Zuo-Guang. Ye Advanced Dielectric Piezoelectric and Ferroelectric Materials: Synthesis, Characterisation and Applications
Подождите немного. Документ загружается.

Handbook of dielectric, piezoelectric and ferroelectric materials40
PMN–PT single crystals of 50–76 mm or larger in diameter and more than
100mm in boule length have been successfully grown by several groups
(Lee et al., 1999; Zhu and Han, 1999; Luo et al., 2000a,b; Xu et al., 2000,
2001; Santailler et al., 2002; Wan et al., 2004; Shin et al., 2004; Nicoara
et al., 2005).
Despite the extensive efforts made in the flux growth of PZN–PT and
PMN–PT and the melt-growth of PMN–PT single crystals, currently grown
PZN–PT and PMN–PT single crystal are plagued with a host of problems.
One of the major issues has been compositional segregation in the grown
crystals. This has led to large scatter in measured dielectric and
electromechanical properties reported by various research groups as well as
within individual groups (Luo et al., 2000a,b; Xu et al., 2000, 2001a,b;
Santailler et al., 2002; Karaki et al., 2002b; Guo et al., 2003; Zawilski et al.,
2003; Shin et al., 2004; Wan et al., 2004; Nicoara et al., 2005). Developing
ways of growing large high-homogeneity PZN–PT and PMN–PT single crystals
is thus one of the major challenges faced by scientists and researchers before
these crystals find wide spread applications.
This chapter describes the results of flux growth of PZN–PT and PMN–
PT single crystals performed in the author’s laboratory over the past seven
years (Kumar and Lim, 2000; Lim, 2004; Lim and Rajan, 2004; Rajan et al.,
2005; Lim et al., 2005). The flux growth mechanisms of PZN–PT and PMN–
PT single crystals and the effects of flux and crystal compositions and growth
conditions on the quality of grown crystals are described and discussed.
Large single crystals (≥35mm edge length for PZN–PT and ≥ 25 mm PMN–
PT) of improved homogeneity have been successfully grown by an improved
flux growth technique which promotes (001) layer growth of the crystals.
The properties of the crystals obtained were evaluated and the results are
presented and discussed.
2.2 Flux growth of PZN–PT and PMN–PT single
crystals
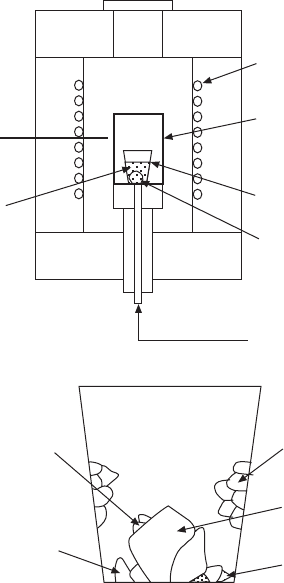
Figure 2.1 shows the typical set-up (a) and growth result (b) of high-temperature
flux growth of PZN–PT and PMN–PT single crystals. The most commonly
used fluxes for the growth of PZN–PT and PMN–PT single crystals are PbO
(or Pb
3
O
4
) and complex fluxes consisting of a mixture of PbO and B
2
O
3
,
respectively, with the solute-to-flux mole ratios varying from 0.55:0.45 to
0.30:0.70.
The component oxide powders, of 99.95% or higher purity, are weighed
to desired proportions and then mixed thoroughly. The charge mixture may
be loaded directly into the Pt crucible, although pre-calcined charge has also
been commonly used. Then, the Pt crucible is covered with the lid and
further placed in a sealed alumina crucible assembly, as shown in Fig. 2.1(a),
WPNL2204
Flux-growth and characterization of PZN–PT and PMN–PT 41
to contain potential PbO loss during the crystal growth run. The assembly is
placed in the crystal growth furnace, which is often equipped with a local
cooling arrangement to eliminate unwanted nucleation. Techniques used for
local point cooling include the use of a thin metal rod (Shimanuki et al.,
1998; Harada et al., 1998, 2001; Matsushita et al., 2002; Babu et al., 2006),
metal wire (Chen and Ye, 2001; Karaki et al., 2002a) and controlled stream
of gas flow (Kobayashi et al., 1997, 1998; Saitoh et al., 1999; Kumar and
Lim, 2000; Benayad et al., 2004; Dabkowski et al., 2004).
The crucible assembly with the charge is then heated to high temperature,
typically between 1150 and 1250°C, and held for a sufficiently long period
of up to 10h to melt and homogenize the solution. Then, the assembly is
cooled at a controlled rate, typically in the range of 0.5–1.5°C/h, to start the
crystal growth process.
When perfect single-crystal nucleation is initiated in the middle of the
Heating
element
Sealed
alumina
crucible
Pt crucible
Crystal
Oxygen
Control
T.C.
Growth
solution
(a)
Parasitic
crystals
Satellite
crystals
Side-wall
nucleation
Main
crystal
Pyrochlore
crystal
(b)
2.1
Schematics showing (a) set-up for flux growth of lead-based
relaxor single crystals from high temperature via spontaneous
nucleation and (b) typical growth results.
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials42
base of the Pt crucible, the crystal will grow in a stress-free manner with
minimum constraints and is totally protected by the PbO-rich solution. The
latter is important because most relaxor–PT systems, in particular PZN–PT,
are unstable at high temperatures even in PbO-rich vapour environments
(Jang et al., 1992; Wakiya et al., 1995; Lim et al., 2002). The entire crystal
growth run may last for one to several weeks. Depending on the crystal type,
slow cooling is stopped after the growth temperature reaches about 800–
1000 °C, whereupon the assembly is cooled at a fast rate to room conditions.
It has also been common practice to use gradually increasing cooling rate in
the slow cooling stage (Mulvihill et al., 1996; Saitoh et al., 1999; Zhang et
al., 2000; Fan et al., 2003; Babu et al., 2006), in order to achieve a more
constant crystal growth rate. This, however, requires the knowledge of the
ternary phase diagram of the material systems concerned. Although relevant
ternary phase diagrams remain unavailable to date, binary phase diagrams
for the PMN–PbO system and for the (PZN–9%PT)–PbO system have been
reported by Ye et al. (1990) and Dong and Ye (2001), respectively.
At the conclusion of the crystal growth run, the crystal is retrieved from
the remaining solidified flux by leaching in boiling concentrated nitric acid.
It has also been common practice to decant the remaining flux solution by
flipping over the alumina crucible assembly at the end stage of the cooling
process. This, however, must be executed with care because the crystal may
crack due to thermal shock, especially for large crystals.
2.3 Effect of flux composition
PbO melts at 886°C and the PbO–B
2
O
3
mixture has a relatively low eutectic
point of 493°C. They are commonly used fluxes in the growth of PZN–PT
and PMN–PT single crystals. Being an end element of the final compound,
the use of PbO in the growth of PZN–PT and PMN–PT is advantageous in
that introduction of foreign ions can be avoided.
PbO melt has a reasonably low viscosity and high solubility for complex
oxides. However, its major drawbacks include its toxicity, volatility above
1100 °C and a tendency to corrode platinum above 1300°C. Lead oxide
(PbO) with additives of Pb
3
O
4
may also be used. The addition of Pb
3
O
4
assures an oxidizing atmosphere during crystallization and it has been reported
that this may help promote the stability of the perovskite phase during the
crystal growth (Park and Shrout, 1997a; Dong and Ye, 1999; Kania et al.,
2005). The addition of B
2
O
3
not only helps lower the crystallization temperature
of the relaxor–PT system but also increases the viscosity of the solution.
Both of these effectively prevent the loss of PbO through volatilization
during the crystal growth run, which is desirable for the growth of certain
relaxor–PT single crystals of relatively high solution temperature, such as
the PMN–PT system.
WPNL2204
Flux-growth and characterization of PZN–PT and PMN–PT 43
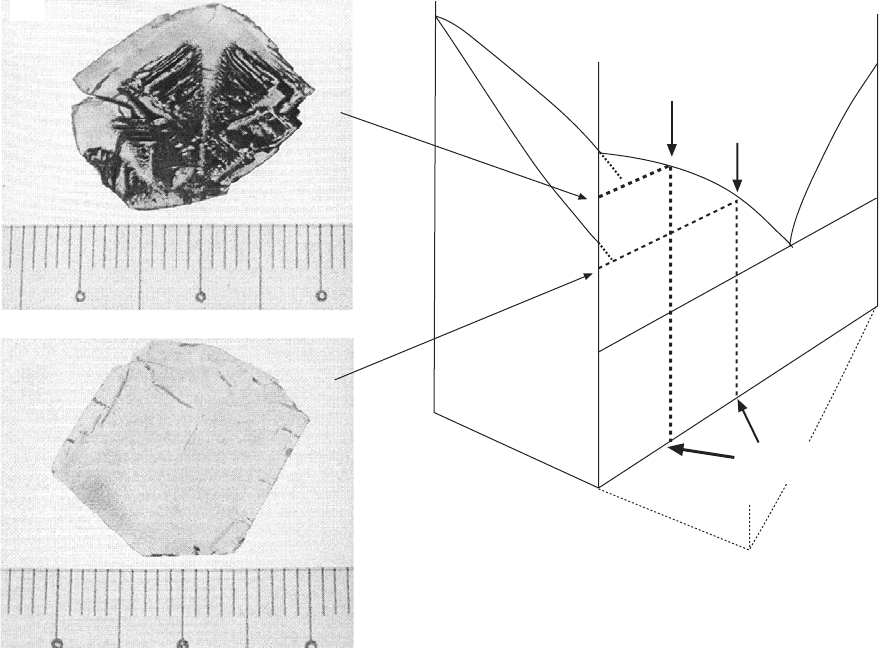
2.2(a)
A hypothetical phase diagram of the PMN–PT–(PbO + B
2
O
3
) system.
The vertical sections of PMN–PT single crystals grown with insufficient
B
2
O
3
and sufficient B
2
O
3
in PbO-based fluxes are given in (b) and (c),
respectively.
L +
PMNT
L
L +
PbO
T
PMNT
PMN
PMNT
+ L
Charge
compositions
PbO +
B
2
O
3
PT
Charge
(PMNT)
(a)
(b)
(c)
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials44
The flux composition strongly affects the outcome of the crystal growth.
For instance, our experience has shown that, on the one hand, the addition of
B
2
O
3
to PbO flux promotes the formation of the pyrochlore phase in the
PZN–PT system, on the other hand, a suitable amount of B
2
O
3
flux is needed
in the growth of PMN–PT to ensure a good growth result.
In addition to flux composition, an appropriate flux-to-solute ratio is essential
in relaxor–PT single crystal growth. Figure 2.2(a) gives a hypothetical phase
diagram of the PMN–PT–PbO(B
2
O
3
) system, which serves to illustrate the
importance of flux composition and content in PMN–PT growth (Lim, 2004;
Lim et al., 2005). This figure shows that when nil or insufficient B
2
O
3
is
present in the solution, the grown PMN–PT crystals always contain a large
amount of flux inclusions, especially in the initially grown part of the crystals
(Fig. 2.2b). This can be attributed to the high crystallization temperature
associated with the growth solution, such that the crystallization temperature
is higher than the solidus of the binary PMN–PT system (Fig. 2.2a). Under
the said conditions, phase separation is expected; the solidified mass is thus
a mixture of a low-PT solid phase and a high-PT liquid phase.
The problem of phase-separated flux inclusion can be avoided by increasing
the flux content in the starting charge to ensure that crystal growth takes
place predominantly below the solidus of the corresponding binary relaxor–
PT system. This is shown schematically in Fig. 2.2(a). A vertical section of
a PMN–30%PT single crystal grown under such a condition is shown in Fig.
2.2(c). The obtained crystal is free of flux inclusions and fully dense.
The above results show that appropriate flux compositions and correct
solute-to-flux mole ratios play a critical role in relaxor–PT single crystal
growth. This is especially so for the growth of relaxor–PT single crystals of
high PT content, such as PMN–PT. As will be shown below, although typical
solute-to-flux ratios of 0.55:0.45 to 0.30:0.70 have been quoted, it may be
necessary to fine-tune the ratio based on the PT content in the solution to
ensure good crystal growth results.
2.4 Growth of relaxor single crystals of low PT
contents: PZN–(4–7)%PT
The PZN–xPT system has a morphotropic phase boundary at x ≈ 0.09–0.10
(Kuwata et al., 1981; La-Orauttapong et al., 2002). PZN–PT single crystals
of PT contents less than 8 mol% are sought after due to their reasonably
good thermal stability and excellent dielectric and piezoelectric properties
after poling. Compared with PMN–PT crystals, the growth of PZN–(4–7)%PT
single crystals appears to be much less complicated due to its lower PT
contents.
The flux used for the growth of PZN–PT single crystals was PbO. Typical
solute-to-flux mole ratio used was 0.55:0.45. When the degree of
WPNL2204
Flux-growth and characterization of PZN–PT and PMN–PT 45
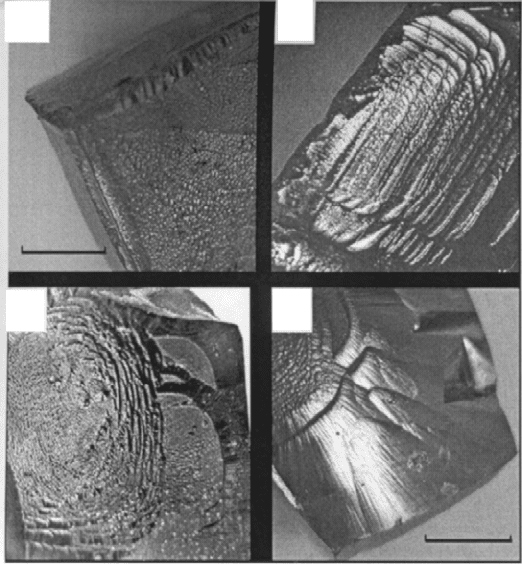
supersaturation in the solution is low (i.e. at low cooling rates), the growth
of PZN–PT single crystals starts by nucleation at <111> corners followed by
spreading down adjacent <001> edges and (001) crystal faces, as evidenced
in Fig. 2.3(a). This is typical of crystal growth from solution of simple ionic
salts (Van Hook, 1961), suggesting that PZN–PT–PbO solutions with ≤7
mol%PT must be composed of simple ions. This remains so even when the
PT content in the solution is close to the morphotropic phase boundary
composition, i.e. about 9–10 mol% PT.
With an increased degree of supersaturation in the solution, growth of
respective single crystals via nucleation at <001> edges and on {001} faces
becomes feasible, as evidenced in Fig. 2.3(b) and (c). The growth remains
faceted in nature until the supersaturation is sufficiently high to promote
profuse nucleation and growth on {001} faces. When this occurs, (001)
growth facets are gradually replaced with smooth, non-crystallographic crystal
faces. An example of such is shown in Fig. 2.3(d).
2.3
(a) Natural (001) growth faces in flux-grown PZN–(4–9)%PT.
(b) and (c) Nucleation of (001) layer growth at <001> edges and (001)
faces, respectively. (d) Non-crystallographic crystal faces produced
by a fast cooling rate.
(a)
(b)
(c)
(d)
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials46
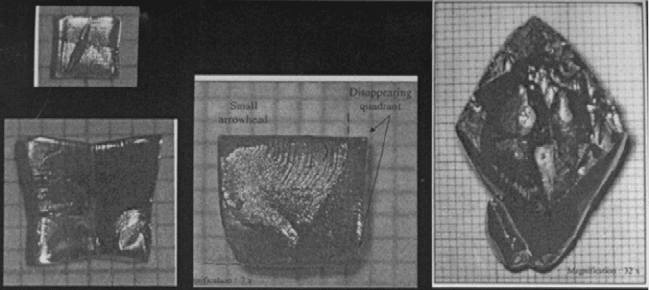
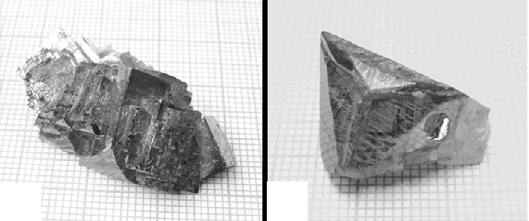
When first nucleated, PZN–PT single crystals are close to cubic in shape
(Kumar and Lim, 2000; Lim and Rajan, 2004). With favourable isotherms in
the solution, preferential nucleation at a certain or given [111] corner is
promoted such that the corner nucleation rate outpaces the spreading rate
onto adjacent {001} crystal faces. As a result, the small cube-shaped crystal
would evolve into various shapes as it grows, as shown in Fig. 2.4.
It is evident that one could engineer the isotherms in the solution to
control [111] corner nucleation while promoting (001) layer growth. The
crystal growth process in this case will be dominated by the layer growth on
certain (001) crystal planes. Since each (001) layer represents a layer of
material grown within a given time interval, and hence of reduced solute
segregation, wafers cut parallel to the prevalent (001) layer growth plane
would show improved homogeneity in composition. Besides, should the
crystal growth occur in an equilibrium or near-equilibrium condition, such
as with suitably low cooling and hence growth rates, compositional uniformity
between wafers can also be enhanced.
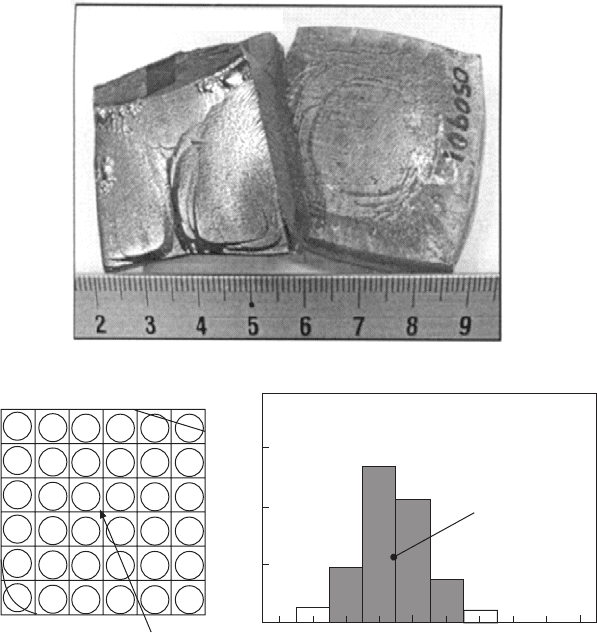
Figure 2.5 shows two PZN–PT single crystals grown using the above
scheme. As-grown PZN–PT single crystals are translucent and light yellow
to brownish-yellow in colour and exhibit prominent (001) facets. Wafers of
0.4mm thickness were sliced from the crystals and the distribution of Curie
temperature (T
C
) were determined by means of the array dot electrode technique
(Kumar et al., 2003). Figure 2.6(a) shows the distribution of T
C
within an as-
cut wafer, while Fig. 2.6(b) gives the statistical distribution obtained from
more than 140 trimmed wafers of >20mm edge length. These figures show
that for PZN–PT single crystals of <7mol% PT grown under optimal conditions,
the compositions of the crystals were fairly uniform such that ∆T
C
< ±2° C
for most part (>75% in volume) of the crystal. This corresponds to <±0.5
2.4
Evolution from (a) near-cubic to (b) star-shaped, (c) arrowhead-
shaped and (d) spearhead-shaped crystal due to fast growth along
certain <111> directions promoted by favorable isotherms in the
solution.
WPNL2204
Flux-growth and characterization of PZN–PT and PMN–PT 47
mol% PT variations in the crystals. The above results show that for relaxor–
PT single crystals of low PT contents, the improved flux growth technique
is a viable means of producing large crystals of high compositional
homogeneity.
2.5 Flux growth of relaxor single crystals of high
PT contents: PMN–(28–34)% PT
PMN–yPT system has a morphotropic phase boundary at y ≈ 0.33–0.35
(Choi et al., 1989; Noblanc et al., 1996). Small PMN–PT single crystals (of
PZN–PT Single Crystals
2.5
Two large PZN–PT single crystals grown from PbO flux.
2.6
Compositional uniformity check of flux-grown PZN–(6–7)%PT
single crystals: (a) distribution of Curie temperature (
T
c
) within an as-
cut wafer determined by the array dot electrode technique, the
separation between the dot electrodes is 5mm; (b) statistical
distribution of
T
c
obtained from more than 140 wafers of >20mm
edge length prepared from about 10 single crystals grown in the
present work.
161 162 163 164 165 166 167 168 169 170
80
60
40
20
0
All with ∆
T
c
< ± 2.5 °C within wafers
90% with ∆
T
c
< ± 2.0 °C within wafers.
162–167 °C (97%)
165.0
165.0
165.1
–
–
–
166.0
163.7
164.8
162.0
164.5
168.3
163.0
163.8
163.8
164.2
165.0
165.6
163.0
163.9
163.7
163.4
163.4
164.3
164.3
164.1
163.8
163.2
163.5
162.5
166.9
165.2
165.7
164.7
164.4
164.4
(a)
(b)
T
c
(°C)
WPNL2204

Handbook of dielectric, piezoelectric and ferroelectric materials48
a few millimetres edge length) can be readily grown from PbO-based fluxes.
They generally exhibit clear (001) growth facets, as shown in Fig. 2.7(a).
However, it may not be as straightforward when growing large PMN–PT
single crystals, because the high PT content of the system produces significant
complications to the flux growth of this material. Our experience has shown
that the growth of PMN–PT single crystals with pure PbO flux often produced
undesirable results (Lim, 2004; Lim et al., 2005). Thus, PbO + B
2
O
3
complex
fluxes were used instead. Even so, the growth results depended sensitively
on the B
2
O
3
content in the PbO solution, as will be detailed below.
First of all, as described earlier in Fig. 2.2, when there is nil or insufficient
B
2
O
3
in the solution, phase-separated flux inclusions are common features in
flux-grown PMN–PT crystals (Fig. 2.2b). With sufficient B
2
O
3
in the PbO
flux to bring the crystallization temperature below the solidus of the binary
PMN–PT system, the phase-separated flux inclusion problem can be
successfully avoided (Fig. 2.2c). Even so, the amount of B
2
O
3
was found to
significantly affect the compositional segregation of the crystal (Lim, 2004;
Lim et al., 2005). For instance, measurements of T
C
distributions from wafers
cut from as-grown PMN–PT crystals showed that with less-than-optimum
amounts of B
2
O
3
in PbO flux, the variations within wafers were acceptable
(i.e.
∆
T
C
≤±3.0°C) but were too large even between adjacent cuts of wafers
(of about 0.5mm thickness). On the other hand, with higher-than-optimum
amounts of B
2
O
3
, the reverse was observed instead.
The amount of B
2
O
3
in PbO flux also affects the growth mechanism of
PMN–PT crystals. Figures 2.7(b) and (c) show the general morphologies of
large PMN–PT single crystals (i.e. those ≥ 20mm edge length) grown in the
present work. Although all crystals exhibit apparent (001) growth facets,
2.7
Different (001) growth morphologies in flux-grown PMN–30%PT
single crystals: (a) small cubic-shaped crystal of prominent (001)
facets of up to 10mm edge length produced from PbO-based fluxes.
Note the evidence of <111> corner nucleation (arrowed) followed by
<011> edge and (001) layer growth; (b) (001) platelet growth in large
crystals (>20mm edge length) produced from pure PbO flux;
(c) microscopic (001) layer growth in large crystals (>20mm edge
length) produced from PbO-based flux containing >5mol% B
2
O
3
.
(a)
(b)
(c)
WPNL2204
Flux-growth and characterization of PZN–PT and PMN–PT 49
those grown without or with insufficient B
2
O
3
in the PbO flux have a platelet
morphology (Fig. 2.7b). An obvious change from (001) platelet growth to
microscopic (001) layer growth is evident even with a few mol% of B
2
O
3
(i.e. about 5mol%) added to the PbO flux (Fig. 2.7c).
Figure 2.8 shows the change in nucleation mechanism during growth
from solution with increasing B
2
O
3
content, revealed by deliberately increasing
the cooling rate at the later stage of the crystal growth run. Apparently, with
a low B
2
O
3
content in the PbO flux, the (001) layer growth is initiated via the
nucleation and growth of (001)-oriented crystal blocks on the (001) growth
facets (Fig. 2.8a). On the other hand, with sufficient B
2
O
3
addition, (001)
layer growth occurs through <111> corner nucleation followed by spreading
down the adjacent <001> edges and (001) faces (Fig. 2.8b).
The above observations suggest that ionic complexes formed in the high
temperature solution of PMN–PT–PbO(B
2
O
3
) system play an important role
in the growth of PMN–PT single crystals. Owing to the strong affinity between
Ti
4+
and O
2–
ions and the substantial amount of PT present in the PMN–yPT
system studied (y = 0.28–0.34), it is likely that Ti
4+
and O
2–
ions may form
various large ionic complexes or clusters (possibly with some covalent nature)
in the solution. The growth of PMN–PT with insufficient B
2
O
3
addition is
thus controlled by cluster growth of Ti
4+
–O
2–
based complexes. Owing to the
lower diffusivity of the latter in the solution, as opposed to other simpler
ions, the adjacent solution becomes enriched with Ti
4+
–O
2–
complexes, leading
to a significant increase in the PT content of successively grown layers as the
crystal grows in size.
On the other hand, owing to the strong B
3+
–O
2–
bonds and the valency
difference, addition of B
2
O
3
helps modify the nature of the Ti
4+
–O
2–
-based
complexes in the solution. With sufficient B
2
O
3
in the PbO flux, a change
(a)
(b)
2.8
Different nucleation mechanisms in flux growth of PMN–PT single
crystals, revealed by higher cooling rates near the end of the growth
runs: (a) cluster nucleation on (001) facets with less-than-optimum
amounts of B
2
O
3
in PbO flux; (b) <111> corner and <001> edge
nucleation and growth with near-optimum amounts of B
2
O
3
in PbO
flux.
WPNL2204
