All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

repeated by the teams of B e rnard B ourdon in the Paris laboratory (Caro et al., 20 03) yield-
ing an excess of
142
Nd in the Isua rocks of Greenland dated 3.8 Ga, but of on ly þ10 to þ 15
ppm (Figure 6. 78). Notice thatthe p recision reported in the measurementof isotopic com-
positions is the extremelimitofwhatcurrenttechniques canachieve.
Dating ofthe same rocksby the convention al
147
Sm^
143
Nd metho d con¢rmed the ageof
3.8 Ma and yielded the initial
143
Nd/
144
Nd ratio expressed in "
CHUR
¼þ2. It is possible,
then, to reason in terms of a two-stage model.The ¢rst stage involves meteoritic material
from4.576GatoT
1
.Then, fromT
1
to 3.8 Ga, the primitive crust (or the pr imitive residual
mantle),T
1
being theageofterrestrial di¡erentiation.
To dothe calculation,wetake 4.576 Gaasthe origin, which is more convenientforextinct
radioactivities. Letus call thetwoisotopicratios of Nd and .
143
Nd=
144
Nd; ¼
142
Nd=
144
Nd:
We note "
143
in10
4
and f
142
in10
6
as relativevalues against astandard:
143
ND
¼
143=144 Nd
sample
143=144 Nd
standard
1
10
4
f
143
ND
¼
143=144 Nd
sample
143=144 Nd
standard
1
10
6
0
¼
147
Sm
144
Nd
;
ε
142
Nd
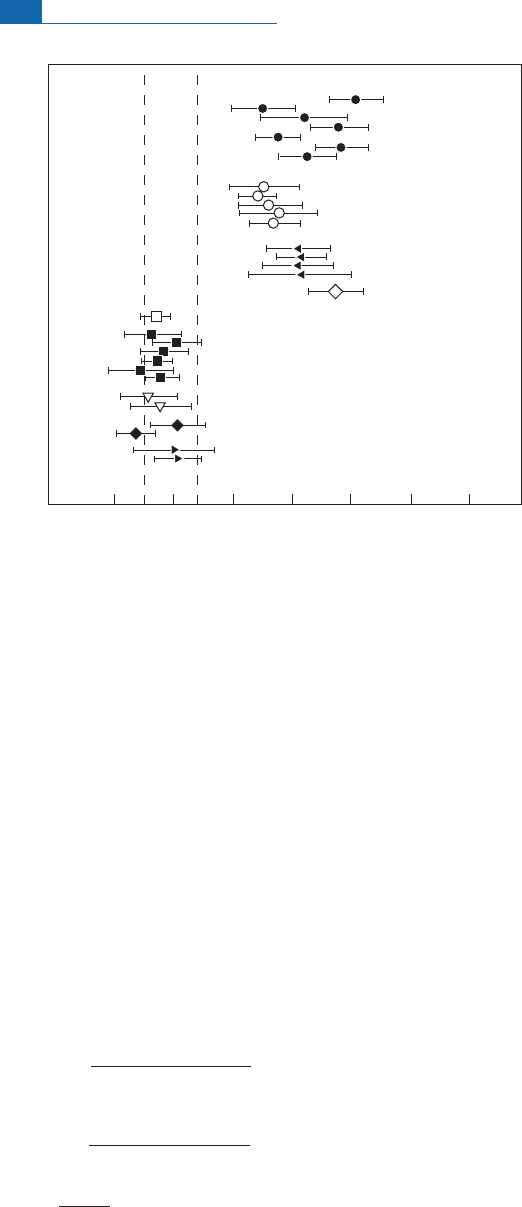
(ppm)
x 100
Metasediments
Orthogneisses
Metabasalts
Amphibolite
enclave
Acasta
Barberton
Pitcairn (EM I)
Societys. (EM II)
MORBs
-5 0 5 10 15 20 25
Figure 6.78 Measurements of
142
Nd anomaly in the Isua rocks of Greenland. After Caro
et
al
. (2005).
349 The early history of the Earth

butis calculatedat 4.576 Ga and not atth e presenttime.
a ¼
146
Sm
147
Sm
at 4:576 Ga:
The two evolution equations are written as multi-stage model with as thefractionation
factorbetweenthe chondritic episodeandth e primitiveEarth episode.
¼
0
þ
0
1 e
l
7
T
1
þ
0
e
l
7
T
1
e
l
7
T
2
¼
0
þ
0
a 1 e
l
6
T
1
þ
a
0
e
l
6
T
1
e
l
6
T
2
;
0
and bei ng the i nitial isotopic ratios, l
7
and l
6
the two radioactive constants, and T
2
being thetime3.8 Ga calculate d since 4.576,thatis,T
2
¼0. 776 Ga.
This non-linear system of two equations with two unknowns and T
1
can be solved gra-
phicallybyconstructing the two curves corresponding to thetwo equations with thevalues
measured for ,
0
, ,
0
, a,and
0
.
A fewvalues areneededfor the calculation.
ð
143
Nd=
144
NdÞ
t¼0
¼ 0:506 677 48
present day
¼ð
147
Sm=
144
NdÞ
Bulk Earth
¼ 0:1966; or
0
¼ 0:201 942 for 4:567 Ga
ð
147
Sm=
144
SmÞ
present day
¼ 4:888 99; or 5:037 316 6 for 4:567 Ga
142
Nd=
144
Nd ¼ 1:141 838 2; or 1:141 478 8 for 4:567 Ga
ð
146
Sm=
144
SmÞ
0
¼ 0:008:
From this it can be deduced that
146
Sm/
147
Sm ¼a ¼0.001588178 and therefore the param-
eter
0
a ¼0.000320 719.
The curves ¼f(T
1
) are therefore drawn for the pairs
147
Sm^
143
Nd and
146
Sm^
142
Nd
takingtwovaluesforobservations at3.8 Ga.
"
143
¼þ1:5 and þ 2f
142
¼ 15 and 10 ppm:
It can be s een that the two curves intersect between 4.46 and 4.35 Ga for (T
1
) (Figure 6. 79).
This is about the age determined for the di¡erentiation of the core and the atmosphere,
with for the present day of 0.216.
The question is what does this age mean? The rocks analyzed by Caro et al.(20 03)
were mostly metamorphic and metasedimentary rocks. They interpreted the results
as being th e value of a primitive depleted mantle which was the complement of di¡eren-
tiation of the ¢rst continental crust. Given the nature of the rocks, it is not obvious that
th is was their origin. An alternative interpretation would be that these values are those
of a lunar-type primitive crust, that is, on e rich in plag ioclase. The residual mantle (not
found) would have a negative "
142
value. As thi ngs stand , both interpretations are possi-
ble. In addition Harrison et al. (2 005) using hafnium isotopes in old zircons claim the
existenc e of a very early crust. The pre cise age of this crust and its nature are still sub-
jects of debate.
350 Radiogenic isotope geochemistry
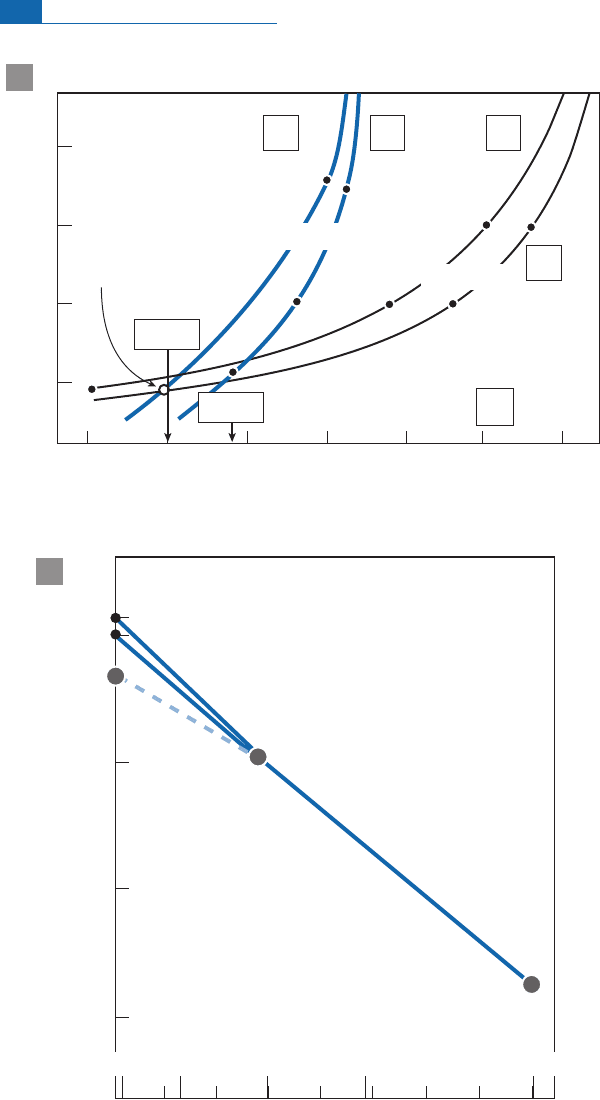
ISUA
CHONDRITES
μ
=
0.196
100x ε
/
142
/
144
146
Sm /
144
Sm
-30
-20
-10
-3.5
0
1
1 2 3 4 5
4.5 Ga 4.4 Ga 4.3 Ga
6 7 8
μ = 0.216
μ = 0.11
4.576 Ga 4 Ga
b
K-1
Time relative to 4.576 (Ga)
4.46
μ = 0.216
present time
0.1
0.2
0.3
0.4
100 200 0 300 400 500 600
4.38
ε
Nd
=
1.5
=
100 ε
Nd
ε
Nd
=
10
ε
Nd
=
15
147
Sm
146
Sm
a
ε
Nd
=
2
ε
Nd
Figure 6.79 (a) Age determination of differentiation of the primitive crust using the two Sm–Nd
systems. (b) Isua anomalies represented on the isochron diagram.
351 The early history of the Earth

Remark
More recently Boyet and Carlson (2005) have asserted that ordinary chondrites have a f
142
value of
–20 ppm compared with the Earth. Unfortunately, there are few C1 and C2 carbonaceous chon-
drites in their measurement sets and the dispersion is quite wide. What are the values for Bulk
Earth? It is best to wait a while, then, before incorporating these results into this textbook although
readers need to know of their existence and should follow the development of this chapter.
6.7.4 The
187
Re–
187
Os system and accretion–differentiation
chronology
Examination of the evolution of the
187
Os/
186
Os ratio of the Earth’s m antle showed us that
theRe/Oschemicalratioofthemantlewasveryclosetothe Re/Osratioofthe carbonaceous
chondrites. Now, this is a surprising result giventhat both Re and Os arehighlysiderophile
elements,thatis,theyenterli quidiron inpreference tosilicates.
Laboratory experiments for these elements give a partition coe⁄cient D ¼C
Fe
/
C
silicate
10
4
^10
5
. Most Re and Os therefore passes intothe iron core, as theyarein the iron
phase ofordinary chondrites and in the metallic iron of iron meteorites. But Re and Os do
nothave exactly thesame a⁄nity for iron: Os is more siderophilethan Re.We speakofafac-
torof10 to100betweenthem.
Letuslookatthiswitha simple model.Fromthe partitionequation oftrace elements:
C
mantle
¼ C
0
=F þ Dð1 FÞ
where Fis the prop ortionofthe mantle (between 0 and1).
Inthe caseof Re andOs,because D is verylarge, F << D (1 F), therefore:
C
mantle
¼ C
0
=Dð1 FÞ:
Accordingly, theratiobetween thetwoele mentsRe and Os iswritten:
C
Re
C
Os
mantle
¼
C
Re
C
Os
0
D
Os
D
Re
:
Therefore:
ðRe=OsÞ
mantle
4
10ðRe=OsÞ:
Now,observation showsthat(Re/Os)
mantle
¼(Re/Os)
initial
.
How can this apparent contradiction be accounted for? By admitting that the accretion
p rocess continued after di¡erentiation of th e core and so contributed enough Re and Os,
incorporated i n the mantle (probablyby primarysubduction processes) for them to domi-
nate the Re an d Os balance of the present-day mantle. Let us try to put this in somewhat
morequantitativeterms. Letuswriteabalance equation:
C
Os
mantle
¼
C
Os
meteorite
m
M
mantle
where C
Os
is the concentration in osmium, M
mantle
the mass of the mantle, and m the
added mass. Now, C
mantle
Os
¼3 ppb and C
chondrites
Os
¼490 ppb. Hence m/M ¼0.6%. Less
352 Radiogenic isotope geochemistry

than 1% of the mass of the mantle was added after 4.4 Ga by the accretion processes.
This in dicates that accretion probably de creased very rapidly after 4 Ga, as the curve
of lu nar craters also indicates.
Exercise
(1) Given that
C
mantle
Re
¼0.26 ppb and
C
chondrites
Re
¼37 ppb, calculate the ratio (
m
/
M
).
(2) If we underestimate the abundance in the mantle by 100%, how is the result altered?
Answer
(1) 0.7%.
(2) The ratio changes to 1.4%, which does not alter the conclusion in qualitative terms.
Ifweattempttomakea(provisional)reviewofthesestudiesoftheprimordialEarth,what
can we say today? First of all, a methodological point we shall return to i n quantitative
terms in the ¢nal chapter: extin ct forms of radioactiv ity do not record pr imordial p heno -
mena i n the same way as long - period forms of radioactivity. Th e former are sensitive to
short- term £uctuations, the latter on th e contrary average var iations out more over the
long term.
Together they give complementary views of primordial phenomena, each of which is
insu⁄cient in itself. This is the case for
40
Ar and
129
Xe for the atmosphere and for
206
Pb,
207
Pb, and
182
Hf for the core, and will be the case for any new form of extinct radioactivity
that mightbeused inthefuture.
Accretion is a process that lasted for quite some time after 4.4 Ga.The formation of the
coreis aphenomenonthatwaspreparedbydi¡erentiationof iron in planetesimalsanditself
occurred rather suddenly (perhaps in less than 100 or 1000 years) sometime between 4.4
and 4.35 Ga.Theatmospherewasproduce d byoutgassingofthe mantle. It¢rstformedvery
suddenly at 4.4 Ga and has been enriched throughout geological time through volc anic
eruptions.
And the continents? The interpretation of the
142
Nd anomalies is still a matter of debate,
but workers agree that if there was a primordial crust dating from the great di¡erentiation
of 4.4 Ga, that crust was destroyed and digested by the mantle, because we have no record
of tha t
142
Nd in rocks as old as 3.4 Ga.The existence of detrital zircons dated 4.3 to 4.4 Ga
seemto carryasimilar message. No continental rocks. Onlydetritalminerals. Allofth is sug-
gests that the primordial continental crust was ephemeral. Apart from this possible occur-
rence of a very primordial continental crust (perhaps a plagioclast crust like that on the
Moon) thegrowth curvesofcontinentsinthegeological and geo chemicalsense oftheterm is
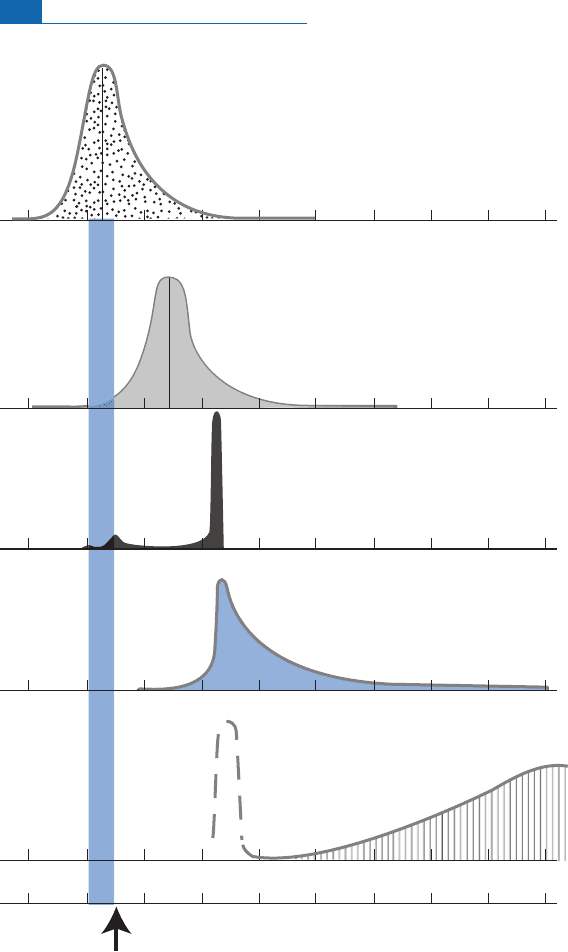
what wehave established. Allofthis is roundedup in a su mmary ¢gure.The PAT (Patterson)
age isthe mythicalageof4.55 GadeterminedbyPatterson(Figure6.80) (see Chapter 5).
6.8 Conclusion
Like the remainder of this textbook, this chapter places more emphas is on methods of rea-
soning than on any would-be ¢rm and ¢nal results. However, it exposes results that s eem
either tobe robustor whichprobablyform a ¢rst approximationofreality.
353 Conclusion
The general method develop ed in this chapter isbased on the idea ofusing radiogenic iso-
topes as tracersofphenomena.Biologistsinjectradioactivetracerstounderstandthephysio-
logyofan animalbodyora cell; hydrologists or chemists use coloringagents to monitor £uid
movement. Like them, we too use tracers to study geologi cal phenomena.This method has
proved extraordinarilye¡ectivefordecipheringthestructureandphysiologyoftheplanet.
4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8
PAT age
Age (Ga)
METEORITES
Accretion
Condensation
Core
Atmosphere
Continents
?
Differentiation
of iron
Figure 6.80 Timescale of the different phenomena that occurred in the early Solar System and the different
phases of early formation and differentiation of the Earth from the isotope results. PAT age, Patterson age.
354 Radiogenic isotope geochemistry

Geological tracers have an original feature compared with other tracers in that they are
chronometers too.They move around with the motion of matter but keep a record of their
history, which is what makes them so valuable. In addition, they c an be used for exploring a
widetime range: notonlythe12billionyearsofthehistoryofthe Universeandthe 4.5billion
years of the Earth’s historybut also the details of planetogenesis some 4.5 billion years ago
or recent phenomenaofth e last millennia.
A second original feature is that the isotopic variations explored here are tiny, ranging
from1 0
3
to1 0
5
.Inotherwords,none ofthis wou ldb efeasiblewithoutth ei ncrediblypre -
ciseand sensitive technique ofmass spect rometry.
Lastly, andthis is nottheleastadvantage, wehaveahigh numberofisotopic tracers(more
than 40).Thus extremely vari ed problems can be addressed with the same techniques an d
the same metho ds.There is no doubtthatthe futurewill see more detailedstudiesofthevar-
ious fundamental phenomena (ocean ridges, subduction) and of the primordial Earth
usingthesamemethodology.
Problems
1 We consider the following geological history of a granite. Some 1500 Ma ago a volcanic–
detrital sediment formed by mixing in the proportions of 1/3 and 2/3. The volcanic
sediment has a
87
Sr/
86
Sr ratio of 0.703, and the Sr and Rb concentrations are 300 ppm and
10 ppm, respectively. For the detrital sediment, the
87
Sr/
86
Sr ratio ¼0.720 and the Sr and
Rb concentrations are 100 ppm and 100 ppm, respectively. The sediment is changed into
rock and sinks progressively over 500 Ma. At that time, caught up in orogenic convulsions,
it undergoes anatexis, which gives rise to a granite by partial melting. The Rb/Sr ratio of
the melt is (Rb/Sr)
melt
¼3(Rb/Sr)
sediment
. The granite then evolves over 1 Ga.
(i) Show the isotope history of the granite on a graph.
(ii) Calculate the initial and present-day
87
Sr/
86
Sr ratios of the granite.
2 In some cases, workers ascribe an important role to the metal core to modify classical
conclusions about the mantle.
(i) We assume the core contains potassium in a concentration of about 100 ppm. How does
that affect the
40
Ar balance as it has been envisaged? Calculate the mass of
40
Ar in the
core and the lower mantle.
(ii) Core–mantle reactions are also evoked to explain the lead isotope compositions of OIB. Take
the
238
U/
204
Pb ratios for the current value of the Earth of 1; the ratio of the primordial
mantle is 7 and that of the core is 0. Calculate the lead concentration in the core, given that
the total lead concentration of the core–lower mantle system is
C
m
Pb
¼0.696 ppm and that
in the lower mantle, after initial differentiation of the core,
C
m
Pb
¼0.1611.
(iii) Suppose that after initial differentiation, the core continues to deplete the lower mantle
of lead. Schematically, let us accept that 20% of the lead disappears (increasing the
mantle
value) and that the phenomenon can be modeled by a two-stage process involving
an extraction episode followed by an episode with no extraction, the age of the change in
regime being 3 Ga. Calculate the
206
Pb/
204
Pb and
207
Pb/
204
Pb ratios of the lower mantle.
Locate them relative to the 4.5 Ga geochron.
(iv) Do the same calculation with
T
diff
¼4 Ga.
(v) Can you draw any geochemical conclusions from these calculations?
355 Problems

3 The Canadian Dick Armstrong (1981) proposed a new model of continent growth. This problem
looks more closely at his idea. Armstrong supposed that all of the continental crust differentiated
say 4.5 Ga ago (like the core and most of the atmosphere) but that since then, during each
mountain-building episode, part of the continent is reinjected into the mantle (as sediment or as
detachment from the lithosphere) and that symmetrically an identical piece of continent is
formed at the expense of the mantle.
In this model, the of the mantle and continental crust remain constant. It is supposed there
were four periods of exchange: at 3.5 Ga, 2.5 Ga, 1.5 Ga, and at the present day. It is assumed
that at present a quarter of the continental crust is swallowed up and regenerated.
(i) Calculate and draw the two (, t) curves of evolution for average continental crust and for
the supposedly isolated upper mantle.
(ii) Calculate and draw the statistical age diagrams for
143
Nd/
144
Nd represented in (
t
),
87
Sr/
86
Sr,
147
Sm/
144
Nd ¼
Sm
,
87
Rb/
86
Sr ¼
Rb
. The subscripts c, m, and 0 represent the
continent, mantle, and Earth, respectively. We note
143
Nd/
144
Nd ¼
Nd
,
87
Sr/
86
Sr ¼
Sr
at
4.4 Ga, the starting point of the calculation.
So:
Nd
0
¼ 0:506 677;
Sr
0
¼ 0:6989
Sm
c
¼ 0:11;
Sm
m
¼ 0:25
Rb
c
¼ 1;
Rb
m
¼ 0:05:
(iii) What do you conclude?
4 The residence time of oceanic lithosphere in the primitive upper mantle is 1 Ga. It can be
assumed that when it goes through the mid-ocean ridge, the corresponding 70 km of mantle is
entirely degassed. The
4
He in the upper mantle is the sum of two terms. The radiogenic part,
formed
in situ
over 1 Ga, and the part from the lower mantle at the same time as
3
He during
the same period. This second part will be ignored.
(i) Calculate the quantity of
4
He accumulated in 1 Ga in the upper mantle, knowing that
U ¼5 ppb and Th/U ¼2.5.
(ii) Given that degassing of
3
He from the ocean ridges is 1 10
3
moles yr
1
and that
(
4
He/
3
He) ¼10
5
, calculate the residence time of
4
He in the upper mantle. What do you
conclude about the processes involved?
5 We consider the continental crust–depleted mantle system, as in Section 6.3.
(i) We wish to calculate the best
143
Nd/
144
Nd and
147
Sm/
144
Nd ratios for the continental
crust ( )
cc
, primitive mantle ( )
pm
, and residual mantle ( )
dm
.
We note the isotope ratios
Nd
and the
147
Sm/
144
Nd ratio ¼
Sm/Nd
.
We give:
Nd
pm
¼ 0:512 62 0:000 01
Nd
dm
¼ 0:513 15 0:000 01:
From which
cc
Nd
is between 0.511 and 0.5120.
Sm=Nd
pm
¼ 0:197 0:01
Sm=Nd
cc
¼ 0:11 0:01:
From which
dm
Sm/Nd
is between 0.227 and 0.280.
356 Radiogenic isotope geochemistry

Calculate the factor
W
Nd
¼
M
CC
C
Nd
CC
m
T
C
Nd
T
where
m
cc
is the mass of the continental crust and
m
T
the mass of the primitive mantle, which
differentiated by extraction of the continental crust.
Calculate the model age of differentiation of the continental crust.
(ii) We also give the values for the
87
Sr/
86
Sr ratio noted
Sr
and
Rb/Sr
for
87
Rb/
86
Sr:
Sr
pm
¼ 0:7047;
Sr
dm
¼ 0:7020;
Sr
cc
¼ variable
Sr
cc
¼ 0:3 0:5;
Rb=Sr
pm
¼ 0:09;
Rb=Sr
dm
Rb=Sr
dm
¼ 0:
Given that
W
Nd
/
W
Sr
¼1.5, calculate
W
Sr
,
cc
Sr
, the strontium model age, and
cc
Rb/Sr
.
357 Problems

CHAPTER SEVEN
Stable isotope geochemistry
When de¢ning the properties of isotopes we invariably say that the isotopes of an element
have the same chemical properties, because theyhave the same electron shell, but di¡erent
physicalp roperties,becausetheyhave di¡erentmasses.However, ifthebehaviorofisotopes
of any chemical element is scrutinized very closely, small di¡erences are noticeable: in the
course of a chemical reaction as in the course of a physic al process, isotope ratios vary and
isotopic fractionation occurs. Such fractionation is very small, a few tenths or hundredths
of1%, and is only well marked for the light elements, let us say those whose atomic mass is
lessthan40. However,thankstothe extremeprecisionofmodernmeasurementtechniques,
values can be measured for almost all of the chemical elements, even if they are extremely
small for theheavyones.
When we spoke of isotope geochemistry in the ¢rst part of this book, we voluntarily
o mitted such phenomena and concentrated on isotopevariations related to radioactivity,
which are preponderant. We now need to look into the subtle physical and chemical
fractionation of stabl e isotopes, the use of which is extremely important in the earth
sciences.
7.1 Identifying natural isotopic fractionation
of light elements
The systematic study of the isotopic composition of light elements in the various naturally
o ccurring compounds brings out variations wh ich seem to comply with a purely naturalis-
tic logic. These variations in isotope composition are extremely slight, and are generally
expressedinaspeci¢ cunit, th e d un it.
d ¼
sample isotope ratio standard isotope ratio
standard isotope ratio
10
3
:
Ultimately,d isarelativedeviationfromastandard,expressedasthenumberofpartsper mil
(ø). Isotope ratios are expressedwiththeheavier isotope inthenumerator.
If d is positivethenthe sampleisricher in theheavy isotopethanthestandard.If d is nega-
tive then the sample is poorer in the heavy isotope than the standard.The term s ‘‘rich’’and
‘‘poor’’are understood as relative to the isotope in the nume ratorof the isotope ratio in the
formula above: by convention it is always the heavy isotope.Thus we speakof the
18
O/
16
O,
D/H,
13
C/
12
C ratio, etc. The standard is chosen for convenience and may be naturally
