All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

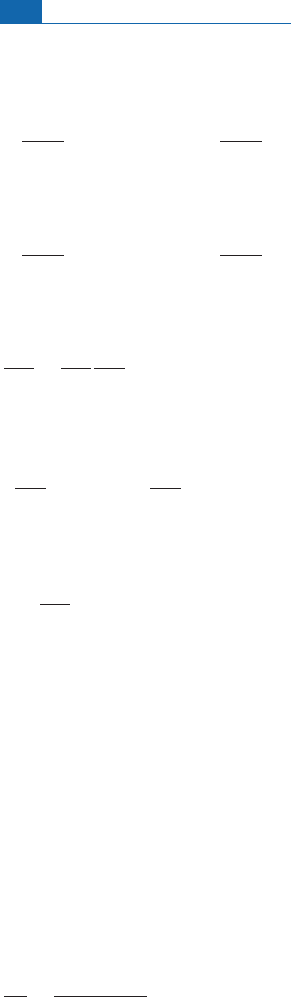
U
16
,giving
16
O
16
O,and noteasY
18
and Y
16
the concentrations ofC
18
O
16
OandC
16
O
2
.W e can
write:
dU
18
dt
¼ K
18
U
18
¼
dY
18
dt
and
dU
16
dt
¼ K
16
U
16
¼
dY
16
dt
:
Ifthe concentration ofinitialproductsiskept constant
Y
18
Y
16
¼
K
18
K
16
U
18
U
16
:
Therefore
18
O
16
O
CO
2
¼
18
O
16
O
O
2
;
or:
¼
K
18
K
16
:
The isotopic fractionation factor is equal to the ratio of th e kineti c constants for each
isotope.
A fuller expression ofthisratio maybeobtained bystatistical mechanicsby using the fact
that the kinetic process consists of two transitions, one towards the activate d complex and
theother towardsthestable compound.Naturally, weusuallyhaveveryfewdataonthisacti-
vate d complex which is very short-lived. Two reactions with two di¡erent isotopes (see
Lasaga,1997) arewritten :
A þ BC!
K
1
AB þ C and A þ BC
0
!
K
2
AB þ C
0
:
It can be shown that
K
1
K
2
¼
Q
ABC
0
Q
BC
Q
ABC
Q
BC
0
;
Q being partition functions corresponding tothe activated co mplex andtothe molecu les.
It should be po ssible to determine the parameters by spectrometry an d so check the
precision of this theory but in fact the problem is so complex that we are far from having
resolved the theoretical approach and having determined th e necess ary spectroscopic
parameter s. But we do understand the general sense of the mechanisms, which is the
mo st important thing. Experimental data are therefore us ed to model natural
ph en o mena.
369 Modes of isotope fractionation

T he temperature e¡ect
Du ring transport, isotopic fractionation is insensitive to temperature as it is in (m
1
/m
2
)
1/2
.
However, collisions and molecular recombinations are a function of energy and therefore
oftemperatureand aretheoreticallyactivated. Itisunderstandable, then, thatisotopic frac-
tionationvarieswithtemperature duringkineticprocesses.
Roughlyspeaking, temperature shouldpromote kinetic fractionation. Having made this
simple observation, things become more complicated. Isotopic exchange, the process by
which equilibrium is attained, is itself a kinetic process and is the refore activated by tem-
perature, so much so that the in creased fractionation because of kinetic e¡ects is progres-
sively cancelled because the equilibrium processes be come dominant and therefore
fractionationwill diminishwiththe increaseintemperature.
This doublegeneral process will thus lead to a lawofkineticfractionation represented by
a bell-shaped curve: fractionation increasing with temperature at ¢rst, and then de clining
beyond a certain temperature.This rule is modulated byspeci¢c kinetic mechanisms.This
is why, d espite many attempts, we have never managed to give a gene ral expression for
k inetic isotopic fractionationbasedon statistical mechanics.
Biologicale¡ects
Many (if not all) biochemical reactions i nvolve isotopic fractionation. A number of these
fractionation phenomena have been studie d in vitro and in vivo, elucidating the i ntimate
mechanisms of certain important biochemical reactions. It is understandable, then, that
somebiological mechanisms, formed by the combination or the succession ofbiochemical
reactions, produce isotopic e¡ects some ofwhich are particularly i mportant in geochemis-
try and so deserve our attention. Let us discus s two of them: sulfate^sul¢de reduction by
Desulfovibrio desulfuricans bacteria and chlorophyll photosynthesis (Harrison and
Thode, 1957,1958).
Sulfate ^ sul¢de reduction by Desulfovibrio desulfuricans bacteria The reaction for the
reduction of sulfate to sul¢de is written SO
2
4
) S
2
. It involves a big change in the
d egree ofoxidation of sulfur ( þ6)to (2), which is made possibl e atlow temperature o nly
by the intervention of the b acteria in question (conversely, the reaction S
2
! SO
2
4
is
easy). This bacterial reduction goes along with isotopic fractionation favoring the light
isotope of sulfur but whose amplitud e is well below that of the sul¢de , sulfate equili-
brium process, governed by the mass action law ( ¼1.025 at 25 8C ve rsus ¼1.075 for
the equil ibrium process). This me ans the sulfate is enriched in the heavy isotope (
34
S)
when there is fractionation with the sul ¢de. This fractionation plays a role in nature and
helps to ¢x the isotopic composition of low-temperature naturallyoccurring sul¢des (see
the end ofthis chapter).
Chlorophyll p hotosynthesis During this process atmospheric CO
2
is ¢xed and
the redu ce d carbon is i nc orp orated i nto organic mol ecul es. An en ri chm ent in
12
Ccom-
p ared with
13
C is observed. The d
13
C value of atmospheric CO
2
is 8 ø. For c arbonate
sediments, d
13
C varies from þ5to5ø. However, plants have d
13
C values ranging,
d epending on varieties, from 15 to 35ø . Park and Eps tein (1960)oftheCalifornia
370 Stable isotope geochemistry

Institute of Technology showed that an important step in
12
C enrichment occurred in
the process of ph otosynthesis. They were even able to attribute partition c oe⁄ci ents to
the di¡erent photosynthetic mechanisms (this is outs ide our ¢eld but is important in
biochemistry).
In short, letus say thatthebiochemi cal e¡ects are important.Theyare even fundamental
in some instances in geochemistry for un derstanding awhole series ofphenomena such as
those related to the CO
2
cycle or the sulfur cycle. But need they be considered as speci¢c
e¡ects of living organisms that are not bou nd by ordinary physical and chemical laws?
Various studies have shown on the contrary that biologic al processes involving enzymes
are in fact a series of chemical reactions.These reactions are associated with isotopic frac-
tionation,generallyofthekinetic type.There donotseemtobe ce rtain speci¢c mechanisms
(such as the spin e¡ect) for biological reactions.Thesebiological fractionations of isotopes
havebeendiscussedindetailbyEricGalimov(1985).
7.2.3 The effects of molecular symmetry: mass-independent
fractionation
All the e¡ects we have examined so far fractionate isotopes according to laws propor-
tion al to the di¡erence in mass of the isotopes.Thus, in carbonate precipitation,
18
O/
16
O
fractionation is twice
17
O/
16
O fractionation. In bacterial reduction of sulfate,
34
S/
32
Sfrac-
tion ation is half
36
S/
32
S fractionation. However, kinetic fractionation has been d iscov-
ered where di¡erences do not depend on the mass di¡erence but on the symmetry of
the m olecule. Thus,
18
O/
16
Oand
17
O/
16
O fractionation is the same. Mark Th ie me ns of
the University of California at San Diego has referred to these phen omena explaining
some fractionation observed by Robert Clayton in meteorites (Figure 7.4). He has proved
the reality of this ph enomenon in the laboratory (Thiemens and Heidenreich, 1983).
These e¡ects also occur in nature, for instan ce, with ozone (O
3
) in the atmosphere and
for sul¢des in meteorites and also in Precambrian rocks. Although their theoretical
explanation is complex ,
6
it does seem that th e decisive parameter in such fractionation is
molecular symmetry.
In this sense, two molecules
16
O
18
Oor
16
O
17
O, both equally asymmetri cal, should
havesimilardegrees offraction ation. Duringthe ozone-forming reaction in thehigh atmo-
sphere (at an altitude of 50 km), which reaction is extremely important as ozone not only
absorbsultravioletradiationand protectsthe Earth,
O þ O
2
! O
3
andthen
O
3
þ M ! O
3
þ M
in wh ich O
3
is the excited molecule, and M is the molecule with which O
3
collides and
becomes de-excite d.
6
This explanation was given by Rudy Marcus’s team at the California Institute of Technology chemistry
department, but is quite complicated. See Gao and Marcus (2001) for an example.
371 Modes of isotope fractionation
0
–10
–20
–30
–40
–40 –30 –20 –10 0 10
δ
17
O(‰)
δ
18
O(‰)
TF
CAI
CAI
C Chondrules
E Chondrules
O Chondrules
Figure 7.4 The d
17
O, d
18
O relation in chondrules and refractory inclusions of various meteorites (CAI,
calcium–aluminum inclusions). For these objects the correlation is of slope 1 whereas the usual
terrestrial fractionation (TF) correlation observed is of slope
1
2
, in line with the mass difference
between
17
O and
16
O and
18
O and
16
O. This discovery made by Robert Clayton
et
a
l
.(1973)is
interpreted by Thiemens (1999) as mass-independent fractionation, unlike Clayton who interpreted it
as a nucleosynthetic effect, and later as a photochemical effect (Clayton, 2002).
60
40
–20
–40
–60
20
20–20 40
δ
18
O
δ
17
O
–40–60 60
Starting
oxygen
Atmospheric O
2
Product ozone
Atmospheric waters
Mass fractionation line
Residual oxygen
Stratospheric
and
mesospheric CO
2
Tropospheric CO
2
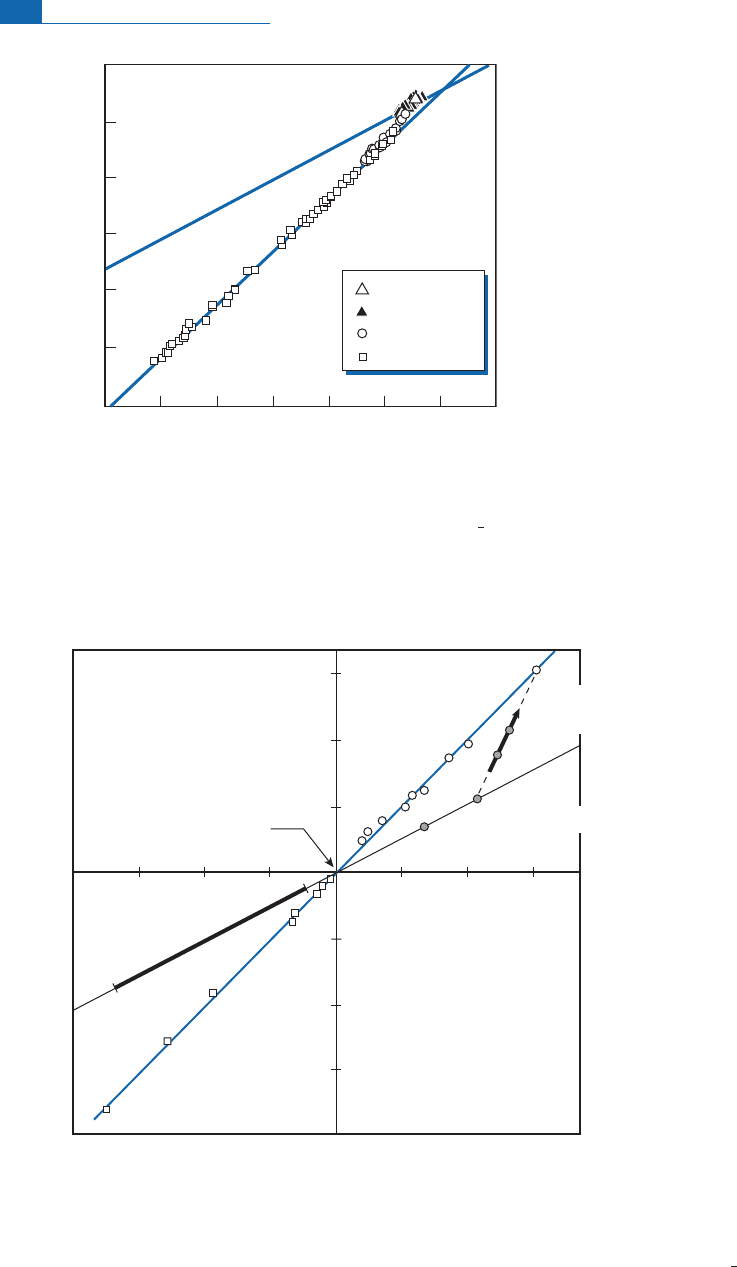
Figure 7.5 Mass-independent fractionation (MIF) for oxygen isotopes in atmospheric material
compared with classical mass-dependent fractionation. The line of slope 1 is MIF; the line of slope
1
2
is
mass-dependent fractionation.
372 Stable isotope geochemistry

It hasbeen shown thatozoneofmass 5 4 (
18
O
18
O
18
O)is not enriched relativeto
16
O
16
O
16
O
ozone of mass 48, whereas the asymmetric al molecule
16
O
17
O
18
O of mass 51is enriched by
200%. It has also been shown that symmetrical ozone molecules
17
O
17
O
17
Oor
18
O
18
O
18
O
are depl eted, whereas all th e asymmetrical molecules
16
O
17
O
17
Oor
17
O
18
O
18
O, etc. are
enriched.This e¡ect, which is called mas s- i n dep en dent fract ionatio n and might be more
appropriately termed the molecular symmetry e¡ect, seems to act with reactions such as
O þCO !CO
2
,OþSiO !SiO
2
,etc.
This is an important process in the atmosphere and seems to have played a role in the
presolar primitive nebu la as a l inear relation of slope1is found in carbonaceous meteor-
ites betwe en
17
Oand
18
O(Figure7.5). T his is an important e¡ect but highly speci¢c to
certain pro cesses. It is just beginn ing to be exploited but already very successfully (see
below).
7.3 The modalities of isotope fractionation
7.3.1 Kinetic effects or equilibrium effects? Isotopic exchange
We have already spoken of this in the earlier chapters. Let us recall a few facts here, as it is a
very important but often neglected phenomenon. L et us bring into contact two chemical
compounds, AO and BO, with at least one element in common, for example, both having
oxygen in their formulas. One of these species has been prepared with
18
Oexclusively,the
other w ith
16
O. Aftera certaintime in contactitcan be seen thatthe
18
O/
16
O composition of
thetwo compounds is suchthat:
ð
18
O=
16
OÞ
AO
ð
18
O=
16
OÞ
BO
¼ KðTÞ
where K (T) is the equilibrium constant. In othe r words, the isotopes
18
Oand
16
Ohave
exchangedsuch that equilibrium hasbeen attained.The rateofthis isotope exchange canbe
measuredand several phenomenaobserved:
(1) Itis fasterathigher temperatures.
(2) It is faster i n gases or liquids than solids. If one of the compounds is a solid it
becomes very slow (in this case the rate of di¡usion in the solid limits th e kinetics
of the process).
(3) It depends largely on the position oxygen oc cupies in th e steric con¢g uration of
compounds AO and BO,
7
that is, the nearer oxygen is to the outside of the
molecular structure, the faster the kinetics
8
^ this isotope exchange is essential in
geochemistry as it provides understanding of variou s fundamental observations
(Figure7.6 ).
7
Which relates to the spatial arrangement of the atoms composing the molecule.
8
For example, in the complex ion SO
4
, oxygen exchanges much faster than sulfur. This is why in sulfate
water S retains the memory of its source but O does not.
373 The modalities of isotope fractionation
Letus suppose we have a reaction A !B together with kinetic isotope frac tionation. If
A and B are left in contact for long enough, the isotopes of A and B swap over, and even-
tu ally the frac tionation betwee n A and B is of the equilibrium fractionation type. To
maintai n kinetic fractionation, the initial product and the end product must not be left
in contact. An example of this is the reductio n of the sulfate ion SO
2
4
to the sul¢de S
2
(by bacteria) which goes along with an out-of-equilibrium isotope e¡ect. I f, after partial
reduction, the sulfate ion remains in conta ct with the sul¢de ion, the system tends to
establish sulfate^sul¢de isotopic equi librium. Conversely, if the sul¢de io n S
2
is in the
presence of a ferrous i on Fe
2þ
, the following reaction occurs: 2S
2
þFe
2þ
!FeS
2
.This
iron sul¢de crystallizes and ‘‘isolates’’ the sul¢de from any further isotopic exchange which
would cancel out the kinetic e¡ect. This is why a number of naturally occurring sul¢des
have isotope compositions re£ecting the kinetic e¡ect (bacterial) related to sulfate
reduction.
Isotope exchange is activated by temperature; therefore, at high temperatures,
only swift and complete isolation of the resulting product can prevent equilibrium
fractionation from tak ing over. In practice, except for the case of gases th at e scape and
become is olated, such as g ases from volcanoes, it is generally di⁄cult to observe kinetic
e¡ects at high temperatures. In these circumst ances, equil ibrium e¡ects are mos tly
preponderant.
15
11
7
3
–1
–5
1
2 3 4 5
Time (days)
C
B
50%exchange
75%exchange
97%exchange
A
Δ
18
O (quartz–H
2
O) at 500 °C
equilibrium
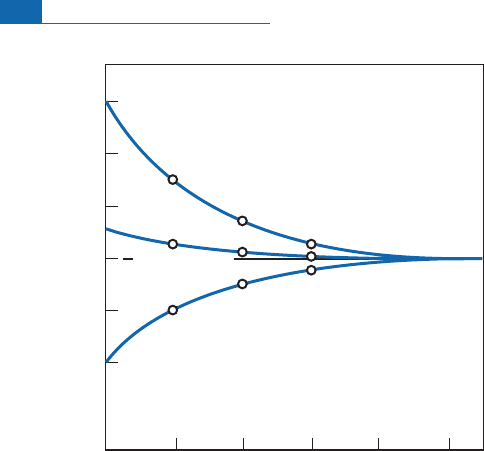
Figure 7.6 Kinetic curve showing the speed of equilibration by water–quartz exchange. The quartz has
a d
18
O value of 10. Three types of water with different compositions are brought into contact with the
quartz at 500 8C. The initial isotope compositions of the waters are expressed in d:A(5), B (þ5), and C
(þ15). The equilibrium value is 3. It can be seen that the three equilibration curves converge towards
the equilibrium value in a matter of days. After O’Neil (1986).
374 Stable isotope geochemistry

7.3.2 A consequence: isotopic memory
As we have already said when discussing radiogenic isotopes, it is fundamental to under-
stand that all isotope geochemistr y, including that of stable light isotopes, is based on the
factthat isotope exchange inthesolidphase atlow temperatures is veryslowandthe system
isnotconstantlyre-equilibrated,other wisetherewouldbenoisotopicmemory.Thisderives
from theissuesofdi¡usion coveredpreviously.
Let us take the example of calcareous fossil shells. A sh ell records the
18
O/
16
O isotope
composition of th e sea water it was formed in and also the ambient temperature. Once
formed, the shell moves around with the animalthat carries itand when the animaldies the
shellfallstothesea£oor.Thereitisincorporated into sedimentsandwiththemwillbepetri-
¢ed in a certain proportion and possibly, mu ch l ater, will be brought to the surface on the
continentsby tectonic processes. It will remain therefor millions ofyearsbefore ageologist
comes along and collects it for analysis. During this time, the fossil shell is in contact with
the grou ndwater thatcirculates in the outer layerofthe Earth. Howdoes the shell behave in
contact withthis new water? If itis isotopically re-equilibrated with thefreshwater whose d
valueisverydi¡erentfrom zero, itlosesitsformer isotopic compositionandsoitspaleother-
mal m emory. Its isotopic co mposition no longer re£ects the conditions of the old ocean
but the conditions of recent aqueous circulation. In fact, in most (but not all!) cases, the
shell remains compact and no isotope exchange occurs.The low rate of di¡usion ofoxygen
in calciteat lowor moderatetemperatureslimitsthemechanism.And allthebetter forg eol -
ogists! Theycan determine the pastte mperature ofth e ocean wherethe animalwhose shell
it was lived.
An important phenomenon is cool ing. Isotopic equilibrium among minerals is
established at high temperature.The mineral assemblage cools and so follows a decreas-
ing thermal trajectory. The isotope equilibrium constant is dependent o n temperature,
and isotope reactions should continue to take place constantly matching temperature
and isotope composition. If this were so, the system would lose all memory of its past
at high temperature and isotope analysis would me rely re£ect the low-temperature
equilibrium. In fact, as isotope exchange at low temperatures occurs very slowly, if
cooling is rapid, the minerals often retain the composition acquired at high temperature.
But this is not always so. Cooling is not always rapid. In metamorphism especially,
exchanges are sometime s accelerated by certain factors and ‘‘initial’’ isotope compo si -
tions are not always maintained. B ut as the oxygen di¡usio n constants of the various sil i-
cate minerals are di¡erent, the temperatures indi cated by the various minerals als o di¡er.
There is a sort of disequilibrium allowing us to detect the occurrence of any secondary
e¡ect.
All of this means that when measuring a compound’s is otopic composition we
must question the meaning of the message it carries and the time it was encoded.
Does it correspo nd to the period when the object formed? Is it the outcome of second-
ary ph enomena? If so, what phenomena? Once again, everything is dominated by iso-
tope exchange mechanisms. The imp ortance of these e¡ects is attested by the answer
to the following general observation. Why is sulfur isotope geochemistry not used
mo re often, since it has substantial natural variations (from þ60 to 40)? Because in
375 The modalities of isotope fractionation
many compounds, and particularly in sul¢de s, secondary isotope exchange occurs
very rapidly. Through this exchange, the compounds lose much of the isotope memory
of their origins. Another reason is the fact that sulfur geochemistry is highly complex
with many degrees of oxidation, etc. However, interesting results have bee n obtained
with sulfur isotopes.
7.3.3 Open system or closed system
T he open system or in¢nite reservoir
When one ofthe reservoirs presentis of in¢nite size (or is in direct contact with aboundless
reservoir) the modalities of isotope fractionation are governed by the initial fractionation
conditions and by conditions related to subsequent isotope exchange. No mass balance
e¡ectdisturbsthe relationb etween and :
¼
equilibrium
;¼
kinetic
; or ¼
mixed
;
depending on the nature ofthe initial fractionation andthe subsequ ent isotope exchange. If
the isotope composition ofthe in¢nite reservoir is R
0
, the‘‘large’’reservoir imposes its iso-
tope compositionthrough thefractionationfactor:
R ¼ R
0
and
0
þð 1Þ1000:
Exercise
Sea water has a d
18
O value of 0. Liquid–vapor fractionation at equilibrium at 20 8Cis
¼1.0098. What is the composition of the water vapor evaporating if it is in equilibrium
with the water?
Answer
The fractionation factor
18
O=
16
OðÞ
vapor
=
18
O=
16
O
liquid
¼ 1= ¼ 0:990 29: Therefore ( 1) ¼
0.0097, or d
18
O ¼9.7ø.
T he closed system
Where the system is closed, a balance e¡ect is superimposed on the modalities described.
We note the isotope composition of the initial system R
0
and assume that from there two
compounds, A and B, are produced with isotopic ratios R
A
and R
B
.We canwritean isotope
fractionation law (without specifying whether it is for equilibrium or not) characterized by
AB
, and an atom con se rvation equation.Th is gives: R
0
¼R
A
x þR
B
(1 x), where x is the
molar fraction ofthe element. In notation,thisgives:
d
0
¼ d
A
x þ d
B
ð1 xÞor d
0
¼ðd
A
d
B
Þx þ d
B
or d
0
¼ D
AB
x þ d
B
:
Exercise
Let us consider bacterial reduction SO
2
4
! S
2
by
Desulfovibrio desulfuric
a
ns
. The kinetic
fractionation factor
34
S/
32
S between sulfate and sulfide at 25 8C is 1.025 (Harrison and
376 Stable isotope geochemistry

Thode, 1958). Let us suppose that bacterial reduction occurred in oceanic sediment that was
continually supplied with sulfate ions. The sulfate stock can therefore be considered infinite.
What is the composition of the S
2
on the ocean floor if the d
34
S of the sulfate is þ24?
Answer
Applying the equation
AB
¼10
3
ln gives ¼þ24.6.
d
sulfate
d
sulfide
¼þ24.6 hence it can be deduced that d
sulfide
¼0.6.
Exercise
Let us suppose now that the sediment becomes isolated from the ocean and is no longer
supplied with sulfate ions and that the same phenomenon occurs. The quantity of organic
matter is such that the proportion of sulfur in the state of sulfate is 1/3. Suppose that, in the
initial state, all of the sulfur was in the sulfate state at d
34
S ¼þ24. What is the isotope
composition of S
2
? What is the isotope composition of the sulfate?
Answer
We apply the equation:
d
0
¼ D
AB
x
þ d
B
; or d
B
¼ d
0
D
AB
x
:
From this we obtain d
S
2
¼ 15:8; d
SO
4
¼ 40:4.
As seen in the previous exercise, the result is markedlydi¡erent foran open system, as the d
value is thenpositive.The e¡ectofthe closed system has shiftedthe isotopevaluesofthe sul-
fate and sul¢de, but not the fractionation factor, of course! (The limiting cases wherex ¼0
and x ¼1shouldbe examined.)
However, a £aw can be found in the foregoing reasoning. If the sul¢des remained in a
closed system as ions long enough, it might be that there was some isotopic exchange and
that the sulfate and sul¢de attai ned thermodynamic equilibrium. In this case ¼1.075 at
25 8C(TudgeandThode,1950). Repeating the calculation with this value gives
d
34
sul¢de
¼0. 14 and d
34
sulfate
¼72.4.
Intermediatescenarios canbe imaginedandtherefore,in nature,thevalueswill probably
beinterm ediateones.
As just seen, then, wi dely di¡erent isotope values are obtained for the same phenom-
enon but di¡erent modalities. It is probably the diversity of modalities that accounts for
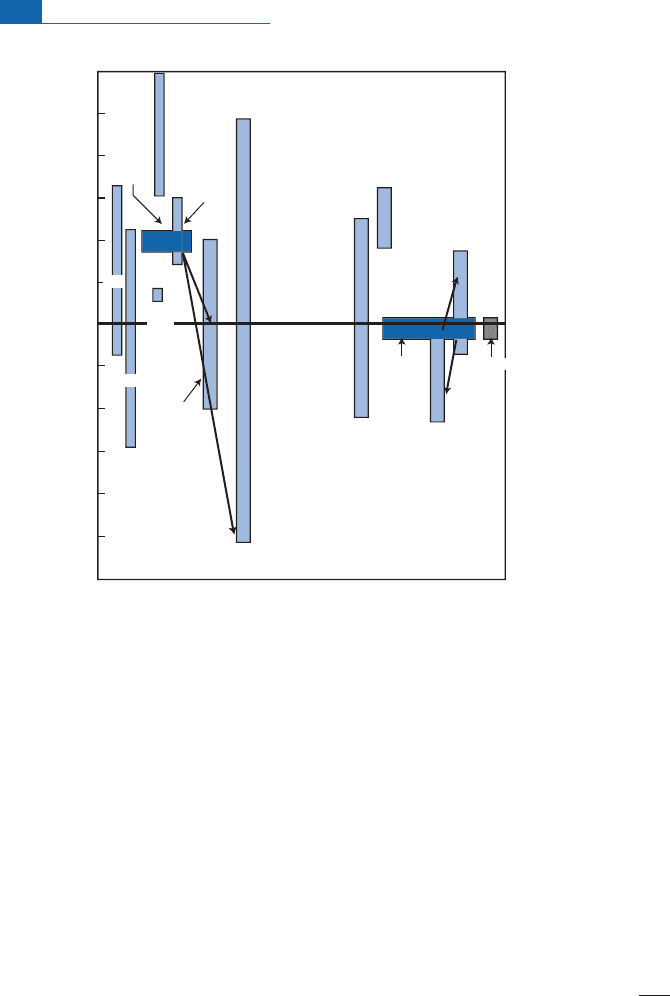
the greatisotopic variation in sul¢des ofsedimentaryorigin (Figure 7.7 ).
Distillation
Herewe look at a rather special (but widely applicable!) case where the system is clos ed but
wherethe product is isolatedas itforms. Let X
2
and X
1
representthe numberofatoms ofthe
twoisotopes. Ateach momentintime, wehave:
dX
2
=dX
1
X
2
=X
1
ðÞ
A
¼
377 The modalities of isotope fractionation
where maybeanequilibriumorkineticvalue,dX
2
isthe quantityof isotope 2 of Awhich is
transformed into B, and dX
1
is the quantityof isotope1of Awhich is transformed into B. By
separating thevariables and integrating, weget:
X
A
2
¼ cX
1
therefore:
X
2
=X
1
ðÞ
A
¼ cX
1
1
:
At time t ¼0 X
2
=X
1
ðÞ
A
¼ X
2
=X
1
ðÞ
0
and X
1
¼X
1,0
, therefore: c ¼ X
2
=X
1
ðÞ
1
X
1
1;0
.
Hence:
X
2
=X
1
ðÞ
A
¼ X
2
=X
1
ðÞ
0
X
1
=X
1;0
1
:
If the transformed remaining fraction of X
1
is called f, we get the famous Rayleigh distil-
lat ion law:
R
A
¼ R
0
f
1
:
Coal
a = 1.075
Biogenic
sulfide
SO
4
fresh
waters
Oil
Sedimentary
sulfide
Granites
Hydrothermal
sulfur
Volcanic S
Meteorites
Basic
igneous
rocks
a = 1.025
Sulfates in
interstitial
waters
Sea
water
sulfates
Old
sediments
Volcanic
SO
2
40
30
50
60
20
10
0
–10
–20
–30
–40
–50
–60
δ
34
S (‰)
Figure 7.7 Analysis of
34
S/
32
S isotope composition in the main terrestrial reservoirs. Notice that the
domains are very extensive for all reservoirs. This corresponds to highly variable reducing conditions to
which sulfur is subjected.
378 Stable isotope geochemistry
