Baggott J. The Quantum Story: A History in 40 Moments
Подождите немного. Документ загружается.

the quantum story
10
Theoreticians had simplifi ed the problem by invoking the idea of a
‘black body’, a hypothetical, completely non-refl ecting (i.e. totally black)
object that absorbs and emits light radiation without favouring any par-
ticular range of frequencies. The intensity of radiation emitted by a black
body is directly related to the amount of energy in the body when it is in
thermal equilibrium with its surroundings.
The theoreticians further realized that they could probe the properties of
a black body by studying the radiation trapped inside a cavity consisting of
perfectly absorbing walls, punctured with a small pinhole through which
radiation can enter and leave. Early examples of such cavities included
rather expensive closed cylinders made from porcelain and platinum.
3
In the winter of 1859–60, the German physicist Gustav Kirchhoff had
demonstrated that the ratio of emitted to absorbed energy depends only
on the frequency of the radiation and the temperature inside the cavity.
It does not depend in any way on the shape of the cavity, the shape of its
walls, or the nature of the material from which the cavity is made. This
implied that something quite fundamental concerning the physics of the
radiation itself was being observed, and Kirchhoff challenged the scien-
tifi c community to discover the origin of this behaviour.
Much progress had been made. Experimental studies of infrared
(or heat) radiation emitted from a radiation cavity had led physicist
Wilhelm Wien in 1896 to devise a relatively simple mathematical relation-
ship between the radiation frequency and cavity temperature. Wien’s
law seemed to be quite acceptable, and was supported by further experi-
ments carried out by Friedrich Paschen at the Technical Academy in
Hanover in 1897. But new experimental results reported in 1900 by Otto
Lummer and Ernst Pringsheim at the Reich Physical-Technical Institute
in Berlin showed that Wien’s law failed at lower frequencies. Wien’s law
was clearly not the answer.
Planck had succeeded Kirchhoff at the University of Berlin in 1889,
rising to full professor in 1892. He was a most unlikely scientifi c revo-
lutionary. Descended from a line of pastors and professors of theology
3
The study of cavity radiation was not just about establishing theoretical principles, how-
ever. It was also of interest to the German Bureau of Standards as a reference for rating electric
lamps.
the most strenuous work of my life
and jurisprudence, at school Planck was diligent and personable but not
especially gifted. Physics was a subject for which Planck himself felt he
had no particular talent, but he had risen through the academic ranks
and established a solid international reputation. Now in his early for-
ties, he worked at a slow, steady, and conservative pace. He preferred the
stability and predictability of a science which refl ected the character of the
bourgeois German society of which he was a part. By his own subse-
quent admission he was ‘peacefully inclined’, and rejected ‘all doubtful
adventures’.
During a visit to Planck’s villa in the Berlin suburb of the Grünewald
on 7 October 1900, experimental physicist Heinrich Rubens had told him
about some new experimental results he had obtained with his associ-
ate Ferdinand Kurlbaum. They had studied cavity radiation at even lower
frequencies. Ruben’s description of the behaviour of the radiation at these
frequencies set Planck thinking. After Rubens had left, Planck continued
to work alone in his study. He adapted Wien’s earlier law and arrived,
largely through some inspired guesswork, at an expression which fi t all
the available experimental data.
Planck had discovered his radiation law.
The law required two fundamental constants, one relating to temperature
and a second relating to radiation frequency. This second constant would
eventually gain the label h and become known as Planck’s constant. When
combined with the speed of light and Newton’s gravitational constant, the
two constants in Planck’s radiation law promised a fundamental underpin-
ning for all physical quantities. Planck wrote that the constants offered:
‘the possibility of establishing units of length, mass, time and temperature
which are independent of specifi c bodies or materials and which necessarily
maintain their meaning for all time and for all civilizations, even those
which are extraterrestrial and nonhuman, constants which therefore can
be called “fundamental physical units of measurement”.’
He sent Rubens a postcard on which he had written details of the new
radiation law, and he presented a crude derivation of the law at a meet-
ing of the German Physical Society on 19 October. He declared: ‘I there-
fore feel justifi ed in directing attention to this new formula, which, from
the standpoint of electromagnetic radiation theory, I take to be the sim-
plest excepting Wien’s.’ The next day, Rubens advised Planck that he had
11
the quantum story
100 200 300 400 500 600 700 800 900 1000
100 200 300 400 500 600 700 800 900 1000
T = 5000 K
Spectral Density
Wavelength/nanometres
T = 4000 K
T
= 3000 K
Planck
Wien
(a)
T = 5000 K
Spectral Density
Wavelength/nanometres
T = 4000 K
T = 3000 K
Planck
Rayleigh-Jeans
(b)
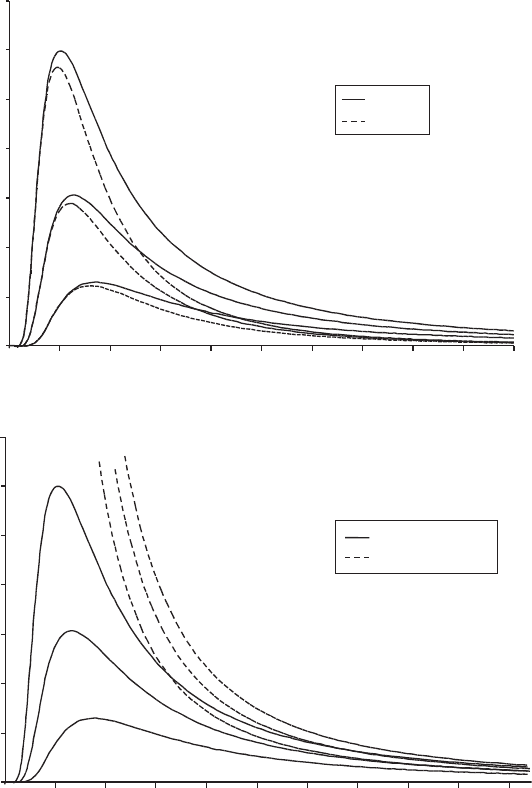
fig 2 a) Comparison of the predictions of Planck’s radiation law and Wien’s law for
three different temperatures. Wien’s law accurately reproduces the behaviour of black-
body radiation at very high frequencies (short wavelengths) but fails at lower frequen-
cies ( longer wavelengths). The discrepancy is most noticeable at higher temperatures.
b) Planck’s radiation law compared to the Rayleigh-Jeans law for the same three temper-
atures. The Rayleigh-Jeans law approaches the behaviour of black-body radiation at very
low frequencies (very long wavelengths) but gives catastrophic results in the ultraviolet.
the most strenuous work of my life
13
compared the experimental results with the new law and found ‘com-
pletely satisfactory agreement in all cases’.
It seemed that Planck’s radiation law was the answer, at least as far as
experiment was concerned. Planck now turned his attention to fi nding
a proper theoretical basis for the law, a task which was to lead to ‘some
weeks of the most strenuous work of my life.’
Planck set out the problem in terms of the interaction between the elec-
tromagnetic fi eld and a set of vibrating ‘oscillators’ in the cavity material.
The primary purpose of these oscillators was to ensure that the energy
was properly equilibrated among the possible radiation frequencies
through a continual, dynamic process of absorption and emission.
4
Being
an expert in entropy and the second law, he began by using the radiation
law to derive an expression for the entropy of an individual oscillator in
terms of its internal energy and its frequency of oscillation, which would
give rise to the same frequency of radiation inside the cavity. This gave
him an expression for the oscillator entropy known to be consistent with
experiment. His challenge now was to derive a similar expression from
‘fi rst principles’, compare the two, and draw appropriate conclusions.
It was this task that proved to be ‘strenuous’. Planck may have tried
several different approaches, but he found that he would always be com-
pelled to return to an expression strongly reminiscent of the statistical
methods of his rival Boltzmann. The mathematics was leading him in a
direction he had not wanted to go.
Boltzmann’s approach to calculating the entropy of a gas was to
assume that its total available energy could be thought of as being organ-
ized into a series of ‘buckets’. The lowest energy bucket was assigned an
energy e, the next an energy 2e, the next 3e, and so on. The gas mol-
ecules would then be distributed among the buckets and the number of
different possible permutations of molecules in the buckets calculated.
In this analysis the energy itself remains continuously variable. All that
4
Planck originally referred to these as ‘resonators’, but by 1909 he had accepted that the
special properties of resonators were not required. Today we would identify these oscillators as
highly excited electrons within the atoms of the cavity material ( but recall that the very exist-
ence of electrons had been confi rmed only three years earlier, in 1897).
the quantum story
14
Boltzmann had done was parcel it up so that he could count the number
of molecules in the energy range zero to e, the range e to 2e, and so on, and
thus calculate the number of different possible permutations.
For example, consider a gas consisting of just three molecules,
which we label a, b, and c. Let’s assume this gas has a total energy of
4e. We can achieve this by putting two molecules in the lowest, e,
energy bucket, and one in the 2e bucket. How many permutations are
possible? There are just three. We can put molecules a and b into the
lowest energy bucket and c in the next, a permutation we can label as
[ab,c]. We can also put molecules a and c in the lowest energy bucket,
and b in the next, labelled [ac,b]. The third possible permutation is
[bc,a].
Boltzmann reasoned that the most probable state of the gas would be
the one with the highest number of possible permutations for the avail-
able energy, representing maximum entropy at that energy. By equating
the maximum number of possible permutations with the most probable
distribution of energy it was a relatively simple step to the calculation of
the entropy itself.
Planck had been fi ghting a losing battle against Boltzmann’s logic for
at least three years. He now succumbed to the inevitable. As he later
explained: ‘I busied myself, from then on, that is, from the day of its
establishment, with the task of elucidating a true physical character for
the [new distribution law], and this problem led me automatically to a
consideration of the connection between entropy and probability, that
is, Boltzmann’s trend of ideas.’
Even though the problem of cavity radiation appeared to be totally
unrelated to the question of whether or not a gas was composed of
atoms or molecules, Planck now reached for the statistical methods of
the atomists. But there was a catch. Because he was working backwards
from the result he was aiming for, the statistical methods he needed were
actually far removed from those used by Boltzmann.
Planck’s statistical distribution was of a subtly different kind. Boltz-
mann had examined the permutations arising from the distribution of
distinguishable molecules over the various possible energy buckets. Planck,
however, examined instead the permutations of indistinguishable energy
elements (which we continue to label as e) over the various oscillators
the most strenuous work of my life
15
in the cavity material. For example, if we use Planck’s methodology to
distribute four energy elements (4e) over three oscillators, then we fi nd
there are now fi fteen possible permutations. We can put all the elements
in the fi rst oscillator, and none in the other two, a permutation which
we can write as (4e,0,0). Other permutations are (3e,e,0), (2e,e,e), (e,2e,e),
and so on.
Moreover, to get the result he wanted Planck found that the energy
elements had to be directly related to the frequency of the oscillators
(and hence the frequency of the radiation) according to his now famous
relation: e = hn—energy element equals Planck’s constant multiplied by
frequency. He further discovered that the energy elements had to be fi xed
in size as integer multiples of hn. In making these choices he was follow-
ing a very different path from Boltzmann.
Many years later Planck described his state of mind as follows:
Briefl y summarised, what I did can be described as simply an act of despera-
tion. By nature I am peacefully inclined and reject all doubtful adventures.
But by then I had been wrestling unsuccessfully for six years (since 1894)
with the problem of equilibrium between radiation and matter and I knew
that this problem was of fundamental importance to physics . . . A theoretical
interpretation therefore had to be found at any cost, no matter how high.
Planck, by now a willing and enthusiastic convert to the atomist view,
presented this new derivation of his radiation law to a regular fortnightly
meeting of the German Physical Society on 14 December 1900 shortly after
5 pm. As he explained to the assembled audience: ‘We therefore regard—
and this is the most essential point of the entire calculation—energy to be
composed of a very defi nite number of equal fi nite packages.’ He submit-
ted a paper to the journal Annalen der Physik in January 1901. About the
physical constant that was to carry his name he had this to say:
. . . since it has the dimensions of a product of energy and time, I called it
the elementary quantum of action or element of action in contrast with
the energy element hn.
The 14 December 1900 is widely acknowledged to be date on which the
quantum revolution began. But, in truth, Planck did not yet recognize
the quantum story
16
that his equation e = hn represented a fundamental unraveling of the
structure of classical physics.
In a possibly apocryphal tale, during a walk in the Grünewald, Planck
is reported to have told his seven-year-old son Erwin that he: ‘felt that he
had possibly made a discovery of the fi rst rank, comparable perhaps only
to the discoveries of Newton.’ If true, it is likely that Planck was referring
to the discovery of the properties of the second constant in his radiation
law—which he had called Boltzmann’s constant, k—and not the discov-
ery of the quantum of action and the fi xed energy elements of electro-
magnetic radiation.
Planck had used a statistical procedure, distributing the fi xed energy
elements over the oscillators, without giving much thought to the
physical signifi cance of this step. If atoms and molecules were real enti-
ties, something Planck was now ready to accept, then in his own mind
energy itself remained determinedly continuous, to fl ow uninterrupted
back and forth between radiation and matter. But in deriving his radia-
tion law, Planck had inadvertently introduced the idea that energy itself
could be ‘quantized’. There the idea sat, in Planck’s lectures and writings,
unarticulated, unnoticed, and unremarked.
It would take true genius to see what everyone else could not.

17
Planck used his radiation law to score some notable successes. In 1901 he used the available
experimental data to obtain estimates for both the Planck and Boltzmann constants. He
went on to use his estimate for Boltzmann’s constant to calculate Avogadro’s number (the
number of atoms or molecules present in a characteristic amount of pure substance called
a mole).
1
He then used his estimate for Avogadro’s number to determine the charge of the
electron. His estimates were all accurate to within one to three per cent of the currently
accepted values for these fundamental constants.
The convert became an ardent atomist: Planck began referring to atoms and molecules
as though they were real.
But whilst Planck’s results appeared to be well-founded, there remained doubts about
his derivation; about the somewhat devious manner in which he had arrived at his result.
Many were puzzled. With the benefi t of hindsight, this should not be surprising. Planck
had sprung a profoundly non-classical concept from within an otherwise entirely classi-
cal formulation. This was not something that could be done without some violence to the
classical prescription.
One young physicist who remained sceptical about Planck’s derivation was in 1905
working in the Swiss Patent Offi ce in Bern, as a ‘technical expert, third class’. His name
was Albert Einstein.
2
Annus Mirabilis
Bern, March 1905
1
A mole is the atomic or molecular weight of the substance in grams.
the quantum story
18
Einstein had joined the Swiss Patent Offi ce on 16 June 1902. This had been
something of a relief. After completing his graduate studies in physics at
the Zurich Polytechnic in August 1900 he had tried, and failed, to fi nd an
academic position at universities in Germany, Holland, and Switzerland.
He had been unemployed for a time before fi nding temporary work as a
high-school teacher.
Growing increasingly desperate about his employment prospects, he
had sought help from his fellow student and good friend Marcel Gross-
mann. Grossmann had become aware of an impending vacancy at the
Patent Offi ce and his father, who knew the director personally, was happy
to suggest Einstein’s name. Einstein had moved to Bern in anticipation of
his appointment to the post and, as he waited on a decision from the
Swiss Council, he had supported himself by offering private tuition in
mathematics and physics.
Later that year Einstein’s father, Hermann, died. On his deathbed
Hermann fi nally yielded to his son’s requests and gave permission for
him to marry his student sweetheart, Mileva Maric´. Einstein had fi rst
met Mileva when they both enrolled as students at the Polytechnic in
1896. She had become his muse; in their tender love letters he called her
‘Dollie’, she called him her ‘Johnnie’. However, it was not initially a match
that had met with approval from Einstein’s parents.
They were married in Bern on 6 January 1903, in a civil ceremony
witnessed by Einstein’s friends Maurice Solovine and Conrad Habicht.
Both had responded to the advert Einstein had placed in a newspaper
on his arrival in Bern, offering tutorials (with trial lessons offered free).
The three had formed a close friendship. They had held frequent discus-
sion meetings on a wide variety of subjects, proclaiming themselves the
‘Olympia Academy’.
Theirs was to be a long and enduring friendship. But there was one
secret that Einstein did not choose to share with even his closest friends.
He had fathered a child with Mileva. Their daughter, Lieserl, was born a
few days after Einstein had fi rst arrived in Bern. Mileva had returned to
her parents’ home in the Serbian city of Novi Sad for the latter stages of
her pregnancy and the birth. Einstein had written of his plans to bring
Mileva and the baby to Bern once things were settled, but then their plans
changed.
annus mirabilis
19
Whilst Einstein’s job at the Patent Offi ce gave him the fi nancial means
to support Mileva and their child, his position in Swiss offi cialdom
demanded a respectability incompatible with a child born out of wed-
lock. Mileva returned to Zurich alone, leaving Lieserl with relatives and
friends eventually, it seems, to be given up for adoption. Einstein never
saw his fi rst child, or held her in his arms. All mention of Lieserl ceased;
her very existence became a close family secret.
2
Her ultimate fate is
unknown.
3
Mileva bore Einstein a second, ‘replacement’ child, Hans Albert, on
14 May 1904. By this time Einstein was settled into his job at the Patent
Offi ce. He found the work interesting, and used it to sharpen his criti-
cal intellect and ground his thinking about physical theories in terms
of their direct practical consequences. His discussions with his friends
in the Olympia Academy provided a rich diet of empiricist philosophy
and the determinism of Dutch philosopher Baruch Spinoza’s impersonal
Deus sive Natura, God or Nature. These early intellectual infl uences were
to remain very powerful for the rest of Einstein’s life.
If Einstein had succeeded in his attempts to fi nd an academic position,
it is quite possible that the challenge of climbing the academic career
ladder would have demanded a certain conformity, a tendency to choose
‘safe’ research topics and churn out admirable, but hardly revolutionary,
research papers. But working quietly outside the strictures of academe
left Einstein free to be rebellious, to think—even suggest—the unthink-
able.
Habicht had moved away from Bern in the spring of 1905. Einstein
wrote to him in late May to tell him of his recent work:
I promise you four papers in return. The fi rst deals with radiation and the
energy properties of light and is very revolutionary . . . The second paper is
a determination of the true sizes of atoms . . . The third proves that bodies
on the order of magnitude 1/1000 mm, suspended in liquids, must already
2
This secret was revealed only in 1986 when researchers discovered a few, scant references to
her in Einstein’s private correspondence.
3
It has been suggested that Lieserl may have died from scarlet fever in September 1903.
Another hypothesis suggests that Lieserl was adopted by Mileva’s friend Helene Savic´. See
Isaacson, p. 87.
