All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

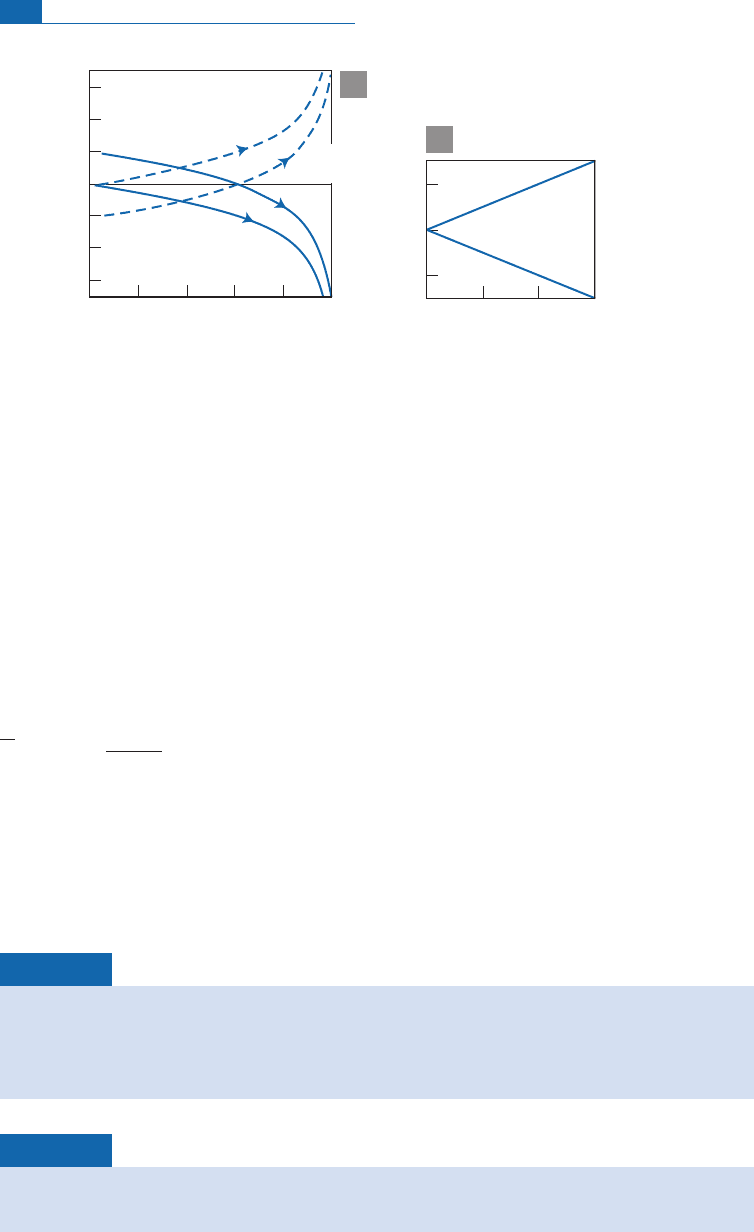
Figure 7.8 shows the Rayleigh law as a function of f where >1and <1.We shall see that
the e¡ects are oppositebut are only extreme whenf isverysmall.We seehow Aevolves, and
alsoB, for wh ich, ofcourse, wehave
R
B
¼ R
0
f
1
:
The mean composition of A iswritten:
R
A
¼ R
A;0
f
1
f 1
:
Itcanb e seenthatwhenf issmall,the compos itionsofthetwocompounds seemtoconverge.
And yet their partition coe⁄cient remains constant! But it is clear that as smal l variations
in f lead to large variations in d, the optical illusion gives the impression of convergence.
Notice too that when f ¼0, R ¼R
A,0
, because of course ‘‘matter is neither created or
destroyed’’asLavoisier said (except in nuclear reactions athigh energy!).
Exercise
Find the Rayleigh formula expressed in d.
Answer
d ¼d
0
þ10
3
( 1) ln
f
. See the next exercise.
Exercise
Let us go back to our example of the formation of sedimentary sulfides. For the time being, we
assume that as soon as the sulfide is formed, it reacts with iron dissolved in solution and
a
b
δ
18
O(‰)
f
20
0.8
0.6
δ
E α
=
1.01
δ
E α
=
0.99
δ
R α
=
0.99
δ
R α
=
1.01
α
=
1.01
α
=
0.99
0.4 0.2
-20
δ
0
R
δ
δ
–
δ
0
lnf
–2–1
20
0
–20
δ
R
=
δ
E
α
=
1
Figure 7.8 Changes in the instantaneous isotopic composition of a reservoir (dR) and an extract (dE)
during a Rayleigh distillation process as a function of the partition coefficient (1.01 and 0.99
respectively). We have
ext-res
> 1,
ext-res
¼1, and
ext-res
< 1 and an initial isotopic composition of
the reservoir d
0
R
¼0;
f
is the remaining fraction of the reservoir and (1
f
) the extent to which the
reaction has progressed. After Fourcade (1998).
379 The modalities of isotope fractionation
forms FeS
2
, without isotope fractionation (in fact, things are more complex than this). Being
heavy in its solid state, the iron sulfide settles out and is removed from contact with the
sulfates. This is a distillation effect. Given that in the end sulfates make up only one-third,
what are the sulfide compositions?
Answer
The initial d
34
S is still þ24. The kinetic coefficient is 1.025. Let us first apply the Rayleigh
equation, which we can use in a handier form with d. Its mathematical form invites us to shift
to logarithms. The formula becomes:
ln
R
¼ ln
R
0
þð 1Þ ln
f
:
Given that
R
¼
R
S
(1 þd/1000) with the logarithmic approximations ln(1 þ") ", and approx-
imating the two terms ln
R
S
, we get:
d ¼ d
0
þ 10
3
ð 1Þ ln
f
:
This is the form we shall use. The final composition of the sulfates is d ¼24 þ25 ln(1/3) ¼
24 þ27.7 ¼51.7.
The sulfides precipitating in the end have a
d
value of þ27.1. The average sulfide is
obtained by the balance equation
d
S average
¼þ10.4.
Exercise
In the first quantitative studies to estimate the degassing rate of magmas, Franc oise
Pineau and Marc Javoy (1983) of the Institut de Physique du Globe in Paris measured the
13
C/
12
C partition coefficient of CO
2
in a magma at 1200 8C and found 4.5ø (CO
2
being
enriched in
13
C). Let us take a basalt with an initial d
13
C value of 7. After degassing we find
d
13
C ¼26ø , with a carbon content of 100–150 ppm. If we assume a Rayleigh distillation,
what is the extent of degassing of the magma? What was the initial carbon content of the
magma?
Answer
We apply the Rayleigh law in d:
d d
0
¼ 1000 ð 1Þ ln
f
:
Hence: 20 ¼4.5 ln
f
and
f
¼0.011, therefore the magma was degassed to 98.8%. Its initial
carbon content was therefore 9000–13 000 ppm.
EXAMPLE
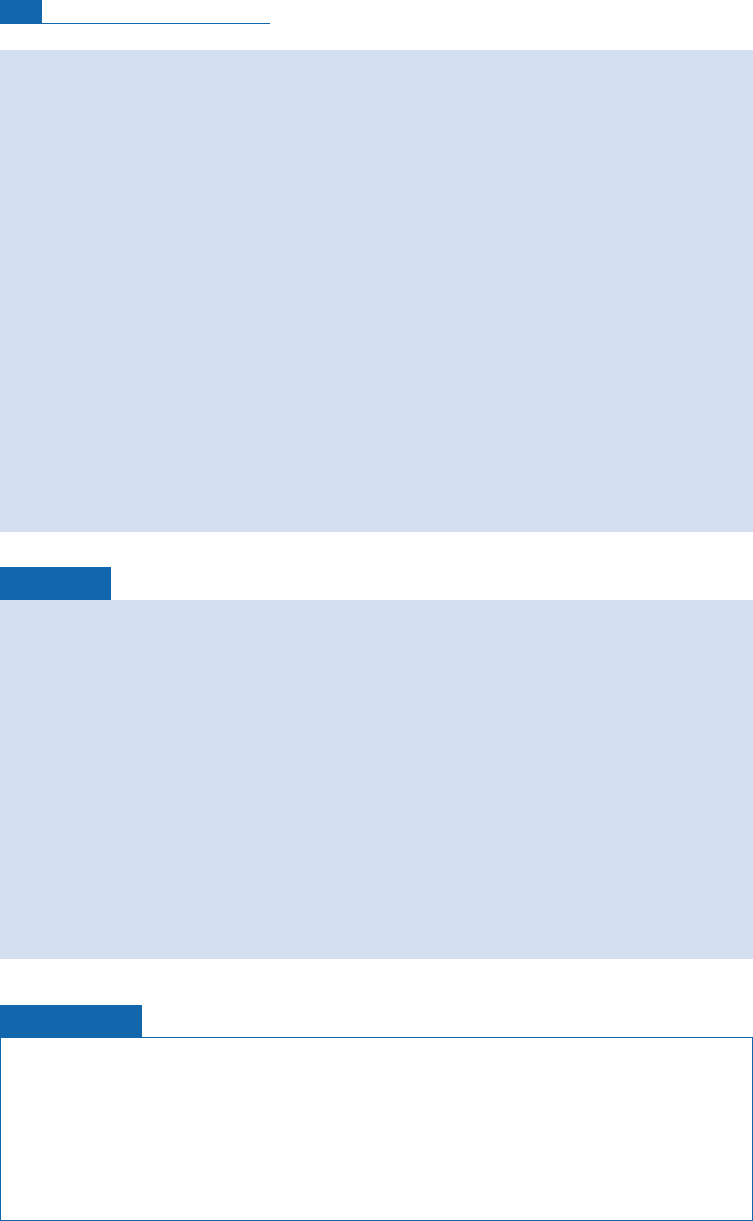
Isotopic evolution of a cloud shedding rain
A cloud forms over the sea. It then migrates over a landmass or migrates to higher latitudes
and loses rain. It is assumed that the cloud formed by the evaporation of sea water and that
the fractionation factor for the oxygen isotopes remains constant at ¼1.008. Figure 7.9
summarizes the isotope evolution of the cloud and of the rain that falls as it evolves. It is
described by a simple Rayleigh distillation.
380 Stable isotope geochemistry
7.3.4 Mixing
Aswehavealreadys een severaltimes,mixingoftwosources is an extremely importantphe-
nomenon ingeochemistry.Forexample,seawater isamixtureofthevariousinputsofrivers,
submarine volcanoes, rain, and atmospheric dust.We have a mixture of two components
A
1
and A
2
with isotop ic compositions:
x
A
y
A
1
and
x
B
y
B
2
:
Theisotope composition ofthe mixtureis:
x
A
y
A
m
¼
x
A
1
þ
x
A
2
y
A
1
þ
y
A
2
¼
x
A
y
A
1
y
A
1
þ
y
A
y
A
2
y
A
2
y
A
1
þ
y
A
2
:
Ifweposit:
y
A
1
y
A
1
þ
y
A
2
¼ x
1
and
y
A
2
y
A
1
þ
y
A
2
¼ 1 x
1
;
and ifwewritethe ratios
x=y
R :
x=y
R
m
¼
x=y
R
1
x
1
þ
x=y
R
2
ð1 x
1
Þ;
then replacing R by the d notationgives:
–30
–25
–20
–15
–10
–5
0
10.5
(1–f )
–35
C
l
o
u
d
R
a
i
n
Vapor
Liquid
(the liquid
is continuously
extracted from
the system)
α = 1.008
δ
18
O
Figure 7.9 Rayleigh distillation between a cloud and rain for d
18
O. The liquid (rain) is continuously
removed. The vapor fraction is 1
f
. After Dansgaard (1953).
381 The modalities of isotope fractionation

d
m
¼ d
1
x
1
þ d
2
ð1 x
1
Þ:
We¢ ndafamiliaroldformula!
Exercise
Carbonates have a
13
C/
12
C isotope composition expressed in d
13
Cof0ø. Organic products
precipitating on the sea floor have a d
13
C value of 25ø. What is the mean value of d
13
Cof
the sediments, given that 80% of the sedimentary carbon is in the carbonates and 20% in the
organic products?
Answer
The main isotopic component of carbon is
12
C. Therefore
x
and (1
x
) are 0.2 and 0.8,
respectively. This gives 0.2 (25ø) þ0.8 0ø ¼5ø. The average composition of the
sediments is therefore 5ø.
Mixinginacorrelationdiagramoftwoisotope ratiosobeystheequationsalreadydevelopedfor
radiogenic isotopes. Let the two elements whose isotopes are under study be A and B.
Remember that ifthe(C
A
/C
B
) ratiois constantforthetwo componentsofthe mixture, the mix-
tureisrepresentedbya straightline. Ifthetwo ratios are di¡erent, the mixture is representedby
ahyperbolawhosedirectionofconcavityisdeterminedby theconcentrationratiosofAandB.
7.4 The paleothermometer
In some sense, paleothermometry is to stable isotopes what geochrono metry is to radio -
genic isotopes, bothan example anda symbol.
7.4.1 The carbonate thermometer
An example ofthis¢eldofresearch hasbecomealegendofsorts. In1947,Haro l d Urey (1934
Nobel Prize winner for his discovery of deuterium, the hydrogen isotope
2
H) and
Bigeleisen and Maye r published two theoretical papers in which they calculated isotope
fractionation occurring in a series of chemical equilibria. In 1951, while professor at
Chicago University, Urey and his co-workers used his method of calculation to determine
the isotope equilibr ium of carbonate ions CO
2
3
and water (H
2
O) and calculated the isoto-
pic fractionation that must a¡ect the
18
Oand
16
O oxygen isotopes whose common natural
abundances are 0.205% and 99.756%, respectively. The (
18
O/
16
O)
carbonate
/(
18
O/
16
O)
water
ratio mustbe a fu nction ofthe temperature at whichthe two species are in equilibrium.The
variations Urey predicted were small but c ould be measured, after converting the CO
2
3
into CO
2
gas, on the double-collection mass spectro meter already developed by Alfred
Nieran d hisstudents attheUniversityofMinnesotaatthetime.Thisfractionationwasmea-
sured exp erimentallyby Urey’s team with the special involvement of Samu e l Epste in ,who
was to become one of the b ig names in the speciality. Together, they developed the simple
thermometric equation (in fact,the original coe⁄cientswereslightlydi¡erent):
T
8C
¼ 16:5 4:3 d
18
CO
3
d
18
H
2
O
þ 0:13 d
18
CO
3
d
18
H
2
O
2
382 Stable isotope geochemistry

where T
8C
is the temperature in degrees centigrade, an d d
18
CO
3
the isotope composition of
the CO
2
extracted from the carbonate, which is expressed bya deviation from the reference
carbonatesample:
9
d
18
CO
3
¼
18
O=
16
O
CO
2
; carbonateX
18
O=
16
O
CO
2
; standard
18
O=
16
O
CO
2
; standard
2
6
4
3
7
5
10
3
:
The standard chosen is a reference limestone known as PDB.The Chicago team decided to
use its carbonatethermo meter to measure g eological temperatures.To do this, theychose a
commo n, robust fossil, the rostrum (the front spike on th e shell) ofa cephalopod known as
a belemnite that lived in the Jurassic (150 Ma) and was similar to present-day squids.
Suppose that in the course of geological time, the isotopic composition of oxygen in sea
water had remained constantat d
18
O ¼0.Thenthe
18
O/
16
Ooxygen isotopic composition of
the carbona te of the fossils re£ects the temperat ure of the sea water in which the shell
formed.This isotope comp osition became ¢xed when th e carb onate was incorporated as
calcite crystals inthe fossilshells (as solid-phase reactions at low te mperature areveryslow,
there is little chance thatthe composition was altered bysecondary processes). By measur-
ing the isotope composition of fossils, it is possible to d etermine the temperature of the
an cient seas.To con¢rm this idea, the Chicago team therefore measured a series of belem-
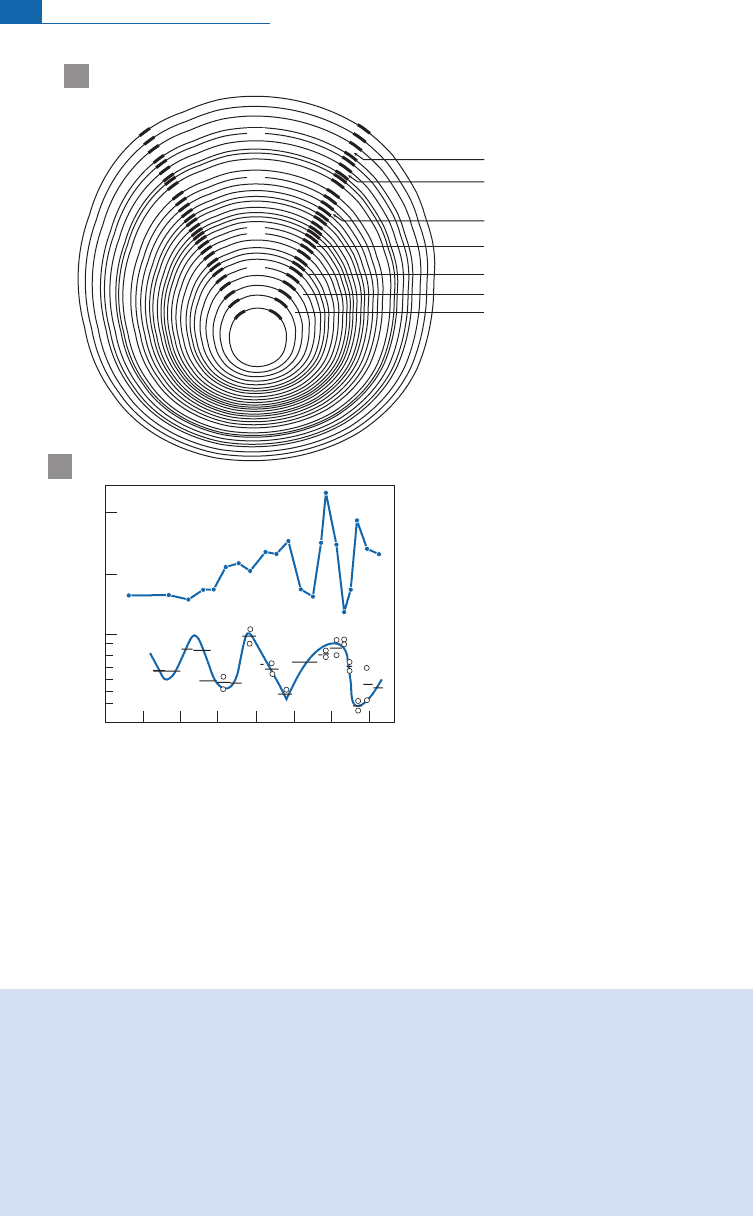
niterostrafromvariousgeographic areas and ofdi ¡erentstratig raphic ages(Figure 7.10).
The results, ¢rst announced in prelim inary form at the 1950 annual meeting of the
Geological Societyof Americawere spectacular and immediately claimed the attention of
the entiregeological community. Letus summarizethem.
At the scale of the planet, for the Jurassic, when belemnites lived, isotope temperature
obtained varied from 12 to 18 8C. These are likely and coherent temperatures; likely because
otherpaleoecological indicators areinagreementwiththem, coherentbecausevariationsover
time in various measurements in various parts of the world concord.Thus it has been deter-
minedthatthe maximumtemperaturewasintheLate Cretaceous,using samples from a single
area (Sweden,Britain) orsamplesincluding fossils collectedfromNorthAmericaand Europe.
Encouraged by these worldwide results, the Chicago scientists set about dissecting i ndi-
vidual rostra. Each rostrum is madeup ofconcentriclayerswhichareevidenceofbelemnite
an nual growth. Layer-by-layer analysis revealed regularly alternating temperatures.There
werethereforesummersandwinters atthetime!Theyeven managedtoshow thatoneparti-
cular individualwasborn in thefall and died inspringtime!
Exercise
The standard chosen for oxygen is SMOW (d
18
O ¼0). McCrea and Epstein’s simplified thermo-
metric equation is:
T
8C
¼ 16:5 4:3 d
18
O
CO
3
:
9
This is an important detail. It is not the isotopic composition of the CO
2
3
that is measured but that of the
CO
2
in equilibrium with the carbonate!
383 The paleothermometer
The precision of measurement of oxygen isotope composition is 0.1 in d units. What is the
power of resolution in temperature of the isotope method defined by Urey?
Answer
Differentiating the formula above gives
T
¼4.3 d. So the precision is 0.43 8C. One
might envisage further increasing the precision when making measurements with the
mass spectrometer to attain 0.01%, but this raises a geochemical problem: what do
the tiny differences revealed signify? We shall get some inkling of an answer in what
follows.
T(°C)
Radius (cm)
0.2 0.4 0.6 0.8 1.0 1.2 1.4
40
30
20
20°C
19
18
17
16
15
δ
13
C
T
20
15
10
5
W
W
S
S
W
S
W
a
b
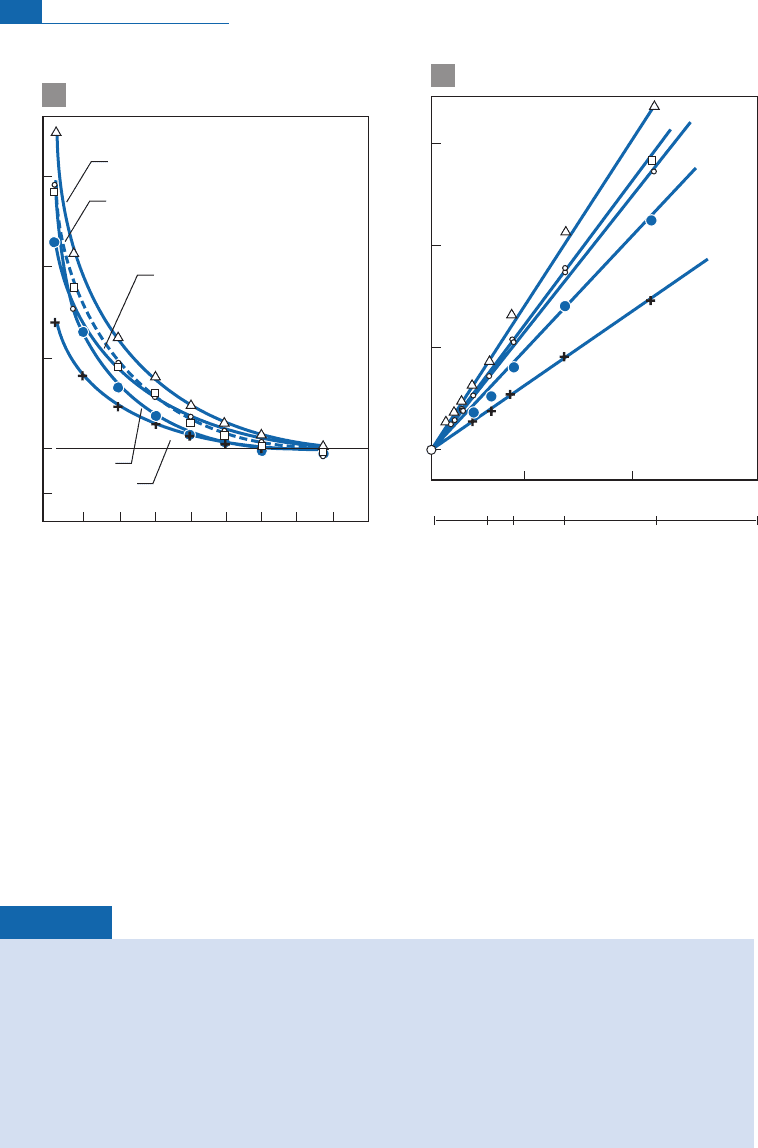
Figure 7.10 Study of a Jurassic belemnite rostrum. (a) A famous figure of a cross-section through a
Jurassic belemnite rostrum. Samples were taken a different radial distances (S, summer; W, winter;
numbers of rings are counted from the outside). (b) Values of d
13
C and below d
18
O converted directly
into temperature. The curve shows that the belemnite was born in the fall and died in spring! After Urey
et
a
l
.(1951).
384 Stable isotope geochemistry

This exceptional s cienti¢c success story opened the way to a new geological discipline,
paleothermometry, or the studyofpasttemperatures on a precise scienti¢c basis, whichgave
tremendous impetus to paleoclimatology. It also encouraged researchers to forge ahead. If
stable isotopes ofoxygen had yielded such signi¢cant results in their ¢ rst application in geol-
ogy, itcould behopedthatthe examinationofother problems,other p roperties,and otherele-
mentswouldbe equ allysuc cessful.Thishopegave risetotheworkthat founded stable isotope
geoch emi stry. However,theChicagoteam’spaleothermometer wasbasedonthe assumption
that
sea water
¼0 hasbeen constantthroughoutgeological times. As we shallsee, this hypoth-
esis probably holds over the average for mi llions ofyears but not on the scale of thousands of
yearswhich isthetimescaleofthe Quaternaryera (Epsteinet al.,1953; Epstein,1959).
7.4.2 The
18
O/
16
O isotope composition of silicates
and high-temperature thermometry
It is relativelyeasy to measurethe isotopic c omposition ofoxygen in carbonates since CO
2
3
reacts with phosphoric acid to transform into CO
2
, which c an be measured directly in
double-collector mass spectrometers. Itis far more di⁄cult to extract oxygen from silicate
minerals. This means using £uorine gas or even the gas BrF
5
and then transforming the
oxygen into CO
2
by bu rning. Of course, all such processes should be performed with no
isotopic fractionation or well -controlled fractionation! These techniques were developed
atthe California In stitute of TechnologybyHugh Taylor and Sa m E ps te in inthelate1960s
(Epstein andTaylor,1967).
Measuring the oxygen isotope composition of silicate minerals reveals systematic
variations with the type of mineral and the type of rock to wh ich the mineral belongs.
These compositions can be characterized by measuring isotope fractionation betwe en
minerals. Now,oneofthegreatfeaturesofisotop es is thatisotopefractionationisverylargely
indep endent of pressure and dependent mainly on temperature.Variations in volume asso-
ciated with exchange reactions are virtually zero. Therefore isotope equilibrium reactions
are very useful for determining the temperatures at which natural mineral associations
formed. Indeed varieswithtemperatureandtendstowardsunityatveryhightemperatures.
Aswehavesaid, thevariationofwithT takestheform:
ln ¼ B þ
C
T
þ
A
T
2
:
The form of this equation is p reserved for and . Between two minerals m
1
and m
2
in
equilibrium:
10
D
m
1
m
2
¼
m
1
m
2
A 10
6
T
2
þ B ¼ 1000 ln :
The term1/T is generally negligible. Oxygen isotopes are especially useful here. Oxygen
is the most abundant element i n silicates and the
18
Oand
16
Oisotopesfractionatein
nature in proportions that can be easi ly measured by mass spectrometry. Experimental
studies conducted mostly by the Chicago University group under Robert Clayton and
10
Tables usually give absolute temperatures so degrees must be converted from Celsius to Kelvin.
385 The paleothermometer

Jim O’Neil and supplemented by theoretical workof Yan Bottinga and Marc Javoy at the
Institutde Physique du Globe in Parishave provided a series ofreliablevalues forcoe⁄cients
A and B (seeO’Neil andClayton,1964;BottingaandJavoy,1975;Javoy,1 977).
In the experimental procedure, the isotope fractionation between minerals and
water is measured ¢ rst.This is a convenient method as isotope equilibration is attained quite
rapidlyat about80^100 8C.Thefractionationbetween minerals isthen calculated.
Tab l e s 7.1 and7.2 showthevaluesofcoe⁄cientsAand B for various m ineral^waterequili-
bria (we shall see the intrinsic importance of such fractionation later) an d then for fractio-
nationbetweenpairsofminerals.
Exercise
What is the d
18
O composition of a muscovite in equilibrium with water at 600 8Cwhosed ¼10?
Answer
The is written:
1:910
6
1
ð873Þ
2
!
3:1 ¼0:6
where ¼d
musc
d
water
.
From this we obtain d
musc
¼10.6.
Table 7.1 Isotope fractionation for mineral–water pairs
Mineral Temperature (8C) AB
Calcite(CO
3
Ca) 0^500 2.78 2.89
Dolomite 300^500 3.20 1.5
Quartz 200^500 3.38 2.90
Quartz 500^800 4.10 3.7
Alkali feldspar 350^800 3.13 3.7
Plagioclase 500^800 3.13 3.7
Anorth ite 50 0^80 0 2.0 9 3.7
Muscovite 500^800 1 .9 3.10
Magnetite (revers edslope)0^5 00 1.47 3.70
Table 7.2 Results of
18
O isotope thermometry based on
18
O/
16
O
fractionation of mineral pairs
Pair AB
Quartz^albite 0.97 0
Quartz^anorthite 2.01 0
Quartz^diopside 2.08 0
Quartz^mag n etite 5.57 0
Quartz^muscovite 2.20 0.6
Diopsid e ^magnetite 5.57 0
Source:AfterO’Neil(1986) modi¢edbyBottinga and Javoy (1975).
386 Stable isotope geochemistry
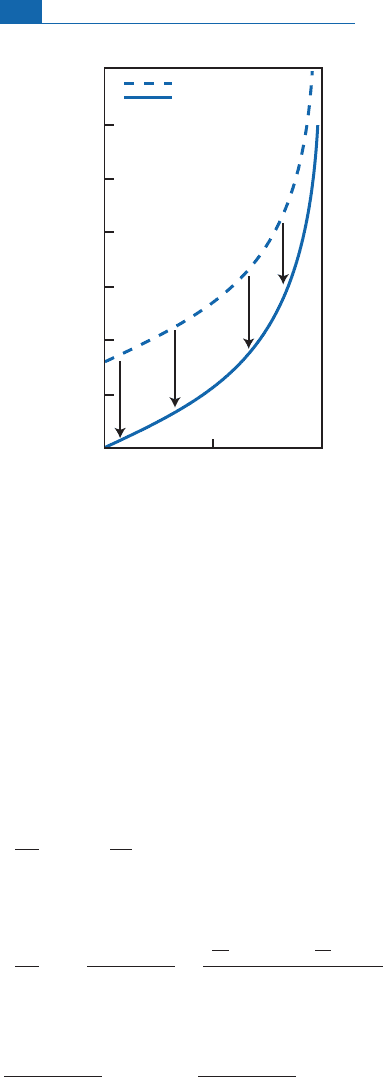
These areshown in Figure 7.11 intwoways: as afunctionoftemperature(8C) and as a function
of1 0
6
/T
2
b ecause the fractionations are linear.We plot1000 ln, that is , on the ordinates,
which means we can calculate
water
¼d
mineral
^ d
water
directly. Notice that fractionation
cancels its elf out at high temperatures. On the experimental curves, this convergence seems
to occur at less than ¼0, but this e¡ect is p robably due to experim ental errors.That would
meanthatminerals andwater wereofthesame composition athightemperatures.
Exercise
Water with d
water
¼10 and rock (composed of several minerals) with an initial d value of
d
(0)rock
¼þ6 are put together. If we mix 100 g of rock and 110 g of water and heat them to
high temperature (500 8C in an autoclave) for which we take a zero overall value, what will
be the composition of the rock and water after the experiment, given that the rock contains
50% oxygen and 90% water?
Answer
d
water
¼d
rock
¼4.29.
So having the values A and B for several minerals, we can calculate fractionation between
mineralpairsfor eachtemperature:
100 200 300 400 500 600 700 800
10
-5
0
20
30
T(°C)
Δ (M–H
2
O)
Quartz
K-spar
Anorthite
Muscovite
Calcite
a
5 10
0
10
Quartz
Calcite
Muscovite
Anorthite
K-spar
20
30
25 0100 800 300
(10
6
/T
2
)
Δ (M–H
2
O)
T(°C)
b
Figure 7.11 Isotope fractionation curves for water and some minerals as a function of temperature
(
T
,or10
6
/
T
2
). Notice that the curve should theoretically converge to zero. The error is the result
of experimental uncertainty. After O’Neil (1986).
387 The paleothermometer
D
m
1
m
2
¼ D
m
1
water
D
m
2
water
:
Letustakethe case ofquartz^muscovitebetween500and 800 8C:
D
quartzmusc
¼ 2:20 10
6
=T
2
0:6:
We can set about geological thermometry using these various pairs of minerals. Havi ng
measured
m
1
m
2
, we return tothe establishedformulaand calculateT.
In this way, th e temperatures ofvarious metamorphic zones havebeen determined. But,
ofcourse,muchaswithconcordanceofagesby various methods,wemustmakesurethevar-
iouspairs ofmineralsyieldthesametemperature.
MarcJavoy,S ergeFo urcade,andthepre s e ntautho r,attheInstitutde PhysiqueduGlobe
in Paris, came up with a graphical discussion method: afte r choosing a reference mineral,
wewriteforeach mineral:
D
quartzmineral
B ¼ A=T
2
:
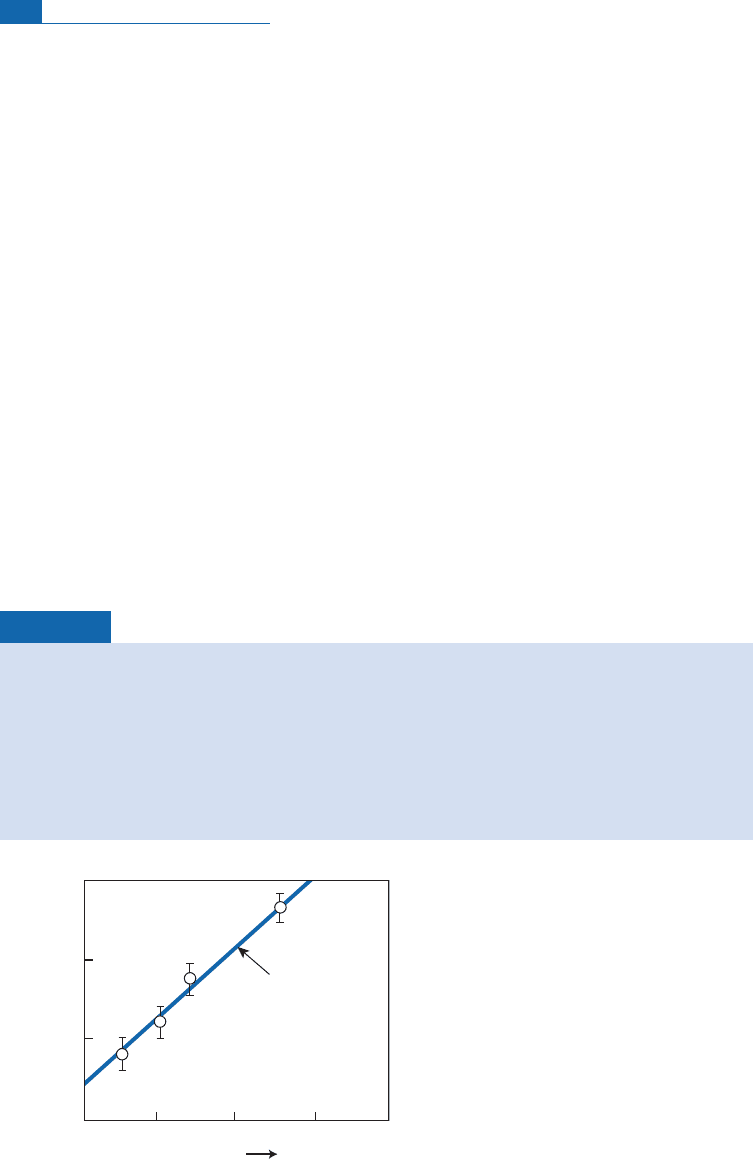
Inaplotof B againstA, thevarious mineralsofarockin isotopic equilibrium arealigned
on a straight line through the origin whose slope (1/T
2
) g ives the temperature at which they
formed (Figure 7.12). Ifthe points are not aligned, the rock is notin equilibrium and the tem-
peraturecannotbe determined.Itwasthuspossibletodraw upatableofthethermaldomains
where the main rocks were formed (Figure 7.13).These ¢ndings are consistent with indirect
evidencefrom mineralsynthesisexperimentsandmetamorphiczoneography.
Exercise
The d
18
O values of the minerals of a metamorphic rock are: quartz þ14.8, magnetite þ5.
(1) Calculate the equilibrium temperature of quartz–magnetite.
(2) Calculate the d
18
O of an aqueous fluid in equilibrium with the rock.
Answer
(1) 481 8C.
(2) þ11.3.
Mg
777 °C
Px
Hb
P
2
2
4
6
4 6
A
Δ–B
Figure 7.12 Javoy’s method of determining paleotemperatures, used here for San Marcos gabbro. P,
plagioclase; Hb, hornblende; Px, pyroxene; Mg, magnetite;
A
and
B
are defined in the text.
388 Stable isotope geochemistry
