observer
is moving in uniform motion, relative to an observed object. In practical
terms, this means that the laws of physics should appear to be identical
in any so-called inertial frame of reference, and so all such frames of ref-
erence are equivalent. An observer stationary in one frame of reference
(standing on the ground, say) should be able to draw the same conclusions
the quantum story
36
from some set of physical measurements as another observer moving
uniformly relative to the fi rst, or stationary in his own moving frame of
reference, such as a moving train or a spaceship.
Einstein also assumed that the speed of light should be regarded as a
fundamental, universal constant, representing an ultimate speed which
cannot be exceeded.
From these assumptions there fl owed a number of bizarre consequences.
Out went any idea of an absolute frame of reference (and hence the idea of
a stationary ether), together with absolute space, time, and simultaneity.
In came all sorts of strange effects predicted for moving objects and clocks
within what came to be recognized as a four- dimensional spacetime. The
theory was ‘special’ only insofar as it treated the special case of observers
moving in constant uniform motion in a straight line.
As Einstein explained it to his Olympia Academy friend Maurice
Solovine:
All movements of bodies were supposed to be relative to the light-carrying
ether, which was the incarnation of absolute rest. But after efforts to dis-
cover the privileged state of movement of this hypothetical ether through
experiments had failed, it seemed that the problem should be restated.
That is what the theory of relativity did. It assumed that there are no privi-
leged physical states of movement and asked what consequences can be
drawn from this.
There was a further, fundamentally important consequence of the principle
of relativity, which Einstein had explored in a short, fi fth paper published in
1905. Suppose a body emits light of total energy E equally in two, opposite
directions. When seen from the perspective of an observer in an inertial
frame of reference moving uniformly with respect to the body, there is a
perceived difference in the amount of energy lost by the body, such that:
. . . its mass decreases by [E/c
2
]. Here it is obviously inessential that the energy
taken from the body turns into radiant energy, so we are led to the more gen-
eral conclusion: The mass of a body is a measure of its energy-content . . .
The theory demanded that energy and mass be recognized as equivalent
and interchangeable, through the approximate relation E = mc
2
.
la comédie française
37
2
Pronounced ‘de Broy’.
This relation suggests that energy can be considered equivalent to mass,
and that all mass represents energy. Einstein’s earlier light-quantum
hypothesis makes the connection between the energy of a light-quantum
and its frequency. Now there were two simple, yet fundamental equa-
tions connecting energy to mass and energy to frequency. This seems to
beg an obvious question. Could these equations be combined?
French physicist Prince Louis de Broglie certainly thought so.
Thirty-one year old de Broglie
2
was the younger son of Victor, fi fth duc
de Broglie. He had originally intended a career in the humanities, and had
studied medieval history and law at the Sorbonne, receiving a degree in
1910. But he gained a passion for physics through the infl uence of his older
brother Maurice and his experiences during World War I serving in the
French Army, in fi eld radio communications, stationed at the Eiffel Tower.
After the war, de Broglie joined a private physics laboratory headed by
his brother which specialized in the study of X-rays. It was whilst he was
working at this laboratory in 1923 that he thought to combine the two
most iconic equations of special relativity and quantum theory:
The notion of a quantum makes little sense, seemingly, if energy is to be
continuously distributed through space; but, we shall see that this is not
so. One may imagine that, by cause of a meta law of Nature, to each por-
tion of energy with a proper mass [m], one may associate a periodic phe-
nomenon of frequency [n], such that one fi nds . . . [hn = mc
2
].
This seemed to imply that a light-quantum with frequency n would pos-
sess a mass, and hence a momentum, calculable as mass times velocity.
Such directed momentum had been revealed in Compton’s experiments,
so this appeared to make sense.
But it was de Broglie’s next step that was breathtaking. He later wrote:
After long refl ection in solitude and meditation, I suddenly had the idea, dur-
ing the year 1923, that the discovery made by Einstein in 1905 should be gen-
eralized by extending it to all material particles and notably to electrons.
If electromagnetic waves, characterized by a frequency n, possessed
associated particle-like properties such as momentum then, de Broglie
the quantum story
38
reasoned, perhaps particles such as electrons, characterized by a mass m,
might possess associated wave-like properties. He went on:
An electron is for us the archetype of [an] isolated parcel of energy, which
we believe, perhaps incorrectly, to know well; but, by received wisdom, the
energy of an electron is spread over all space with a strong concentration
in a very small region, but otherwise whose properties are very poorly
known. That which makes an electron an atom of energy is not its small
volume that it occupies in space, I repeat: it occupies all space, but the fact
that it is undividable, that it constitutes a unit.
De Broglie was suggesting that the electron could be thought of as a wave. A
beam of electrons could be diffracted, just like a beam of light. The connec-
tion could be reduced to a simple equation, l = h/p, the wavelength of the
wave is equal to Planck’s constant divided by the momentum, p (mass times
velocity), of the particle. That this wave nature of particles is not apparent
in everyday macroscopic objects is due to the very small size of Planck’s
constant h.
3
The dual wave–particle nature of matter is apparent only in the
microscopic world of fundamental particles, atoms, and molecules.
Whatever they were, these ‘matter waves’ could not be considered to be
in any way equivalent to more familiar wave phenomena, such as sound
waves or ripples on the surface of a pond. De Broglie was able to show that
the velocity of such matter waves would be greater than the speed of light—
forbidden in Einstein’s special theory of relativity—and could not therefore
be waves carrying energy. He therefore concluded that the matter wave:
‘represents a spatial distribution of phase, that is to say, it is a “phase wave”.’
The concept of phase was therefore critical to de Broglie’s work right
from the beginning. We can think of phase in terms of a simple sinusoi-
dal oscillation, with ‘peaks’ and ‘troughs’ in the height or amplitude of
the wave. A series of waves in which the peaks and troughs coincide in
space and in time are said to be ‘in phase’.
De Broglie’s interest in chamber music now led him to a major break-
through. Musical notes produced by string or wind instruments are
the result of so-called standing waves, vibrational patterns which ‘fi t’
3
If Planck’s constant were very much larger, the macroscopic world would be an even more
peculiar place than it is.
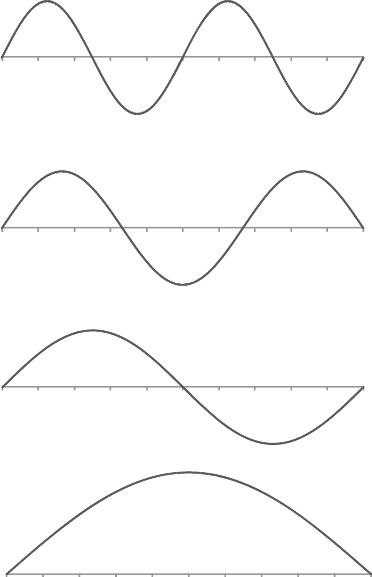
la comédie française
39
within the stopped length of the string or the length of the pipe. A vari-
ety of standing wave patterns is possible provided the patterns meet the
requirement that they fi t between the string’s secured ends or the ends
of the pipe. This means that they must have zero amplitude at each end.
This is possible only for vibrational patterns which contain an integral
number of half-wavelengths.
(d)
(c)
(b)
(a)
n=4
n=1
n=2
n=3
fig 3 De Broglie made a connection between the physics of musical notes and
quantum numbers. Musical notes generated by string or wind instruments are
derived from standing wave patterns. These wave patterns must have zero amplitude
at each end of the string or pipe, and so the only possible patterns contain an integral
number of half-wavelengths. In this fi gure, pattern (a) has no nodes (points where
the wave passes through zero), ( b) has one node, (c) has two and (d) has three. If we
defi ne the quantum number n as the number of nodes +1 (or the number of half-
wavelengths) then these patterns correspond to n = 1, 2, 3 and 4.
