All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

abundant such as sea water for
18
O/
16
O and D/H, a given carbonate for
13
C/
12
C, or even a
commercialchemical (Craig,1965).
Exercise
Oxygen has three stable isotopes,
16
O,
17
O, and
18
O, with average abundances of 99.756%,
0.039%, and 0.205%, respectively. The
16
O/
18
O ratio in a Jurassic limestone is 472.4335. In
average sea water, this same ratio is
16
O/
18
O ¼486.594. If average sea water is taken as the
standard, what is the
d
of the limestone in question?
Answer
By convention, d is always expressed relative to the heavy isotope. We must therefore invert
the ratios stated in the question, giving 0.002 116 7 and 0.002 055 1, respectively. Applying
the formula defining d
18
O gives d
18
O ¼þ30.
Exercise
The four naturally occurring, stable isotopes of sulfur are
32
S,
33
S,
34
S, and
36
S. Their average
abundances are 95.02%, 0.75%, 4.21%, and 0.017%, respectively. Generally, we are interested
in the ratio of the two most abundant isotopes,
34
S and
32
S. The standard for sulfur is the
sulfide of the famous Canyon Diablo meteorite
1
with a
32
S/
34
S value of 22.22. We express d
relative to the heavy isotope, therefore:
d ¼
ð
34
S=
32
SÞ
sample
ð
34
S=
32
SÞ
standard
1
!
10
3
:
If we have a sample of sulfur from a natural sulfide, for example, with
32
S/
34
S ¼23.20, what is
its d
34
S?
Answer
Given that the standard has a
34
S/
32
S ratio of 0.0450 and the sample a ratio of 0.0431,
d
34
S ¼42.22. Notice here that the sign is negative, which is important. By definition, the
standard has a value d ¼0.
7.1.1 The double-collection mass spectrometer
Variations in the isotope comp osition oflightelementsaresmall, evenverysmall. A precise
instrument is required to detect them (and a fastone, if we wantenough results to represent
natural situations).We have already seen the principle of how a mass spectrometer works.
Remember that in a scanning spectrometer, the magnetic ¢eld is varied and the ion beams
correspondingtothe di¡erentmasses(ordi¡erentisotopes)arepickedupinturnin ac ollec-
tor.The collector picks up the ions and provides an electric current which is fed through a
resistortogiveavoltageread-out.
As we have already said, in multicollector mass spectrometers, th e collectors are ¢xed
andthebeamsofthevariousisotopesarereceivedsimultaneously. In thisway wegetaround
1
Canyon Diablo is the meteorite that dug Meteor Crater in the Arizona desert.
359 Natural isotopic fractionation of light elements

the temporary £uctuations that may occur during ionization. However, the recording cir-
cuitsfor thevarious collectors mustbe identical.
Since1948, the double-collection mass spectrometer invented by Ni er has been used for
measuring slight isotopic di¡erences for elements which can be measured in the gaseous
state and wh ich are ion ized by electron bombardment (Nier, 1947;Nieret al., 1947).
2
The
two electri cal currents, picked up by two Faraday cups, are computed using aWheatstone
bridge arrangement, which we balance (we measure the resistance values required to bal-
an cethebridge).TheratioofelectricalcurrentsI
a/b
isthereforedirectlyrelatedtotheisotope
ratio R
a/b
by the equation:
I
a=b
¼ KR
a=b
where K is a fractionation factor and re£ectsb ias that may occur during measurement. It is
evaluated with an instantaneous calibration system using a standard.The standard sample
is measured immediatelyafter the unkn own samplex.Thisgives:
I
s
¼ KR
s
:
EliminatingK from the two equationsgives:
I
x
I
s
¼
R
x
R
s
:
The measurementofthe relativedeviation is then introducedquiten atu rally:
D
x
¼
R
x
R
s
R
s
¼
R
x
R
s
1
I
x
I
s
1
:
Aswearehandli ngsmallnumbers,thisnumber ismultipliedby1000forthesakeofconveni-
ence. This is wh ere th e de¢nition of the d unit comes from, which is therefore provided
directlybythe mass spectrometer measurement,since d ¼
x
10
3
.
This gas-source, double-coll ection mass spectrometer automatically corrects two types
of e¡ect. First, it eliminates time £uctuations which mean that when we‘‘scan’’ by varying
the magnetic ¢eld (see Chapter1), the emission a t time t when isotope1is recorded may be
di¡erentfrom emission attime(t þt)whenisotope2isrecorded.Second,itcorrectserrors
generatedbythe appliancebythe sample^standard switching te chnique.
The measurement sequence is straightforward: sample measurement, standard meas-
urement,samplemeasurement, etc.Theoperation is repeatedseveraltimestoensure meas-
urement reproducibility. Fortunately, many l ight elements can enter gas compounds.This
is the case of hydrogen in the form H
2
(or H
2
O), of carbon and oxygen as CO
2
, of sulfur
(SO
2
)or(SF
6
), of nitrogen (N
2
), of chlorine (Cl
2
), and so on. For other elements such as
b oron, lith ium, magnesium, calcium, and iron, it was not until advances were made in
solid-source mass spectrometry or the emergence of inductively coupled plasma mass
spectrometry (ICPMS), originally d eveloped for radiogenic isotope studies, that an
2
Multicollector mass spectrometers for thermo-ionization or plasma sources have been routinely used
only since the year 2000 because of electronic calibration difficulties.
360 Stable isotope geochemistry
e¡ective multic ollection technique could be u sed. This domain is booming today and we
shall touchupon itatthe endofthis chapter.
3
7.1.2 Some isotope variations and identifying coherence
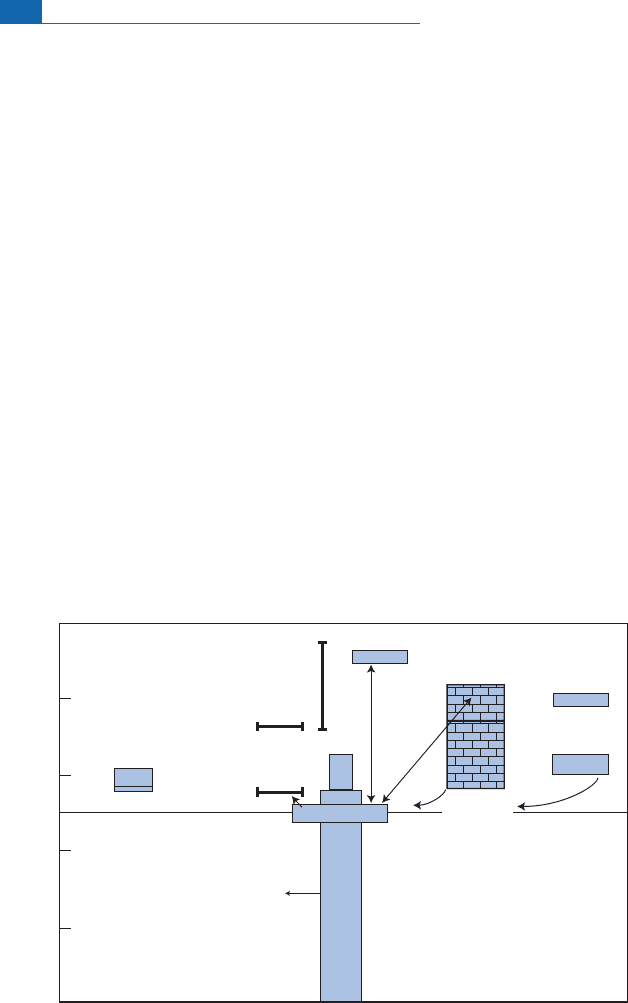
Oxygen
This is the most abundant chemical element on Earth, not only in the ocean but also
in the silicate Earth (Figure 7.1). Its isotope composition varies clearly, wh ich is a
godsend!
Oxyg en has three isotopes:
18
O,
17
O, an d
16
O (the most abundant).We generallystudy var-
iations in the
18
O/
16
O ratio expressed, of course, in units, taking ordinary seawate r as the
benchmark(with d ¼0 byde¢nition).
4
Systematic measurementofvarious naturallyoccur-
ring compounds (molecules, minerals, rocks, water vapor, etc.) reveals thatthey have char-
acteristic isotope compositions that are peculiar to their chemical natu res and th eir
geochemical origins, whatever their geological ages or their geog raphical origins. For
igneous or metamorphic silicate rocks d is positive, ranging fro m þ5toþ13. Such rocks
are therefore enriched in
18
O (relative to sea water). Limestones are even more enriched
sincetheird values vary from þ25 to þ34. Ofcourse, we mayaskwhat‘‘o¡sets’’such enrich-
ment in
18
O.
50
30
10
-10
-30
-50
Fresh
water
O
2
by
dissociation
O
2
dissolved
in ocean
water
Atm.O
2
Photosynthetic
ocean - O
2
α = 1.005
α = 1.041 at 25°C
α = 1.03 to 25°C
Carbonates
Sandstones
Shales
Diatomites
Exchange with
fresh water at
high temperature
Igneous
rocks
Granites
Basalts
Meteorites
12
45
41
34
30
15
10
25
7
23
7
5
Ocean water
Atm.CO
2
Volcanic water
δ
18
O (‰)
Limestones
(exchange)
Figure 7.1 Distribution of oxygen isotope compositions in the main terrestrial reservoirs expressed in
d
18
O. The isotope fractionation factors are shown for various important reservoirs. The smaller
numbers indicate extreme values. Values are of d
18
O expressed relative to standard mean ocean
water (SMOW). After Craig and Boato (1955).
3
The technique of alternating sample and standard used with electron bombardment of gas sources is
difficult to implement whether with sources working by thermo-ionic emission or by ICPMS because of
the possible memory effects or cross-contamination.
4
It is called standard mean ocean water (SMOW).
361 Natural isotopic fractionation of light elements

Which compoundshavenegative d values? We observe thatthose offreshwater are nega-
tive, ranging from 10 to 50. A few useful but merely empirical observations can be
inferred from this. As we know that limestones precipitate from s ea water, enrichment in
18
O suggests that limestone precipitates with enrichment in the heavy isotope. Conversely,
we know that fresh water comes from evaporation and then condensation of a u niversal
source,theocean.Itcanthereforebededucedthatthereis depletionin
18
Oduringthehydro-
logicalcycle(evaporation^condensation).Theseobservations suggestthereisac onnection
between certain n atu ral phenomena, their physical and chemical mechanisms, the origin
ofthe products,and isotope fractionation.
Hydrogen
Letusnowlookatthenaturalisotopicvariationsofhydrogen,thatis,variationsinthe(D/H)
ratio (D is the symbol for deuteriu m). Taking mean ocean water as the standard, it is
observed that organic products, trees, petroleum, etc. and rocks are enr iched in deuterium
whereasfreshwate r containslessof it (Figure 7.2).
We ¢nd similar behavior to that observed for oxygen, namely depletion of the heavy iso-
tope in fresh water and enri chment in rocks an d organic products. The product in which
hydrogen and oxygen are associated is water (H
2
O). It is important therefore to know
whether the variations observed for D/H and
18
O/
16
O in natural water are ‘‘coherent’’ or
not. Coherence in geochemistry is ¢rst re£ected by correlation. Epstein and Mayeda
(1953) from Chicago and then Harmon Craig (1961) of the Scripps Institution of
Oceanographyatthe University of C alifornia observed excellent correlation for rainwater
between D/H and
18
O/
16
O, which shows that there is‘‘coherence’’ in isotopic fractionation
related to the water cycle (Figure 7.3). This invites us th erefore to look more closely at any
quantitative relationsbetween isotopefractionationandthem ajor naturalphenomena.
500
300
100
-300
-100
-500
F resh
wa ter
10
–16
+4
Ocean w ater
Net prog ressiv e D
enrichment of ocean
T rees
fruits
petroleum
etc
ω
Organic
products
Meteorites
carbonaceous
chondr ites
Atm. H
2
Photo
dissociation
Escape from atmosphere
J uv enile w ater
?
δD (‰)
0%
Figure 7.2 Distribution of isotope compositions of hydrogen expressed in dD in the main terrestrial
reservoirs. After Craig and Boato (1955).
362 Stable isotope geochemistry
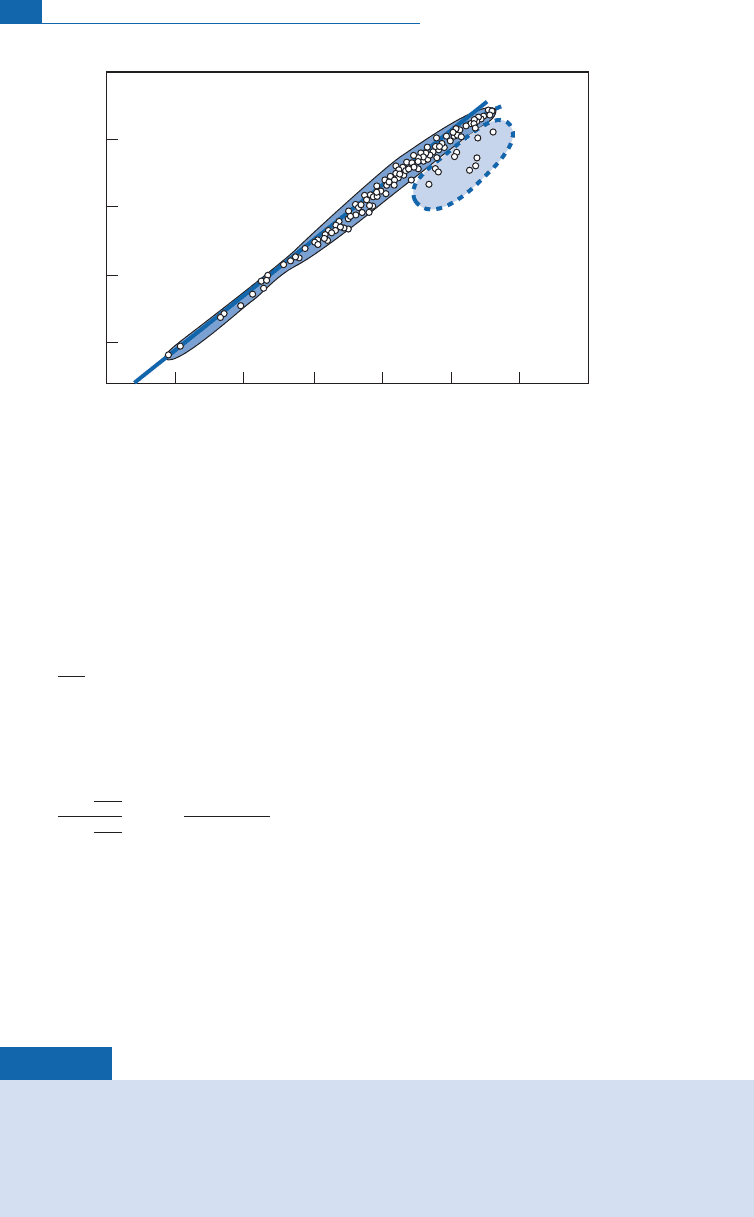
7.1.3 Characterization of isotope variations
Between two geological products A and B, related by a natural process, and whose isotope
ratios are n otated R
A
and R
B
, we canwrite:
AB
¼
R
A
R
B
where
AB
is the overall fractionation factor between A and B. With d
A
and d
B
being
de¢ned as previously, we can write:
AB
¼
1 þ
A
1000
1 þ
B
1000
1 þ
ðd
A
d
B
Þ
1000
following the approximation 1 þ "ðÞ= 1 þ "
0
ðÞ1 þ " "
0
ðÞ.
We note
AB
¼d
A
d
B
. This yields a fundamental formula for all stable isotope
geochemistry:
1000ð
AB
1ÞD
AB
:
Exercise
Given that the d
18
O value of a limestone is þ24 and that the limestone formed by precipita-
tion from sea water, calculate the overall limestone–sea water fractionation factor .
Answer
D
LimH
2
O
¼ d
Ca
d
H
2
O
¼ 24 0. We deduce that ¼1.024.
0
–100
100
–200
–300
–40 –30 –20 –10 0 +10 +20–50
δD(‰)
δO
18
(‰)
δD
= 8 × δ
18
O + 10
Closed
basins
Craig's meteoric water line
Figure 7.3 Correlation between (D/H,
18
O/
16
O) of rainwater. After Craig (1961).
363 Natural isotopic fractionation of light elements

It is possible, then, to c alculate the overall fractionation factors for various geological
p rocesses: the transition from granite to clay by weathering, the evaporation of water
between ocean and clouds, the exchange of CO
2
in the atmosphere with that dissolved in
the ocean or with carbon ofplants,and so on.
This is a descriptive approach, not an explanatory one. Various chemical reactions
and physical processes have been studied in the laboratory to determine the variations
in thei r associate d isotope compositions.Thus, for instance, ithasbeen obs erved that when
water evaporates, the vapor is enriched in light isotopes for both hydrogen and oxygen.
Fractionation factors havebeen de¢ned for each process fro m careful measurements made
in the laboratory.These elementary fractionation factors willbe denoted .
Geochemists have endeavored to synthesize these two types of information, that is,
to con nec t
q
an d
a
, in other words, to break down natural phenomena into a series of
elementary physical and chemical processes whose isotope fractionations are measured
experimentally. This approach involves m aking models of natural processes. We then
calculate from measurementsof made in thelaboratory.When the agreementbetween
so calculated and observed in nature is ‘‘good,’’ the model proposed can be considered a
‘‘satisfactory’’ i mage of reality.Thus, wh ile the studyofthe isotopic compositions of natural
compounds is interesting in itself, it also provides insight into the underlying mechan isms
ofnatural phenomena. Hencethe role oftracers ofphysical^chemical mechanisms ingeo-
logicalprocessesthat areassociatedwithstudies oflight-isotope fractionation.
In attempting to expose matters logically, we shall nottrace its historical development.We
shall endeavor ¢rstto present isotope fractionation associated withvarious types ofphysical
andchemicalphenomenaandthentolookatsome examplesofnaturalisotopefractionation.
7.2 Modes of isotope fractionation
7.2.1 Equilibrium fractionation
As a consequence of elements having several isotop es, combinations between chemical ele-
ments, that is molecules and crystals, have many isotopic varieties. Let us take the molecule
H
2
Oby wayofillustration.There are di¡erentisotopicvarieties:H
2
18
O, H
2
17
O, H
2
16
O, D
2
18
O,
D
2
17
O, D
2
16
O, DH
18
O, DH
17
O, DH
16
O (omitting combinations with tritium,T).These di¡er-
ent molecules are known as isotopologs. Ofthese, H
2
16
O accounts for 97%, H
2
18
O for 2.2%,
H
2
17
O for about 0.5%, and DH
16
O for about 0.3%.When the molecule H
2
Oisinvolvedina
ch emical process, all of its varieties contribute and we should write the various equilibrium
equationsnotjustforH
2
Oalonebutforallthe corresponding isotopic molecules.
Chem ical e qui l ibria
Letus consider, for example, the reaction
Si
18
O
2
þ 2H
16
2
O
!
Si
16
O
2
þ 2H
18
O;
which correspondstoa mass action law:
ðH
2
18
OÞ
2
ðSi
16
O
2
Þ
ðH
2
16
OÞ
2
ðSi
18
O
2
Þ
¼ KðTÞ:
364 Stable isotope geochemistry
Harol dUrey(1947),andindependentlyBigeleisen andMayer(1947),showedusingstatisti-
cal quantum mechanics thatthis kind ofequilibrium constant, although close to1, is di¡er-
entfrom1.
Moregenerally, for an isotope exchange reaction aA
1
þbB
2
!
aA
2
þbB
1
,whereB and A
are compounds and the subscripts1and 2 indicate the existence of two isotopes of an ele-
ment common to both c ompounds, we can write in statistical thermodynamics, following
Urey (1947) and Bigeleisen and Mayer (1947):
K ¼
QðA
2
Þ
QðA
1
Þ
a
QðB
1
Þ
QðB
2
Þ
b
:
Functions Q aretermedpartition functions ofthe molecule and aresuchthatforagiven sin-
gle che micalspecieswe canwrite:
Q
2
Q
1
¼
1
2
M
2
M
1
3=2
P
exp
E
2i
kT
P
exp
E
1i
kT
I
1
I
2
:
Inthis equation
1
and
2
arethe symmetry numbers ofmolecules1and2, E
2i
and E
1i
arethe
di¡erent rotational or vibrational energy levels of the mole cules, M
1
and M
2
are their
masses, andI
1
and I
2
aretheir momentsofinertia.
The greater the ratio M
1
/M
2
thegreater thefractionationbetweenisotopespecies,all else
being equal. It can alsobe shownthatln K, as foranyequilibrium constant, canbeputin the
form a
0
+ b
0
/T +c
0
/T
2
, which induces the principle of the isotopi c thermometer. It can be
deduc e d from theformulathat asT increases K tends towards1. At very high temperatures,
isotope fractionation tends tobecome zero and at low temperature it is much greater.
5
Ifwe
de¢ne the isotope fractionation factor associated with a process by the ratio
A
2
=A
1
ðÞ= B
2
=B
1
ðÞ¼
AB
, and K are related by the equation ¼ K
1=n
,wheren is the
numberofexchangeableatoms.Thus, inthe previous example, n ¼2 as therearetwooxygen
atomstobe exchanged, butusually ¼K.
Let us now writethe fractionation factor
AB
in d notation, noting each isotope ratio R
A
and R
B
:
d
A
¼
R
A
R
S
1
10
3
d
B
¼
R
B
R
S
1
10
3
;
R
S
being thestandard.
¼
1 þ
A
1000
1 þ
B
1000
!
1 þ
ðd
A
d
B
Þ
1000
;
sinced
A
and d
B
are small.
5
Remember that isotope geology studies phenomena from 80 8C (polar ice caps) to 1500 8C (magmas)
and in the cosmic domain the differences are even higher.
365 Modes of isotope fractionation

We come back to the equation (
AB
1) 100 0 ¼d
A
d
B
¼
AB
, which we met for the
factor.
Exercise
We measure the d
18
O of calcite and water with which we have tried to establish equilibrium.
We find d
cal
¼18.9 and d
H
2
O
¼5. What is the calcite–water partition coefficient at 50 8C?
Calculate it without and with the approximation ð1 þ d
1
Þ=ð1 þ d
2
Þ1 þðd
1
d
2
Þ.
Answer
(1) Without approximation:
calH
2
O
¼ 1:024 02.
(2) With approximation:
calH
2
O
¼ 1:0239.
Physical equilibria
Such equilibrium fractionation is not reserved for the sole case where chemical species are
di¡erent, but also appli es when a phase change is observed, for instance.The partial pres-
sure of a gas is Pg ¼P
total
Xg, where Xg is the molar fraction. Moreover, the gas^liquid
equilibrium obeys Henry’s law. Thus, when water evaporates, the vapor is enriched in the
light isotope. If the mixture H
2
18
OandH
2
16
O is considered perfect, and i f the water vapor
is aperfectgas, we canwrite:
PðH
2
16
OÞ¼X
e
H
2
16
O
P
0
ðH
2
16
OÞ
PðH
2
18
OÞ¼X
e
H
2
18
O
P
0
ðH
2
18
OÞ
where P isthetotalpressure,X designatesthemolarfractionsintheliquid, andP
0
(H
2
O) the
saturatedvapor pressure.Then (proveit as an exercise):
ðvaporliquidÞ¼
P
0
ðH
2
18
OÞ
P
0
ðH
2
16
OÞ
;
the denser liquidbeing thelessvolatile P
0
(H
2
18
O) < P
0
(H
2
16
O) and <1. Likeall fractiona-
tion fact ors, is dependentontemperature.UsingClapeyron’s equation,itcanbeshownthat
ln canbewritten in theform ln ¼ða=TÞþb. For waterat 20 8C (this is thevapor^liquid
coe⁄cient,nottheopposite!),
18
O
¼ 0:991 and
D
¼ 0:918.At208Cfractionationisthere-
fore about eight times greater for deuterium than for
18
O. (Rememberthisfactorof 8 forlater.)
Exercise
What is the law of variation of with temperature in a process of gas–liquid phase change?
We are given that ¼
P
0
(X
1
)/
P
0
(X
2
), where X
1
and X
2
are the two isotopes.
Answer
Let us begin from Clapeyron’s equation:
d
P
d
T
¼
L
vapor
TV
vapor
366 Stable isotope geochemistry

where
T
is the temperature,
V
the volume, and
L
vapor
the latent heat of vaporization.
1
P
d
P
d
T
¼
L
TVP
:
Since
PV
¼
nRT
(Mariotte’s law):
1
P
d
P
d
T
¼
L
RT
2
hence
d
P
P
¼
L
RT
2
d
T
:
Integrating both terms gives ln
P
¼
L
RT
þ
C
.
Since ¼
P
0
ðX
1
Þ=
P
0
ðX
2
Þ, we have:
ln ¼ ln
P
0
ðX
1
Þln
P
0
ðX
2
Þ¼
L
X
1
L
X
2
RT
þ
C
:
Exercise
The liquid–vapor isotope fractionation is measured for oxygen and hydrogen of water at three
temperatures (see table below):
Temperature (8C)
D
18
O
þ20 1.0850 1.0098
0 1.1123 1.0117
20 1.1492 1.0141
(1) Draw the curve of variation of with temperature in (,
T
), [ln(), 1/
T
], and [ln(), 1/
T
2
].
(2) What is the d value of water vapor in deuterium and
18
Oat208C and at 0 8C, given that
water has d ¼0 for (H) and (O)?
(3) Let us imagine a simple process whereby water evaporates at 20 8C in the temperate zone and
then precipitates anew at 0 8C. What is the slope of the precipitation diagram (d D, d
18
O)?
Answer
(1) The answer is left for readers to find (it will be given in the main text).
(2) At þ20 8C, d D ¼85 and d
18
O ¼9.8, and at 0 8C, d D ¼112.3 and d
18
O ¼11.7.
(3) The slope is 14.3. In nature it is 8, proving that we need to refine the model somewhat (the
liquids have as starting values at 20 8C, d D ¼0 and d
18
O ¼0 and at 0 8C, d D ¼27.3 and
d
18
O ¼1.9).
7.2.2 Kinetic fractionation
Forag eneralaccountofkineticfractionation see Bigeleisen(1965).
Transport phenomena
Du ring transport, as isotopic species have di¡erent mass es, they move at di¡erent speeds.
The fastest isotopes are the lightest ones. Isotopic fractionation may result from these
367 Modes of isotope fractionation
di¡erences in speed. Suppose we have molecules or atoms with the same kinetic energy
E ¼
1
2
mv
2
. For twoisotopic molecules1and 2 of masses m
1
and m
2
, we canwritev
1
, v
2
being
thevelo cities:
v
1
v
2
¼
m
2
m
1
1=2
:
The ratio of the speed of two‘‘isotopic molecules’’ is proportional to the square root of the
inverse ratio of their mass.This law corresponds, for example, to the isotopic fractionation
that occurs duri ng gaseous di¡usion for which the fractionation factor between two iso-
topes of
16
Oand
18
Ofor the moleculeO
2
iswritten:
¼
32
34
1=2
¼ 1:030:
Note in pas sing thatsuch fractionation is ofthe same orderas the fractionation we encoun-
tered during equilibrium processes! Such fractionation is commonplace during physic al
transportphenomena.Forexample, whenwaterevaporates, vapor is enriched in molecules
containing light isotopes (H rather than D,
16
O rath er than
18
O). In the temperate zone
(T ¼20 8C), for water vapor over the ocean
18
O ¼13, whereas for vapor in equilibrium
thevalue is closer to
18
O ¼9, as seen.
Chemical re actions
Isotopically di¡erent molecules react chemically at di¡erent rates. Generally, the lighter
molecules react more quickly. Lighter molecules are therefore at a kinetic advantage.This
is due to two combined c auses. First, as we have just seen, light molecules move faster than
h eavy molecules. Therefore light moleculeswill collide more. Second, heavy molecules are
morestable than lightones. During collisions, they willbe dissociated less often and willbe
less chemicallyreactive.The details ofthe mechan isms aremore complex. During a chemi-
cal reaction, thereis avariation in isotopic composition between the initialproduct andthe
endproduct.Letus consi der, for example,the reaction:
C þ O
2
! CO
2
:
Interms ofoxygen isotopes,there aretwo main reactions:
C þ
16
O
18
O ! C
16
O
18
O
C þ
16
O
16
O ! C
16
O
2
:
Remark
The other possible reactions are not important. The reaction C þ
18
O
16
O !C
16
O
18
O is identical to
the first in terms of its result. The reaction C þ
18
O
18
O !C
18
O
2
yields a molecule of very low
abundance as
18
O is much rarer than
16
O.
Thesetworeactionsoccuratdi¡erentspeeds,withtwokinetic constants,K
18
and K
16
.Letus
note the in itial con centrations of the pro duct containing the isotopes 18 and 16 as U
18
and
368 Stable isotope geochemistry
