All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

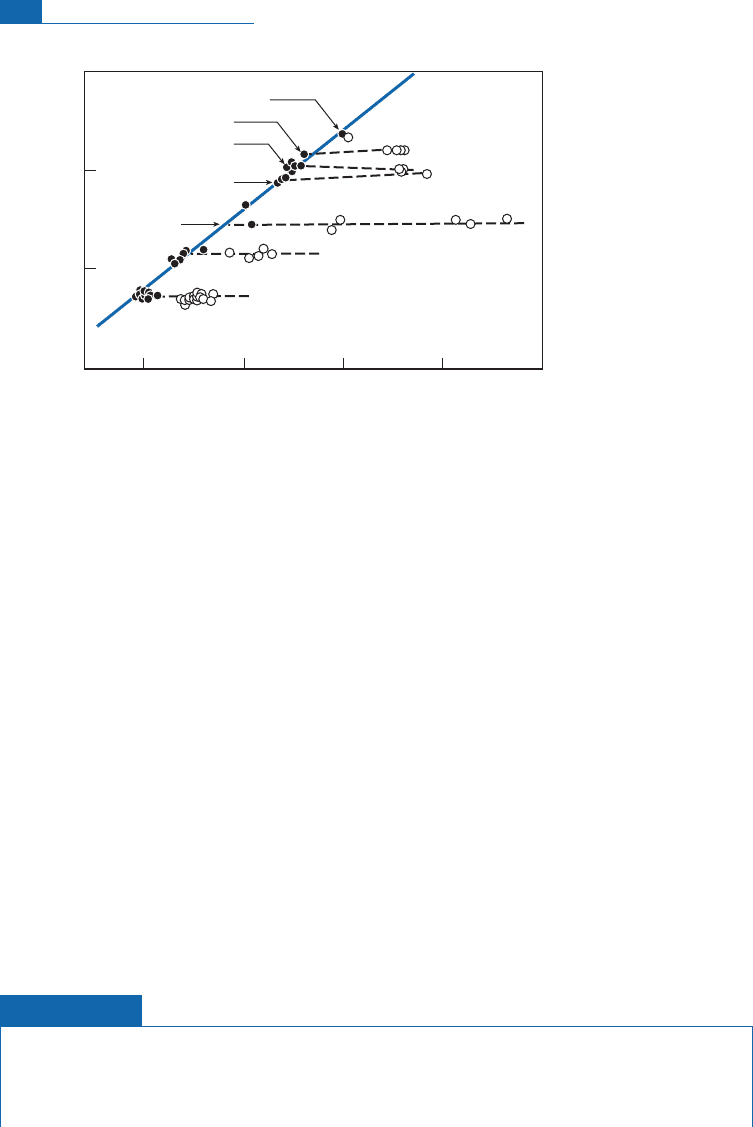
is still referred to as juvenile water. Har mon C rai g (he again) studied geothermal water to
determine the isotope composition of any juven ile water. He showed that the d values of
geothe rmalwaters from the same source canbe plotte d on a (d D, d
18
O)diagram on straight
linespretty wellparallelto the d
18
O axis and which cutthe straightline of precipitation cor-
responding to the composition of rainwater for the region. And so the composition of
geothe rmalwater canbe explain ed by th e evolution ofmeteoricwater viaisotope exchange
ofoxygen with the country rock.There is no need to invoke juvenile water from the mantle
to explaintheseisotope c ompositions (Figure 7. 21).
As these relations are systematic for allthe geothermal regions studied, Craig concluded
thatthe inputof juvenile water intoth e currentwater cycle is negligible and thatgeothermal
waters are only recycled surface water. The same go es for water from volcanoes. This
hypothesis has been con¢rmed by more elaborate studies ofvariations in the isotope com-
position of geothermal water over time. In many cases, it has been shown that variations
trackedthose observed in thesame place for rainwater, withatime lagc orresponding to th e
transittimewhichvaried from monthstoyears.
12
EXAMPLE
Iceland’s geysers
In some instances, such as the geysers of Iceland, the straight line of (dD, d
18
O) correlation is
not horizontal but has a positive slope (Figure 7.22).
0
–50
–100
–150
–15 –10 –5 0 5
Wairakei
Laederello
The Geysers
Iceland
Niland
Hekla
Lassen Park
Steamboat Springs
δD
δ
18
O
Figure 7.21 Correlation diagram for (
18
O/
16
O, D/H) in geothermal waters. They form horizontal lines
cutting the meteoric straight line at the point corresponding to local rainwater. This is interpreted by
saying that water has exchanged its oxygen isotopically with the rock but the hydrogen of water does
not change because it is an infinite hydrogen isotope reservoir compared with rocks that are relatively
poor in hydrogen. After Craig (1963).
12
A spa water company signed a research contract with a Parisian professor to study the isotopic
composition of the water it sold to prove it was ‘‘juvenile’’ water, a name whose advertising value can
be well imagined. As the studies showed the water was not juvenile, the company terminated the
contract and demanded that the results should not be published!
399 The isotope cycle of water
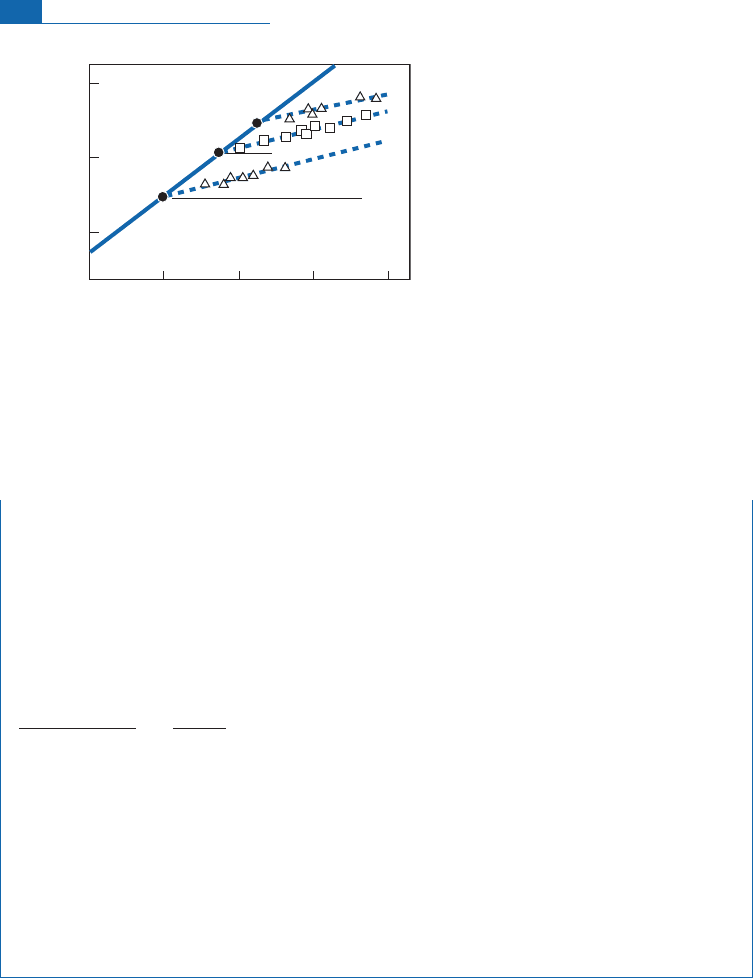
Suppose we begin with rainwater of local composition and that this water undergoes
distillation by evaporating. Then:
d
D
d
O;D
þ 10
3
ð
D
1Þln
f
:
d
18
O d
18
O; O þ 10
3
ð
18
O 1Þln
f
:
Eliminating ln
f
gives:
d
D
d
O;D
d
18
O d
18
O; O
D
1
O
1
:
We know that at 100 8C, for water–vapor fractionation,
D
¼1.028 and d
18
O ¼1.005. The
slope corresponds to 5.6, a lower value than that of equilibrium fractionation (8). The effect is
therefore a combination between exchange and distillation.
In fact, in nature, isotopic compositions of geothermal water or vapor are combinations
between Rayleigh distillation and the water–rock oxygen isotope exchange, between kinetic
fractionation and equilibrium fractionation. A horizontal slope indicates that isotope
exchange has been possible and so the transit time is long. When the slope is identical to
that of the Rayleigh law, the transit time has been short.
7.6 Oxygen isotopes in igneous processes
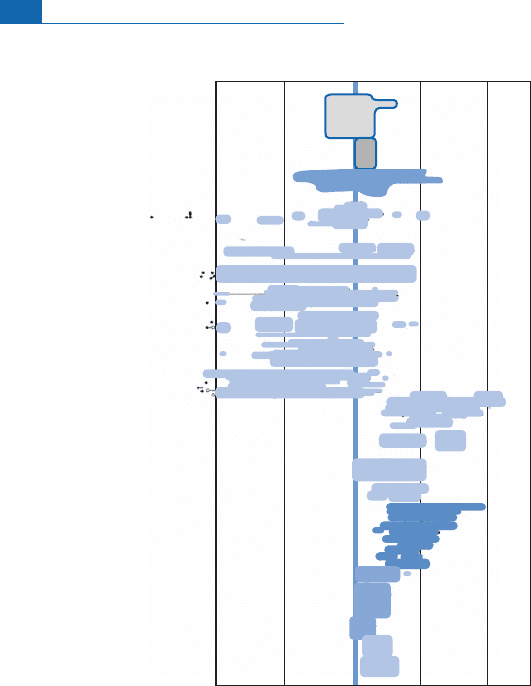
Examination shows thatthe
18
O/
16
O isotope composition ofunaltered rockof deep origin,
whether o cean basalts or ultrabasic rocks, is extraordinarily constant at d
18
O ¼þ5.5
(Figure 7.23).This value is analogous to the meanvalue of meteorites. It has th erefore been
agreed that this value is the reference value for the mantle.When taking stockof measure-
ments on basic or acid, volcanic or plutonic igneous rocks, the results are found to divide
between:
0
-10
-20
-30 -20 -10 0 +10
δD (%)
δ
18
O (‰)
Meteoric line
Geysers
Figure 7.22 Correlation diagram for (
18
O/
16
O, D/H) in acidic geothermal waters and geysers. The diagram
is identical to the previous one except that these are acidic geothermal waters with a high sulfate content
whose pH is close to 3 and for which the correlated enrichment in D and
18
O results mostly from more
rapid evaporation of light molecules with kinetic fractionation into the bargain. After Craig (1963).
400 Stable isotope geochemistry
igneousrockswitha d
18
O valuegreater than5.5;
igneousrockswitha d
18
O valueless than 5.5, andsomewith negative values.
These twotrends correspondtotwo types ofphenomena a¡ecting igneous rocks: contami-
nationbycrustalrocks and postsolidus exchanges with hydrothermal £uids.
7.6.1 Contamination phenomena
These phenomena are classi¢ed under two types: those involving mixing at the magma
source where melting a¡ected both acidic and basic metamorphic rocks, and those
where contamination occur red when the mag ma was emplaced. The latter process,
known as assimilation, obeys a mechanism already accounted for by Bowen (1928).
Mineral crystallization in a magma chamber releases latent h eat of crystallization.
This latent heat melts rock around the edges of the magma chamber leading to their
assimil ation.
–5
Syenites and trachytes
Anorthosites
Ultramafic and rocks
Basalts and gabbros
Andesites
Granites,
granodiorites and tonalites
Ash flow and
rhyolitic tuffs
Rhyolites and dacites
Italian volcanic rocks
Border zone of
granitic bodies
Scottish Hebrides
Skaergaard intrusion
San Juan Mountains
Western Cascade Range
Western Nevada Au-Ag
Boulder batholith
Iceland
Eclogites
Lunar rocks
Meteorites
0+5+10 +15
–5 0 +5 +10 +15
δ
18
O (‰)
Figure 7.23 Values of d
18
O in rocks and minerals. After Taylor (1974).
401 Oxygen isotopes in igneous processes
m # L ¼ m " C
P
DT;
where L is thelatentheat, m#the mass ofcrystalsprecipitatingper unittime, m"the massof
rock assimilated, C
P
thespeci¢cheatofthesurrounding rocks, and T thetemperaturedif-
ference between th e wall rock and the magma. If we can write m#¼kM,thenkML ¼m#
C
P
T, therefore:
m"
M
¼
kL
C
P
DT
:
The magma is contaminated isotopically toobythe mixing law:
ðd
18
OÞ
Hy
¼ðd
18
OÞ
magma
ð1 xÞþðd
18
OÞ
country rock
ðxÞ
with x ¼ m #=MðÞ, because the oxygen contents of the country rock and the magma are
almost identical. This was shown by Hugh Taylor (1968) of the California Institute of
Technology (see also Taylor, 1979).
Exercise
What is the d
18
O value of a basaltic magma whose d
18
O ¼0 and which assimilates 1%, 5%,
and 10% of the country rock whose d
18
O ¼þ20?
Answer
The contamination effect therefore increases the d
18
O value because sedimentary and
metamorphic rocks have positive d
18
O values. An interesting approach to studying the
contamination of magmas by continental crust is to cross the studies of oxygen isotopes
with those of strontium isotopes. The (O–Sr) isotope diagram can be calculated quite simply
because it is assumed that the oxygen content is analogous in the different rocks. The mixing
diagram depends only on the Sr contents of the two components of the mixture. Figure 7.24 is
the theoretical mixing diagram.
Such combined studies have been made of volcanic rocks of the Japan arcs and the
Peninsular Range batholith in California (Figure 7.25).
Exercise
A basaltic magma is emplaced and assimilates 1%, 5%, and 10% of the country rock. The d
18
O
values are those of the previous exercise. The
87
Sr/
86
Sr values are 0.703 for the magma and
0.730 for the country rock. The Sr content of the magma is 350 ppm and that of the country
rock is 100 ppm. Calculate the isotopic compositions of the mixture and plot the (d
18
O,
87
Sr/
86
Sr) diagram.
Assimilation
1% 5% 10%
d
18
O 5.64 6.22 6.95
402 Stable isotope geochemistry
Answer
The d
18
O values have already been given.
It is left to the reader to plot the diagram.
6.0
0.703
0.705 0.707 0.709 0.711
8.0
10.0
12.0
x = 5.0
x = 1.0
δ
18
O (‰)
87
Sr/
86
Sr
Crustal
contamination
C
M
Source
contamination
1:10
2:1
1:1
1:2
S
r
m
S
r
c
=
5
:
1
x = 0.2
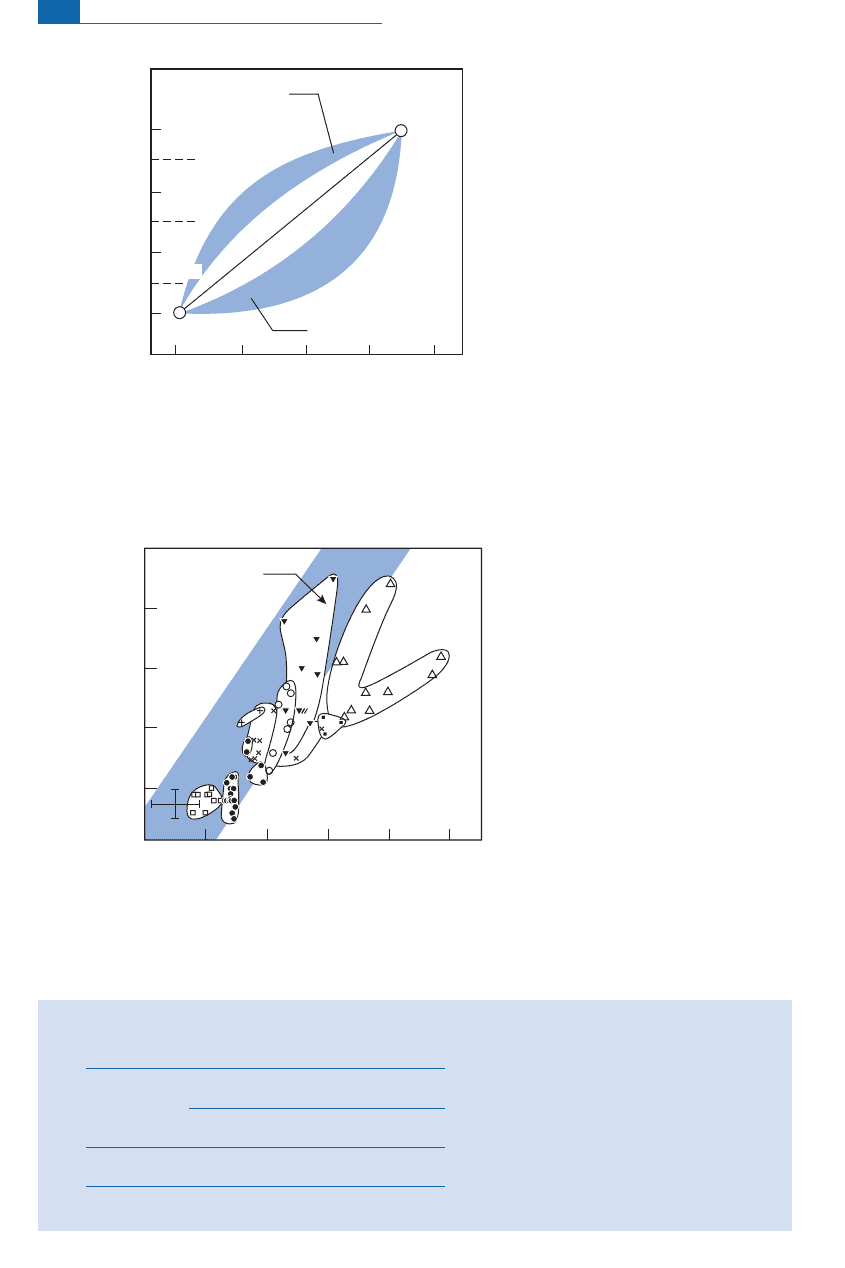
Figure 7.24 Theoretical O–Sr isotope mixing plots. The
x
values show the proportion of country rock
relative to the magma. The Sr
magma
/Sr
country rock
parameter varies from 5 to 0.1. M, magma; C, crust.
After James (1981).
0.704
Marianas
arc
California
batholith
MORB
Japanese arc
0.702 0.706
9
8
7
6
δ
18
O whole rock
87
Sr/
86
Sr whole rock
Figure 7.25 Example of an O–Sr isotope correlation diagram showing the Japan arcs and batholith
granites in California. After Ito and Stern (1985).
Assimilation
1% 5% 10%
87
Sr/
86
Sr 0.703 08 0.7034 0.703 94
403 Oxygen isotopes in igneous processes

7.6.2 Water–rock interaction
As we have said, isotope memory is retained if no exchange occurs after crystallization.
When th is is not the c ase, secondary isotopi c disturbances can be turnedto account. Hu gh
Tay l o r and his studentsobserved when examining variousgranitemas sifsorhydrothermal
mineral deposits that the
18
O/
16
O isotope compositions had been disrupted after their
initial crystallization by water^rock exchanges. The calibration made on water^mineral
fractionationwas thereforeturn ed directlyto account.
Whereas the d
18
O values of minerals and rocks of deep origin are generally positive
(between þ5andþ8), these rocks had negative d
18
Ovaluesof6to7. In the same c ases,
relative fractionation as can be observed between minerals, such as quartz^potassium
feldspar fractionation, was reversed.Taylor remembered Craig’s results on thermal waters
and postulated that, rather than observing the wate rs, he was obse rving rock with which
the waters had swapped isotopes. From that point, he was able to show that the emplace-
mentofgranite plutons, especially those with associated mineral deposits, involves intense
£uid circulation inthe surrounding rock.Ofcours e, the existence ofsuch £uids wasalready
k nownbecause theygive rise toveins ofaplite and quartz pegmatitean d theyengender cer-
tain forms of mineralization around granites, but their full importance was not
u nderstood.
In a closedsystem, we canwritethe massbalance equation:
Wg
w
d
0;W
þ Rg
r
d
0;R
¼ Wg
w
d
W
þ Rg
r
d
R
where W is the mass of water and R the mass of rock, g
w
is the proportion of oxygen in
the water and g
r
the proportion of oxygen in the rock, d
0,W
and d
0,R
are the initial
compositions of water and rock, and d
W
and d
R
are the ¢nal compositions thereof.
W
R
¼
g
r
g
w
d
R
d
0;R
d
0;W
d
W
;
sinc e d
W
and d
R
are related by fractionation reactions d
W
¼d
R
. This gives:
W
R
¼
g
r
g
w
d
R
d
0;R
d
0;W
ðd
R
DÞ
g
r
g
w
0:5:
Indeed,g
r
¼0.45 and g
w
¼0.89.We estimate d
0,R
from thenature ofthe rockand the catalog
of sound rock (close to þ5), and we estimate by calibrating and estimating temperature
by fractionation among minerals.This temperature canbe compared with thetemperature
obtained by theheatbudget.
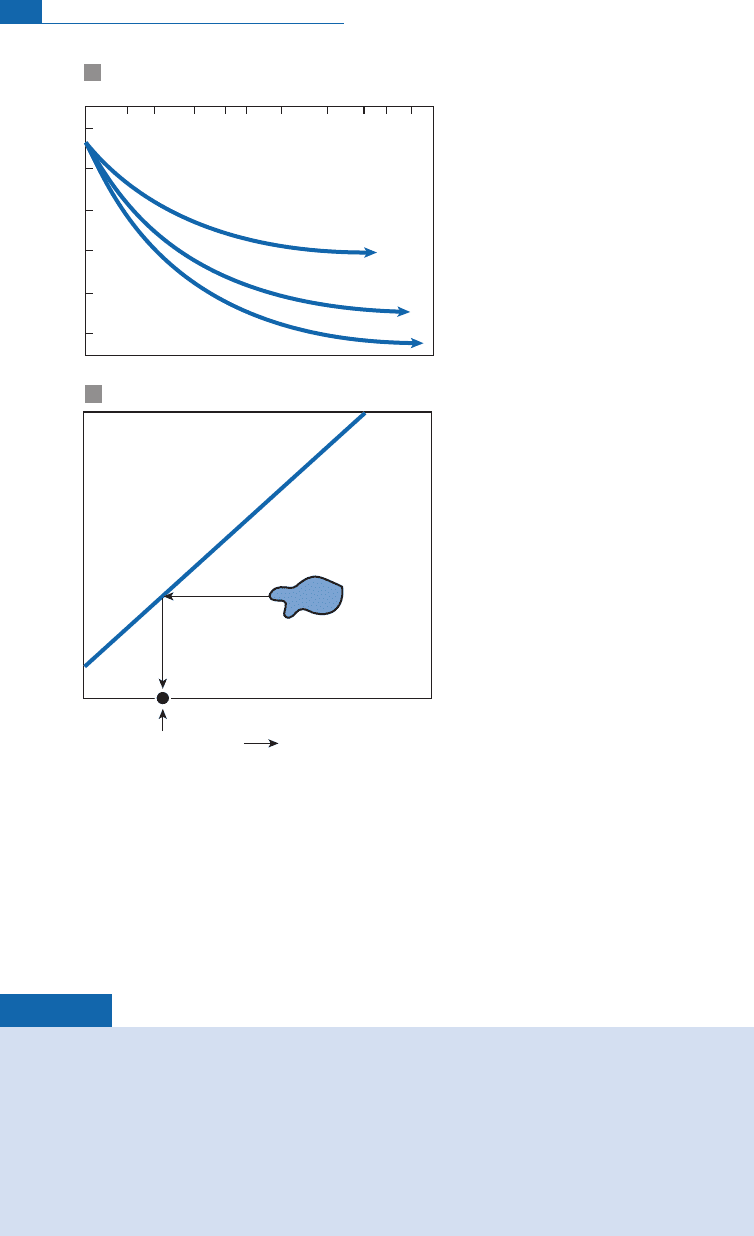
Acalculation maybe made, for example, forafeldspar with d
18
O ¼þ8andd
W;
18
O
¼16
at various temperatures (Figure 7.26a). It shows that in a closed system, theW/R ratios may
be extremely variable.
404 Stable isotope geochemistry
Exercise
What is the W/R ratio of a hydrothermal system supposedly working in a closed system at
400 8C?
The initial d
18
O value of feldspar is þ8, that of the water determined by the meteoric
straight line is d
0
,
W
¼20. The
feldspar–water
fractionation factor is 3.13 10
6
T
2
3.7. The
d
18
O of feldspar is measured as d
R
¼2.
Answer
The fractionation factor D ¼ 3:13 10
6
=ð673Þ
2
3:7 ¼ 3:21 ¼ d
R
d
W
ð
W=R
Þ¼0:34:
δ
18
O
δ
D
Meteoric line
Measured
point
Initial composition
of the fluid
Computation of
W/R
10
6
2
-2
-6
-10
1
0.50 2 3 4 6 10 15 20 25
150 °C
TO –3.3
TO –8.9
TO –12.0
250 °C
350
°C
(W/R) closed system
δ
18
O
rock
δ
R
R = 8.5
δ
R
W = –16
a
b
Figure 7.26 Variation in d
18
O composition and (dD, d
18
O) correlation diagram. (a) The variation in the d
18
O
composition of a feldspar with an initial composition d ¼þ18 is calculated as a function of (W/R) for
various temperatures, with the initial composition of water being d ¼16. (b) It is assumed the altered
rocks are represented by the blue area in the (dD, d
18
O) diagram. We can try to determine the initial
composition of the fluid by assuming, as a first approximation, that the dD values of the rock and water
are almost identical. The intersection between the horizontal and the (dD, d
18
O) correlation diagram of
rainwater gives the value of water involved in alteration. Reconstructed from several of Taylor’s papers.
405 Oxygen isotopes in igneous processes
Exercise
Let us now suppose the
W
/
R
ratio ¼5, that is, there is much more water. All else being equal,
what will be the d
18
O value of the feldspar measured?
Answer
d
18
O ¼14.54.
Theprocess describedinthe previous exercise involves adouble exchange anditis either the
water or the rock that in£uences the isotopic composition of the other depending on the
W/R ratio.
Allowing for the pointthat varies with temperature, a whole range of scenarios canbe
generated.
Exercise
Let us pick up from where we left off in the previous exercise. Imagine an exchange between
sea water and oceanic crust whose d
0,
R
¼þ5.5. The exchange occurs at
W
/
R
¼0.2. What will
the isotope composition of the water and rock be?
Answer
At high temperature ¼0; maintaining
g
r
/
g
w
¼0.5 gives d
rock
¼þ3.64 and d
water
¼þ3.64.
Exercise
Let us imagine now that the water is driven out of the deep rock and rises to the surface and
cools to, say, 200 8C. It attains equilibrium with the country rock and its minerals. If the rock
contains feldspar, what will the isotope composition of the feldspar be?
Answer
At 100 8C,
feldspar–water
¼10. Therefore the feldspar of the rock will have a d value of
13.92 þ14.
It can be seen from the water cycle in the previous exercise thathydrothermal circulation
reduc es the d value of deep rocks and increases the d value of surface rocks. (This is what is
observedinophiolite mas sifs.)
7.7 Paleothermometry and the water cycle:
paleoclimatology
We have just seen how hydrothermalism can be studied by combin ing information on the
isotope cycle of water and that of isotope fractionation.We are going to see how these two
e¡ects combine to give fundamental information on the evolution ofour planet and its cli-
ma te. After the initial impetus from Cesare Emiliani at Miami Universit y and Sam
Epstein attheCali forniaInstituteofTechnology,Europeanteamshavebeenthe moreactive
406 Stable isotope geochemistry

ones inthis ¢eld: for sediment paleothermometry, the teams from Cambridge and Gif-sur-
Yvette; forglacial records, thosefrom Copenhagen, Berne, Grenoble,and Saclay.
7.7.1 The two paleoclimatic records: sediments and polar ice
Carbonate paleoclimatology
In order to use oxygen isotopes as a thermometer, we must, strictly, know the d
18
Ovaluesof
two compounds in equi librium: water and carbonate.The formula established by Urey and
his team f or the carbonate thermometer dra w s on d
18
O
CaCO
3
and d
18
O
H
2
O
. In a ¢rst approach, the
Chicagoteam hadconsidered that dH
2
O,thatisthe ofseawater, wasconstantovergeologi-
caltim e andtherefore thatthe d
18
O
CaCO
3
measurementgave paleotemperatures directly.The dis-
covery of extreme d
18
O values for Antarctic ice challenged this postulate. If the amount of
Antarctic ice lost every year into the ocean varies, the d
18
O value of the ocean must vary
too, since this ice mayhave d
18
Ovaluesaslowas50. In this case, thehypothesisofconstant
dH
2
O is untenable and it seems that temperatures cannotbe calculated simply. On the other
hand, ifthe dissolution ofAntarctic ice in the oceanvaries involume, thisphenomenon must
berelatedto climateandtherefore,tosomeextent,mustre£ecttheaverageglobaltemperature.
The ¢rst idea developed by Emiliani in1955 was therefore to measure the d
18
Ovaluesof
carbonate foraminifera in Q uaternary sediment cores for which (gl acial and interglacial)
climatic £uctuationshavelongbeenknown.Variationsin isotope compositionareobse rved
(Figure 7. 27 ) and seem to be modulated by g lacial and interglacial cycles and more
Depth in the core (cm)
Zones
Stages
500
0
1
2
Z
Y
X
W
V
U
3
Cold Warm Temperature (°C)
Ericson curve
for G. menardi
abundance
600
300
200
100
400
20 25 30
1
Isotopic curve
from
Emiliani
2
3
4
5
6
7
8
9
10
11
12
13
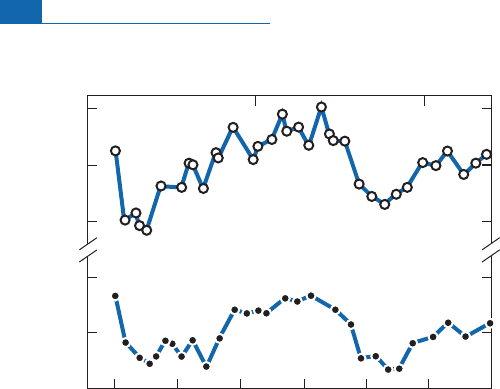
Figure 7.27 The first isotope determination using
18
O of Quaternary paleotemperatures by Emiliani
(1955) compared with glacial–interglacial divisions by Ericson.(
Globorot
a
li
a
men
a
rdi
is a foraminifer.)
407 Paleoclimatology
speci¢callytofollow the theoreticalpredictions oftheYugoslavastronomerMilankovitch.
Are these variations a direct e¡ect of temperature on (carbonate^water) fractionation or
arethey the e¡ectof
18
O dilutionbypolar i ce?Thequestion remainedunanswered.
Theformula:
T
C
¼ 16:9 4:2 d
18
O
CaCO
3
d
18
O
H
2
O
þ 0:13 d
18
O
CaCO
3
d
18
O
H
2
O
2
shows us that that two e¡ects work in the same direction. When T increas es,
dCaCO
3
dH
2
OðÞfraction ation decreases and so dCaCO
3
decreases if dH
2
O remains
constant. If, with constant local fractionation, d
18
O
H
2
O
decreases for wantof polar ice then
d
18
O
CaCO
3
also declines. Ni ck Shackl eton of the University of Cambridge suggested that the
18
O/
16
O variations measured in foram s were the result of £uctuations in the volu me of
p olar ice, a climate-relatedphenomenon.
Jean-Claude Duplessy and his colleagues in the Centre National de la Recherche
Scienti¢que (CNRS) at Gif-sur-Yvette had the idea of comparing d
18
O £uctuations of
surface-living (pelagic) foraminifera and bottom-dwelling (benthic) foramini fera. It is
k nownthatthetemperatureofthe deep oceanvaries l ittle around þ4 8C.
In conducting their study theyrealizedthat d
18
O£uctuationsofpelagic andbenthic spe-
cies were very simil ar (Figure 7.28). At most, extremely close scrutiny reveals an additional
£uctuation of 2ø inthe d
18
O ofpelagic species, whereas no great di¡erence appears for the
same comparison with d
13
C. This means, then, that th e d
18
O variations in foraminifera
re£ ect just as much variation in the d value of sea water as variations in local temperature.
Thesignal recorded istherefore meaningful for the global climate(Emiliani,1972).
In fact,morerecentstudieshave con¢rmedthat, for pelagic spec ies,some 50%ofthe sig-
nal re£ects a Urey-type local temperature e¡ect (remembe r that when temperature rises
d
18
O falls), above all in the temperate zones, and 50% the e¡ect of melting of the polar ice
δ
18
O
0
-1
1
3
4
5
0
50 100
10050 200
150 200 250 300
Globigerinoides sacculifer
Planulina wuellestorfi
Depth in the core (cm)
Time (Ma)
Figure 7.28 Variation in d
18
O in samples of two species of foraminifer. Top: a pelagic (ocean surface)
species. Bottom: a benthic (ocean floor) species. Modified after Duplessy
et
a
l
.(1970).
408 Stable isotope geochemistry
