Журнал - Проблемы криобиологии 2010 №4
Подождите немного. Документ загружается.

овтсещеВ
ecnatsbuS
,арутарепмеТ
C°
,erutarepmeT
C°
f
4
, × 01
6-
f
3
, × 01
5-
f
2
, × 01
3-
f
1
f
0
R
2
нозапаиД
йицартнецнок
%.ссам,С
noitartnecnoC
)w/w(%,egnar
кинчотсИ
ecnerefeR
sedimAыдимА
АФМД
AFMD
62000 4230,0-941,737999,001 ÷ 001]54[
sremylopelbulosretaWыремилопеымировтсародоВ
КЭОГ
SEH
420 88,71-596,95861,0-21,274758,01÷ 04]09[
нартскеД
nartxeD
42000 610,0-627,072448,01÷ 03]09[
1=nГЭО
1=nGEO
02484,961,78-88,929445,0-19,077589,05,0 ÷ 03]2,1[
2=nГЭО
2=nGEO
0241,515,811-366,639276,0-49,074689,05,0 ÷ 03]2,1[
3=nГЭО
3=nGEO
020 45,72-54,818535,0-64,072689,05,0 ÷ 03]2[
5=nГЭО
5=nGEO
020 72,54-90,521575,0-3,079999,05,0 ÷ 03]12,71[
01=nГЭО
01=nGEO
020 44,83-28,518082,0-99,56209,01÷ 03]2,1[
02=nГЭО
02=nGEO
020 30,13-15,317582,0-90,569669,05,0 ÷ 03
]2[
03=nГЭО
03=nGEO
0276,463,344-85,692438,0-72,663678,05÷ 03]12,1[
ПВП
)00001.м.м(
PVP
thgiew.lom(
)00001
4203,96-49,421913,0-68,862548,01÷ 02]09[
429
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
,анирецилгяицартнецноK
%еынмеъбо
,noitartnecnoclorecylG
)v/v(%
e
1
e
0
R
2
нозапаиД
С°,рутарепмет
,egnarerutarepmeT
С°
кинчотсИ
ecnerefeR
018540,0-532,170,11- ÷ 04]91[
026160,0-916,075899,05- ÷ 04]91[
036250,0-55,967899,001- ÷ 04]91[
049840,0-880,860,151- ÷ 04]91[
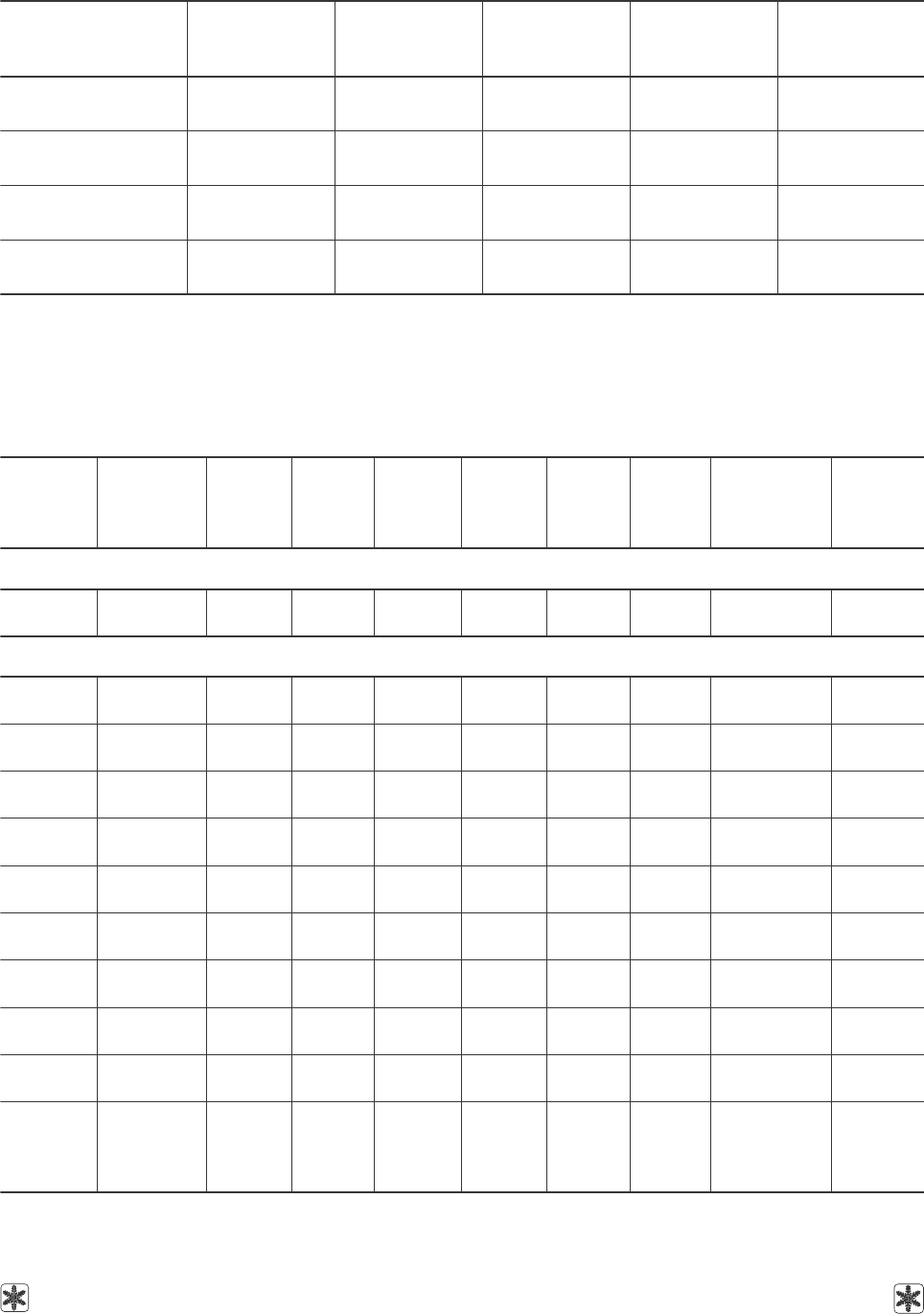
Таблица 11. Коэффициенты уравнения (5) для расчета поверхностного натяжения водных растворов
глицерина при заданной объемной концентрации в зависимости от температуры; дисперсии
аппроксимаций и диапазоны температур применения уравнения
Table 11. Coefficients of equation (5) to calculate the surface tension of aqueous solutions of glycerol at given volumetric
concentration depending on temperature; approximation dispersion and temperature ranges of equation application
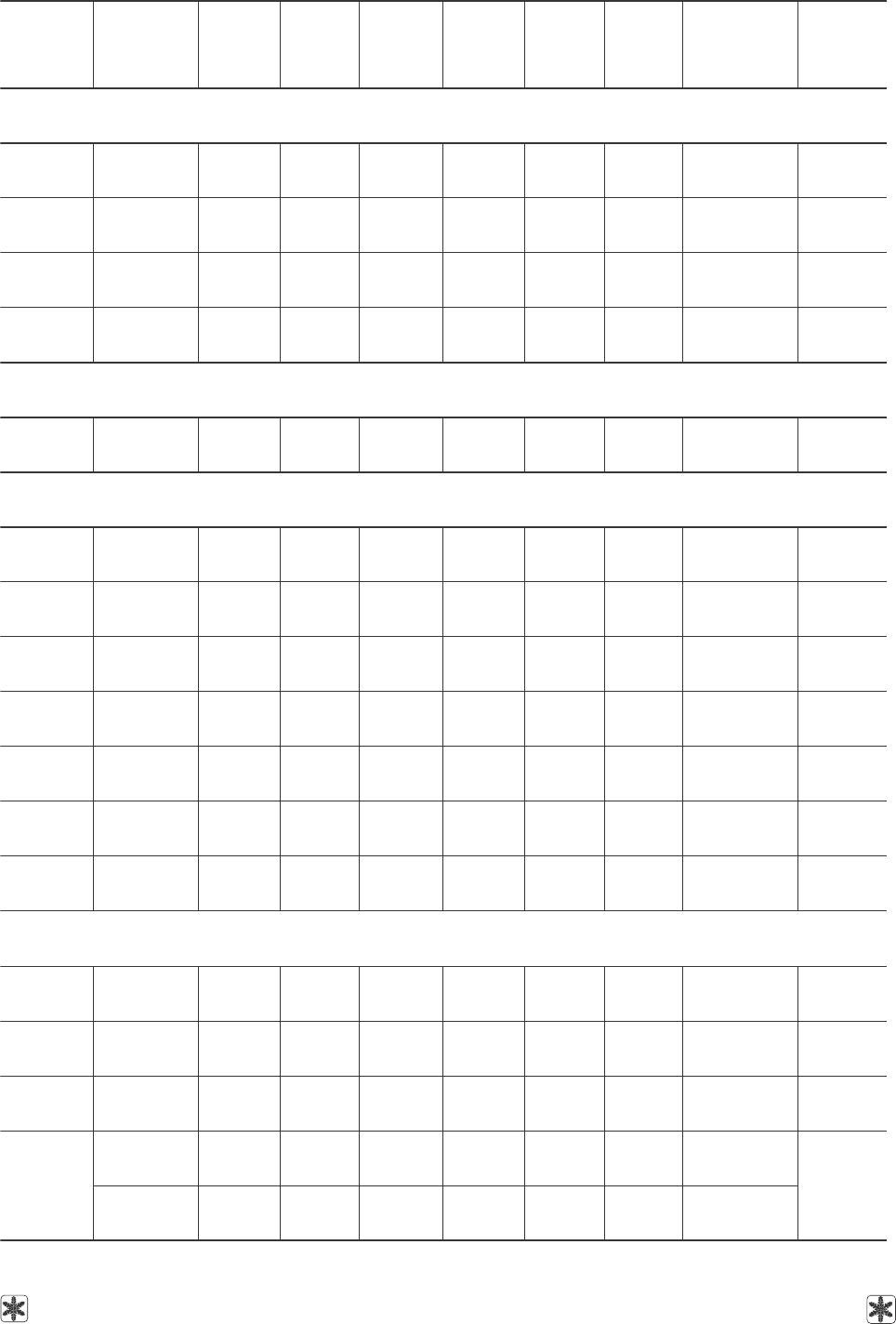
Таблица 12. Коэффициенты уравнения (6) для расчета поверхностного натяжения ряда водных растворов
криопротекторов в зависимости от концентрации при фиксированных температурах; дисперсии аппроксимаций
и диапазоны концентраций применения уравнения
Table 12. Coefficients of equation (6) to calculate the surface tension of some aqueous solutions
of cryoprotective agents depending on concentration at fixed temperatures; approximation
dispersion and concentration ranges of equation application
Продолжение на следующей странице.
Next page to be continued.
овтсещеВ
ecnatsbuS
,арутарепмеТ
C°
,erutarepmeT
C°
f
4
, × 01
6-
f
3
, × 01
5-
f
2
, × 01
3-
f
1
f
0
R
2
нозапаиД
йицартнецнок
%.ссам,С
noitartnecnoC
)w/w(%,egnar
кинчотсИ
ecnerefeR
sremylopelbulosretaWыремилопеымировтсародоВ
ОЭП - 001
OEP - 001
020013,311429,0-39,275799,01÷ 03]41,1[
ОЭП - 004
OEP - 004
0200791,1903,0-967869,01÷ 001]12,41,1[
ОЭП - 005
OEP - 005
020087,715436,0-72,272859,01÷ 02]41[
ОЭП - 0004
OEP - 0004
0200266,41752,0-74,768589,01÷ 03]12,41,1[
sedixOыдискО
ОСМД
OSMD
42000 7852,0-601,076599,01÷ 001]09[
slohoclAытрипС
нирецилГ
lorecylG
4200 7623,0-42,5-278399,01÷ 001]09[
ГЭТ
GET
520 225,1-9932,43435,0-1,27999,00÷ 001]28[
ГЭ
GE
030 242,1-88,2414,0-2,175189,00÷ 001]75[
3,1 - ДБ
3,1 - DB
03678,155,64-68,04426,1-2,174299,00÷ 001]75[
4,1 - ДБ
4,1 - DB
03784,13,63-80,13632,1-2,176599,00÷ 001]65[
2,1 - ДП
2,1 - DP
030 638,5-56,21840,1-2,176299,00÷ 001]75[
3,1 - ДП
3,1 - DP
03510,119,62-67,42340,1-2,174889,00÷ 001]75[
setardyhobraCыдовелгУ
001М 52004,0-8000,0-34,177899,05÷ 02]32[
051М 5200 75820,075710,093,174499,05÷ 53]32[
002М 52003,0-1230,034,179999,05÷ 02]32[
азорахаС
esorcuS
420008340,0354,178799,01÷ 08
]09,32[
520004530,0823,178579,05÷ 04
Продолжение табл. 12
Table 12. (Continued from the previous page)
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
430
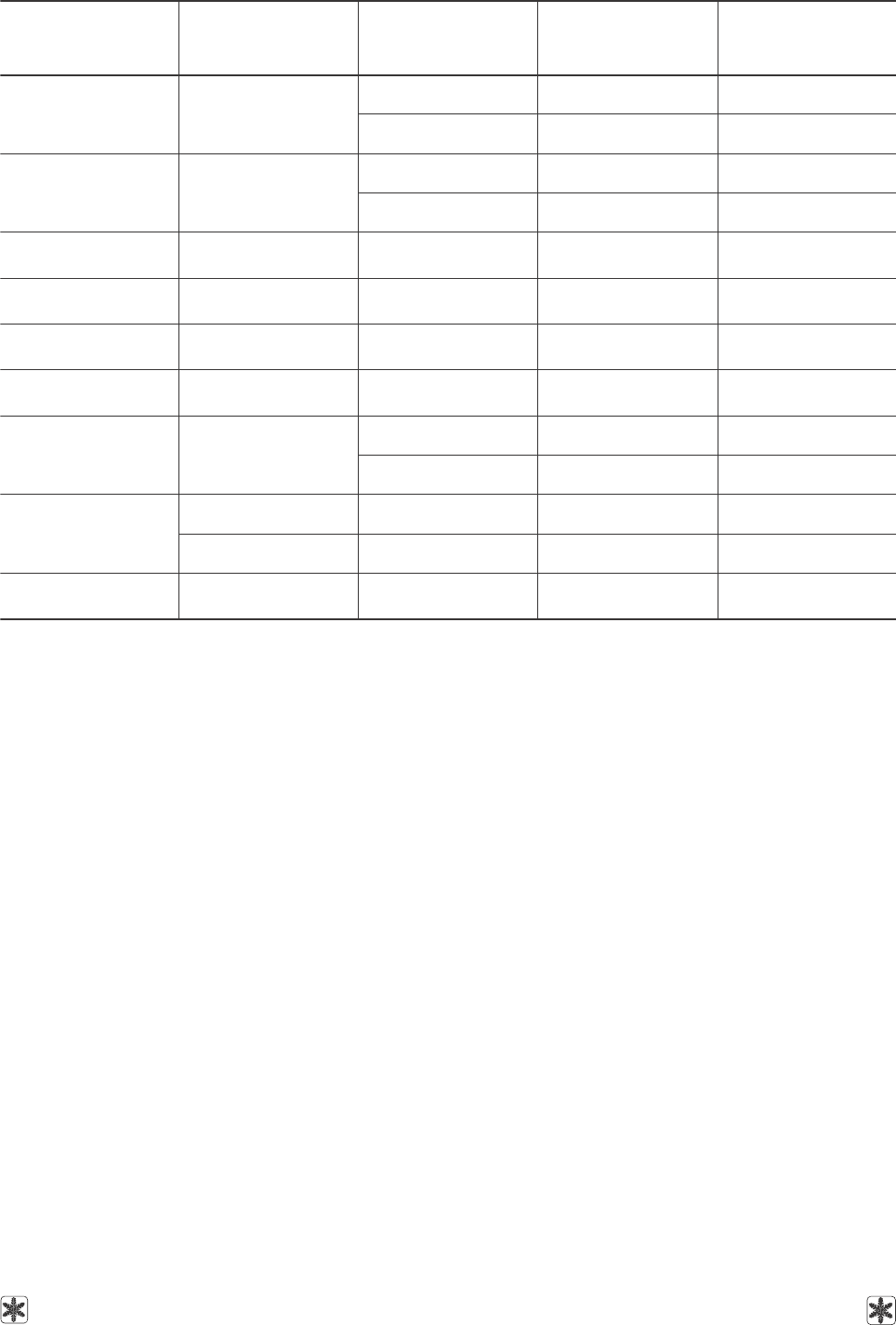
Таблица 13. Поверхностное натяжение некоторых криопротекторов и их растворов
Table 13. Surface tension of some cryoprotective agents and their solutions
Литература
Актуальные проблемы криобиологии / Под общ. ред.
Н.С. Пушкаря и А.М. Белоуса.– Киев: Наук. думка, 1981.–
608 с.
Белоус А.М., Шраго М.И., Пушкарь Н.С. Криоконсерван-
ты.– Киев: Наук. думка, 1979.– 198 с.
Богданов Н.С., Иванов О.П., Куприянова А.В. Холодиль-
ная техника. Свойства веществ. Справочник.– М.: Агро-
промиздат, 1985.– 208 с.
Варгафтик Н.Б., Волков Б.Н., Воляк Л.Д. О международ-
ных таблицах поверхностного натяжения воды // Тепло-
энергетика.– 1979.– N5.– С. 73–74.
Кавешников А.И., Суев А.В. Измерения вязкости крови
при разведении ее различными кровезаменителями в
условиях гипотермии // Патологическая физиология и
экспериментальная терапия.– 1971.– Т. 15, N1.– С. 70–
75.
Карапетян Ю.А., Эйчис В.М. Физико-химические свойст-
ва электролитных неводных растворов.– М.: Химия,
1989.– 256 с.
Коверда В.П., Скрипов В.П. О температурной зависимос-
ти теплоемкости переохлажденной воды // Журнал
физической химии.– 1978.– Т. 52, N4.– С. 1041–1043.
Компаниец А.М., Николенко А.В., Кощий С.В., Ивано-
ва И.А. Монометиловый эфир глицерина: цитотоксич-
ность и криозащитная эффективность при заморажива-
нии тромбоцитов // Физико-химические свойства и
биологическое действие криопротекторов: Сб. статей /
Под ред. В.И.Лугового.– Харьков, 1990.– С. 59–63.
Кошкин Н.И., Ширкевич М.Г. Справочник по элементарной
физике.– М.: Наука, 1982.– 208 с.
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
431
References
Actual tasks of cryobiology / Ed. by N.S. Pushkar and
A.M. Belous.– Kiev: Naukova dumka, 1981.– 608 p.
Belous A.M., Shrago M.I., Pushkar N.S. Cryopreservatives.–
Kiev: Naukova dumka, 1979.– 198p.
Bogdanov N.S., Ivanov O.P., Kupriyanova A.V. Refrigerating
apparatuses. Properties of substances. Reference book.–
Moscow: Agropromizdat, 1985.– 208 p.
Vargaftik N.B., Volkov B.N., Volyak L.D. About international
tables of surface tension of water // Teploenergetica.– 1979.–
N5.– P. 73–74.
Kaveshnikov A.I., Suev A.V. Measurements of blood viscosity
at its dilution with different blood substances under
hypothermia // Patologicheskaya fiziologiya i eksperimen-
tal'naya terapiya.– 1971.– Vol. 15, N1.– P. 70–75.
Karapetyan Yu.A., Eychis V.M. Physical and chemical proper-
ties of electrolyte non-aqeuous solutions.– Moscow: Khimiya,
1989.– 256 p.
Koverda V.P., Skripov V.P. About temperature dependence
of thermal capacity of overcooled water // Zhurnal Fizicheskoy
Khimii.– 1978.– Vol. 52, N4.– P. 1041–1043.
Kompaniets A.M., Nikolenko A.V., Koschiy S.V., Ivanova I.A.
Monomethyl ether of glycerol: cytotoxicity and cryoprotective
efficiency during freezing of platelets // In: Physical and
chemical properties and biological effect of cryoprotectants /
Ed. by V.I. Lugovoy.– Kharkov, 1990.– P. 59–63.
Koshkin N.I., Shirkevich M.G. Reference book on elementary
physics.– Moscow: Nauka, 1982.– 208 p.
Brief Reference Book of Physical and Chemical Values / Ed.
by A.A. Ravdel' and A.M. Ponomareva.– Leningrad: Khimiya,
1983.– 232 p.
1.
2.
3.
4.
5.
6.
7.
8.
9.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
овтсещеВ
ecnatsbuS
,яицартнецноK
%еынмеъбо
,noitartnecnoC
)v/v(%
С°,арутарепмеТ
C°,erutarepmeT
еонтсонхревоП
мс/нид,еинежятан
mc/nid,noisnetecafruS
кинчотсИ
ecnerefeR
цАМД
cAMD
001
0253,53]97[
0237,44]41,01[
ОСМД
OSMD
001
7,3234]42[
5289,24]41,01[
АФМД
AFMD
001027,63]58,97,6[
цАМ
cAM
001737,33]6[
ГЭП - 004
GEP - 004
001025,34]97[
ГЭТ
GET
001521,54]6[
димамроФ
edimamroF
001
024,85]58,97,6[
7,3275]42[
2,1 - ДП
2,1 - DP
0015273]08,27[
055254]08[
ГЭ
GE
055265]08[

problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
432
Краткий справочник физико-химических величин / Под ред.
А.А. Равделя и А.М.Пономаревой.– Л.: Химия, 1983.– 232 с.
Куликов М.В., Крестов А.Г., Сафонова Л.П., Колкер А.М.
Избыточные термодинамические функции систем вода
N-метилформамид вода - N,N - диметилформамид при
308,15 К // Термодинамика растворов неэлектролитов.–
Л.: Химия, 1989.– С. 27–32.
Нефть-газ. Электронная библиотека [Электронный
документ] // [веб-сайт] www.oglib.ru/tabl/index.html
(17.05.2010)
Павлов К.Ф., Романков П.Г., Носков А.А. Примеры и
задачи по курсу процессов и аппаратов химической
технологии.– Л.: Химия, 1987.– 576 с.
Пушкарь Н.С., Шраго М.И., Белоус А.М. Криопротекторы.–
Киев: Наук. думка, 1978.– 204 с.
Рид Р., Праусниц Дж., Шервуд Т. Свойства газов и жид-
костей: Справочное пособие.– Л.: Химия, 1982.– 592 с.
Справочник по физико-техническим основам криоге-
ники / Под ред. М.П. Малкова.– М.: Энергоатомиздат,
1985.– 432 с.
Таблицы физических величин. Справочник / Под ред.
И.К. Кикоина.– М.: Атомиздат, 1976.– 1008 с.
Теплотехнический справочник. Т.2 / Под ред. В.Н. Юре-
нева и П.Д. Лебедева.– М.: Энергия, 1976.– 896 с.
Тодрин А.Ф. Об измерении поверхностного натяжения
водных растворов глицерина и плазмы крови // Моде-
лирование криобиологических процессов: Сб. статей.–
Харьков, 1988.– C. 142–145.
Тодрин А.Ф., Попивненко Л.И., Коваленко С.Е. Теплофизи-
ческие свойства криопротекторов. II. Динамическая вяз-
кость ряда криопротекторов, их растворов и смесей //
Проблемы криобиологии.– 2010.– Т. 20, №3.– С. 266–281.
Шраго М.Й., Гучок М.М., Калугін Ю.В. Спрямований синтез
і комплексне вивчення кріопротекторів // Вісник АН УРСР.–
1980.– N9.– С. 35–40.
Brodkey R.S., Hershey H.C. Transport phenomena: a unified
approach.– Columbus: Brodkey Publishing, 2003.– 516 p.
Carvajal P.A., MacDonald G.A., Lanier T.C. Cryostabilization
mechanism of the fish muscle proteins by maltodextrins //
Cryobiology.– 1999.– Vol. 38, N1.– P. 16–26.
Chatterjee D., Hetayothin B., Wheeler A.R. et al. Droplet-
based microfluidics with nonaqueous solvents and solutions //
Lab Chip.– 2006.– Vol. 6, N2.– P. 199–206.
Chem Group Incorporated [Электронный документ] // [веб-
сайт] www.chem-group.com (20.01.2011).
Chemical.net. [Электронный документ] // [веб-сайт]
http://chemical.net/home/Defaults.asp (20.01.2010).
Cobos D. Using the KD2 to measure thermal conductivity of fluids
[Электронный документ] // [веб-сайт] www.decagon.com/
appnotes/UsingtheKD2toMeasureThermalConductivity.pdf
(25.10.2009).
Cowie J.M.G., Toporowski P.M. Association in the binary
liquid system dimethyl sulphoxide - water // Can. J. Chem.–
1961.– Vol. 39, N11.– P. 2240–2243.
CRC handbook of chemistry and physics. 69
th
edition, 1988-
1989 / Ed. by R.C. Weast.– Boca Raton, Florida: CRC Press,
Inc., 1988.– 427 p.
Crombie D.S., Hipkins M.F., Milburn J.A. Gas penetration
of pit membranes in the xylem of Rhododendron as the cause
of acoustically detectable sap cavitation // Aust. J. Plant
Physiol.– 1985.– Vol. 12, N5.– P. 445–453.
Diller K.R. The influence of controlled ice nucleation on
regulating the thermal history during freezing // Cryobiology.–
1985.– Vol. 22, N3.– P. 268–281.
Dimethylacetamide [Электронный документ] // [веб-сайт]
http://chemicalland21.com/industrialchem/solalc/n,n-
DIMETHYLACETAMIDE.htm (25.04.2009).
Elert G. The physics hypertexbook [Электронный доку-
мент] // [веб-сайт] http://hypertexbook.com/physics
(03.06.2010).
Kulikov M.V., Krestov A.G., Safonova L.P., Kolker A.M. Exces-
sive thermo-dynamical functions of water N-methyl formamide
and water-N,N -dimethyl formamide systems at 308;15 K // In:
Thermodynamics of non-electrolytes solutions.– Leningrad:
Khimiya, 1989.– P. 27–32.
Oil-gas [Electronic resource] // [web-site] www.oglib.ru/tabl/
index.html (17.05.2010).
Pavlov K.F., Romankov P.G., Noskov A.A. Examples and tasks
on the course of the processes and apparatuses of chemical
technology.– Leningrad: Khimiya, 1987.– 576 p.
Pushkar N.S., Shrago M.I., Belous A.M. Cryoprotectants.–
Kiev: Naukova dumka, 1978.– 204 p.
Reid R., Prausniz J, Shervood T. Properties of gases and
fluids: Reference book.– Leningrad: Khimiya, 1982.– 592 p.
Reference Book on Physical and Technical basics of cryoge-
nics / Ed. by M.P. Malkova.– Moscow: Energoatomizdat,
1985.– 432 p.
Tables of physical values. Reference book / Ed. by I.K. Ki-
koin.– Moscow: Atomizdat, 1976.– 1008 p.
Thermotechnical reference book. Vol. 2 / Ed. by V.N. Yurenev
and P.D. Lebedev.– Moscow: Energiya, 1976.– 896 p.
Todrin A.F. About change of surface tension of aqueous
solutions of glycerol and blood plasma // In: Simulation of
cryobiological processes.– Kharkov, 1988.– P. 142–145.
Todrin A.F., Popivnenko L.I., Kovalenko S.E. Thermophysical
properties of cryoprotective agents. II. Dynamic viscosity of
some cryoprotective agents, their solutions and mixtures//
Problems of Cryobiology.– 2010.– Vol. 20, N3.– P. 266–281.
Shrago M.I., Guchok M.M., Kalugin Yu.V. Directed synthesis
and complex study of cryoprotectants // Visnyk AN UkrSSR.–
1980.– N9.– P. 35–40.
Brodkey R.S., Hershey H.C. Transport phenomena: a unified
approach.– Columbus: Brodkey Publishing, 2003.– 516 p.
Carvajal P.A., MacDonald G.A., Lanier T.C. Cryostabilization
mechanism of the fish muscle proteins by maltodextrins //
Cryobiology.– 1999.– Vol. 38, N1.– P. 16–26.
Chatterjee D., Hetayothin B., Wheeler A.R. et al. Droplet-
based microfluidics with nonaqueous solvents and solutions //
Lab Chip.– 2006.– Vol. 6, N2.– P. 199–206.
Chem Group Incorporated [Electronic resource] / [web-site]
www.chem-group.com (20.01.2011).
Chemical.net. [Электронный документ] // [веб-сайт]
http://chemical.net/home/Defaults.asp (20.01.2010).
Cobos D. Using the KD2 to measure thermal conductivity of fluids
[Electronic resource] // [web-site] www.decagon.com/appnotes/
UsingtheKD2toMeasureThermalConductivity.pdf (25.10.2009).
Cowie J.M.G., Toporowski P.M. Association in the binary
liquid system dimethyl sulphoxide - water // Can. J. Chem.–
1961.– Vol. 39, N11.– P. 2240–2243.
CRC handbook of chemistry and physics. 69
th
edition, 1988-
1989 / Ed. by R.C. Weast.– Boca Raton, Florida: CRC Press,
Inc., 1988.– 427 p.
Crombie D.S., Hipkins M.F., Milburn J.A. Gas penetration
of pit membranes in the xylem of Rhododendron as the cause
of acoustically detectable sap cavitation // Aust. J. Plant
Physiol.– 1985.– Vol. 12, N5.– P. 445–453.
Diller K.R. The influence of controlled ice nucleation on
regulating the thermal history during freezing // Cryobiology.–
1985.– Vol. 22, N3.– P. 268–281.
Dimethylacetamide [Electronic resource] // [web-site] http://
chemicalland21.com/industrialchem/solalc/n,n-
DIMETHYLACETAMIDE.htm (25.04.2009).
Elert G. The physics hypertexbook [Electronic resource] //
[web-site] http://hypertexbook.com/physics (03.06.2010).
Eliassi A., Modarress H. Densities of poly(ethylene glycol) +
water mixtures in the 298.15–328.15 K temperature range //
J. Chem. Eng. Data.– 1998.– Vol. 43, N5.– P. 719–721.
Figura L.O., Teixeira A.A. Food physics. Physical properties –
measurement and applications.– New York: Springer, 2007.–
550 p.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.

problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
433
Eliassi A., Modarress H. Densities of poly(ethylene glycol) +
water mixtures in the 298.15–328.15 K temperature range //
J. Chem. Eng. Data.– 1998.– Vol. 43, N5.– P. 719–721.
Figura L.O., Teixeira A.A. Food physics. Physical properties –
measurement and applications.– New York: Springer, 2007.–
550 p.
Flick E.W. Industrial solvent handbook.– Westwood: William
Andrew, 1998.– 963 p.
Foroutan M. Density dependence of the viscosity and excess
volume of aqueous solutions of polyvinylpyrrolidone // Acta
Chim. Slov.– 2006.– Vol. 53, N2.– P. 219–222.
Gaylord Chemical Company LLC. Dimethyl Sulfoxid (DMSO).
Physical properties [Электронный документ] // [веб-сайт]
www.gaylordchemical.com/bulletins
van Gelder M.F. A thermistor based method for measurement
of thermal conductivity and thermal diffusivity of moist food
materials at high temperatures: Thesis … of doctor of
philosophy in Biological Systems Engineering.– Blackburg,
Virginia, 1998.– 160 p.
Goel M., Roy S.K., Senguptas S. Laminar forced convection
heat transfer in microcapsulated phase change material
suspensions // Inf. J. Heal Mass Transfer.– 1994.– Vol. 37,
N4.– P. 593–604.
Haddadin R. Accuracy of Brookfield and capillary viscome-
ters for newtonian viscosity determination [Электронный
документ] // [веб-сайт] www.seas.upenn/edu/courses/
belab/LabProjects/2002/be309f02m3p1.doc (7.12.2009).
Han F., Zhang J., Chen G., Wei X. Density, viscosity, and
excess properties for aqueous poly(ethylene glycol) solutions
from (298.15 to 323.15) K // J. Chem. Eng. Data.– 1998.–
Vol. 53, N11.– P. 2598–2601.
Hare D.E., Sorensen C.M. The density of supercooled water.
II. Bulk samples cooled to the homogeneous nucleation limit //
J. Chem. Phys.– 1987.– Vol. 87, N8.– P. 4840–4845.
Huck J., Dufour J., Bondeau A. Density of supercooled
glycerol-water solutions // 9th International conference on
conduction and breakdown in dielectric liquids.– 1987.– New
York, 1987.– P. 240–244.
Jarusuwannapoom T., Hongrojjanawiwat W., Jitjaicham S.
et al. Effect of solvents on electro-spinnability of polysterene
solutions and morphological appearance of resulting electro-
spun polysterene fibers // Eur. Рolym. J.– 2005.– Vol. 41,
N3.– P. 409–421.
Josens R.B., Farina W.M. Nectar feeding by the hovering
hawk moth Macroglossum stellatarum: intake rate as a function
of viscosity and concentration of sucrose solutions // J. Comp.
Physiol. A.– 2001.– Vol. 187, N8.– P. 661–665.
Kawaizumi F., Ohno M., Miyahara Y. Ultrasonic and volumetric
investigation of aqueous solutions of amides // Bull. Chem.
Soc. Jpn.– 1977.– Vol. 50, N9.– P. 2229–2233.
Kaye & Laby Tables of physical and chemical constants.
[Электронный документ] // [веб-сайт] www.kayelab.npl.co.uk/
toc/ (12.08.2010).
Kestin J., Khalifa H.E., Correia R.J. Tables of the dynamic
and kinematic viscosity of aqueous NaCl solutions in the
temperature range 20–150°C and the pressure 0,1-35 MPa //
J. Phys. Chem. Ref. Data.– 1981.– Vol. 10, N1.– P. 71–87.
Kratochvil A., Hrncir E. Correlation between the blood surface
tension and the activity of some enzymes // Physiol. Res.–
2001.– Vol. 50, N4.– P. 433–437.
Laque W.E., Ronneberg C.E. A study of the decarboxylation
of trichloroacetic acid in solution of water and dimethyl-
sulfoxide // Ohio Journal of Science.– 1970.– Vol. 70, N2.–
P. 97–106.
Martinez I. Thermodynamics of solution [Электронный
документ] // [веб-сайт] http://webserver.dmt.upm.es/
~isidoro/bk3/c07sol/solutions.htm (4.04.2010).
McDonald E.J., Turcotte A.L. Density and refractive indices
of lactose solutions // J. Res. Natl. Bur. Stand.– 1948.– Vol. 41,
N7.– P. 63–68.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
Flick E.W. Industrial solvent handbook.– Westwood: William
Andrew, 1998.– 963 p.
Foroutan M. Density dependence of the viscosity and excess
volume of aqueous solutions of polyvinylpyrrolidone // Acta
Chim. Slov.– 2006.– Vol. 53, N2.– P. 219–222.
Gaylord Chemical Company LLC. Dimethyl Sulfoxid (DMSO).
Physical properties [Electronic resource] // [web-site]
www.gaylordchemical.com/bulletins.
van Gelder M.F. A thermistor based method for measurement
of thermal conductivity and thermal diffusivity of moist food
materials at high temperatures: Thesis … of doctor of
philosophy in Biological Systems Engineering.– Blackburg,
Virginia, 1998.– 160 p.
Goel M., Roy S.K., Senguptas S. Laminar forced convection
heat transfer in microcapsulated phase change material
suspensions // Inf. J. Heal Mass Transfer.– 1994.– Vol. 37,
N4.– P. 593–604.
Haddadin R. Accuracy of Brookfield and capillary viscome-
ters for newtonian viscosity determination [Electronic
resource] // [web-site] www.seas.upenn/edu/courses/belab/
LabProjects/2002/be309f02m3p1.doc (7.12.2009).
Han F., Zhang J., Chen G., Wei X. Density, viscosity, and
excess properties for aqueous poly(ethylene glycol) solutions
from (298.15 to 323.15) K // J. Chem. Eng. Data.– 1998.–
Vol. 53, N11.– P. 2598–2601.
Hare D.E., Sorensen C.M. The density of supercooled water.
II. Bulk samples cooled to the homogeneous nucleation limit //
J. Chem. Phys.– 1987.– Vol. 87, N8.– P. 4840–4845.
Huck J., Dufour J., Bondeau A. Density of supercooled
glycerol-water solutions // 9th International conference on
conduction and breakdown in dielectric liquids.– 1987.– New
York, 1987.– P. 240–244.
Jarusuwannapoom T., Hongrojjanawiwat W., Jitjaicham S.
et al. Effect of solvents on electro-spinnability of polysterene
solutions and morphological appearance of resulting electro-
spun polysterene fibers // Eur. Рolym. J.– 2005.– Vol. 41,
N3.– P. 409–421.
Josens R.B., Farina W.M. Nectar feeding by the hovering
hawk moth Macroglossum stellatarum: intake rate as a function
of viscosity and concentration of sucrose solutions // J. Comp.
Physiol. A.– 2001.– Vol. 187, N8.– P. 661–665.
Kawaizumi F., Ohno M., Miyahara Y. Ultrasonic and volumetric
investigation of aqueous solutions of amides // Bull. Chem.
Soc. Jpn.– 1977.– Vol. 50, N9.– P. 2229–2233.
Kaye & Laby Tables of physical and chemical constants.
[Electronic resource] // [web-site] www.kayelab.npl.co.uk/toc/
(12.08.2010).
Kestin J., Khalifa H.E., Correia R.J. Tables of the dynamic
and kinematic viscosity of aqueous NaCl solutions in the
temperature range 20–150°C and the pressure 0,1-35 MPa //
J. Phys. Chem. Ref. Data.– 1981.– Vol. 10, N1.– P. 71–87.
Kratochvil A., Hrncir E. Correlation between the blood surface
tension and the activity of some enzymes // Physiol. Res.–
2001.– Vol. 50, N4.– P. 433–437.
Laque W.E., Ronneberg C.E. A study of the decarboxylation
of trichloroacetic acid in solution of water and dimethyl-
sulfoxide // Ohio Journal of Science.– 1970.– Vol. 70, N2.–
P. 97–106.
Martinez I. Thermodynamics of solution[Electronic resource] //
[web-site] http://webserver.dmt.upm.es/~isidoro/bk3/c07sol/
solutions.htm (4.04.2010).
McDonald E.J., Turcotte A.L. Density and refractive indices
of lactose solutions // J. Res. Natl. Bur. Stand.– 1948.– Vol. 41,
N7.– P. 63–68.
METTLER TOLEDO [Electronic resource] // [web-site]
www.us.mt.com/home (23.05.2010).
Mexal J., Fisher J.T., Osteryoung J., Reid C.P.P. Oxygen
availability in polyethylene glycol solutions and its implications
in plant-water relations // Plant physiol.– 1975.– Vol. 55, N1.–
P. 20–24.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.

problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
434
METTLER TOLEDO [Электронный документ] // [веб-сайт]
www.us.mt.com/home (23.05.2010).
Mexal J., Fisher J.T., Osteryoung J., Reid C.P.P. Oxygen
availability in polyethylene glycol solutions and its implications
in plant-water relations // Plant physiol.– 1975.– Vol. 55, N1.–
P. 20–24.
Molinero V., Cagin T., Goddard W.A. III. Sugar, water and
free volume networks in concentrated sucrose solution //
Chem. Phys. Lett.– 2003.– Vol. 377, N3–4.– P. 469–474.
Nakanishi K., Matsumoto T., Hayatsu M. Surface tension of
aqueous solutions of some glycols // J. Chem. Eng. Data.–
1971.– Vol. 16, N1.– P. 44–45.
Noda K., Ohashi M., Ishida K. Viscosities and densities at
298.15 K for mixtures of methanol, acetone, and water // J.
Chem. Eng. Data.– 1982.– Vol. 27, N3.– P. 326–328.
Okutomi T., Nemoto M., Mishiba E., Goto F. Viscosity of
diluent and sensory level of subarachnoid anaesthesia
achieved with tetracaine // Can. J. Anaesth.– 1998.– Vol. 45,
N1.– P. 84–86.
Polyvinylpyrrolidone [Электронный документ] // [веб-сайт]
www.camd.lsu.edu/msds/p/polyvinylpyrrolidone.htm
(9.08.2009).
Radhakrishnan S. Measurement of thermal properties of
seafood / Thesis … Master of Science in Biological Systems
Engineering.– Blackburg, Virginia, 1997.– 83 p.
Rao B.G., Singh U.C. A free energy perturbation study of
solvation in methanol and dimethyl sulfoxide // J. Am. Chem.
Soc.– 1990.– Vol. 112, N10.– P. 3803–3811.
Rastogi P.P. Ion-solvent interaction of tetraalkylammonium
and some common ions in N-methylformamide from viscosity
data // Bull. Chem. Soc. Jpn.– 1970.– Vol. 43, N12.– P. 2442–
2444.
Regmi S. Study and estimation of temperature dependent
physical parameters of poly(vinylidene fluoride) and poly(1,4
butylene adipate) dissolved in N,N-dimethyl formamide // J.
Sci. Eng. Tech.– 2007.– Vol. I, NIII.– P. 1–10.
Roy R.N., Baker G.E., Hoffman T. et al. Standard electrode
potentials of silver-silver chloride electrodes in 20, 30, and
50% (w/w) ethylene glycol/water from 25°C to –20°C pK2
and pH* values of the physiological buffer "BES" in 50%
(w/w) ethylene glycol/water // Cryo-Letters.– 1988.– Vol. 9,
N3.– P. 172–185.
Roy R.N., Gibbons J.J., Baker G., Bates R.G. Standard
electromotive force of the H2-AgCl; AgCl in 30, 40, and 50
mass.% dimethyl sulfoxide/water from –20 to 25°C: pK2 and
pH values for a standard "Bicine" buffer solutions at subzero
temperatures // Cryobiology.– 1984.– Vol. 21, N6.– P. 672–
681.
Saleh J.M., Khalil M., Hokmat N.A. Investigation of some
physical properties of glycerol- water mixtures at 298.15 K //
J. Iraqi Chem. Soc.– 1986.– Vol.11, N1.– P. 89–104.
Saluja A., Kalonia D.S. Measurement of fluid viscosity at
microliter volumes using quartz // AAPS Pharm. Sci. Tech.–
2004.– Vol. 5, N3.– P. 1–14.
Schonherr J., Bucovac M.J. Penetration of stomata by liquids.
Dependence on surface tension, wettability, and stomatal
morphology // Plant Physiol.– 1972.– Vol. 49, N5.– P. 813–819.
Sears P.G., Siegfried W.D., Sands D.E. Viscosities, densi-
ties, and related properties of solutions of some sugars in
dimethyl sulfoxide // J. Chem. Eng. Data.– 1964.– Vol. 9, N2.–
P. 261–263.
Sengers J.V., Kamgar-Parsi B. Representative equations for
the viscosity of water substance // J. Phys. Chem. Ref. Data.–
1984.– Vol. 13, N1.– P. 185–205.
Shell Chemical [Электронный документ] // [веб-сайт]
www.shell.com (11.06.2010).
Shih W.Y., Li X., Gu H., Shih W.-H., Aksay I.A. Simultaneous
liquid viscosity and density determination with piezoelectric
unimorph cantilevers // J. Appl. Phys.– 2001.– Vol. 89, N2.–
P. 1497–1505.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
Molinero V., Cagin T., Goddard W.A. III. Sugar, water and
free volume networks in concentrated sucrose solution //
Chem. Phys. Lett.– 2003.– Vol. 377, N3–4.– P. 469–474.
Nakanishi K., Matsumoto T., Hayatsu M. Surface tension of
aqueous solutions of some glycols // J. Chem. Eng. Data.–
1971.– Vol. 16, N1.– P. 44–45.
Noda K., Ohashi M., Ishida K. Viscosities and densities at
298.15 K for mixtures of methanol, acetone, and water // J.
Chem. Eng. Data.– 1982.– Vol. 27, N3.– P. 326–328.
Okutomi T., Nemoto M., Mishiba E., Goto F. Viscosity of
diluent and sensory level of subarachnoid anaesthesia
achieved with tetracaine // Can. J. Anaesth.– 1998.– Vol. 45,
N1.– P. 84–86.
Polyvinylpyrrolidone [Electronic resource] // [web-site]
www.camd.lsu.edu/msds/p/polyvinylpyrrolidone.htm
(9.08.2009).
Radhakrishnan S. Measurement of thermal properties of
seafood / Thesis … Master of Science in Biological Systems
Engineering.– Blackburg, Virginia, 1997.– 83 p.
Rao B.G., Singh U.C. A free energy perturbation study of
solvation in methanol and dimethyl sulfoxide // J. Am. Chem.
Soc. – 1990.– Vol. 112, N10.– P. 3803–3811.
Rastogi P.P. Ion-solvent interaction of tetraalkylammonium
and some common ions in N-methylformamide from viscosity
data // Bull. Chem. Soc. Jpn.– 1970.– Vol. 43, N12.– P. 2442–
2444.
Regmi S. Study and estimation of temperature dependent
physical parameters of poly(vinylidene fluoride) and poly(1,4
butylene adipate) dissolved in N,N-dimethyl formamide // J.
Sci. Eng. Tech.– 2007.– Vol. I, NIII.– P. 1–10.
Roy R.N., Baker G.E., Hoffman T. et al. Standard electrode
potentials of silver-silver chloride electrodes in 20, 30, and
50% (w/w) ethylene glycol/water from 25°C to –20°C pK2
and pH* values of the physiological buffer "BES" in 50%
(w/w) ethylene glycol/water // Cryo-Letters.– 1988.– Vol. 9,
N3.– P. 172–185.
Roy R.N., Gibbons J.J., Baker G., Bates R.G. Standard
electromotive force of the H2-AgCl; AgCl in 30, 40, and 50
mass.% dimethyl sulfoxide/water from –20 to 25°C: pK2 and
pH values for a standard "Bicine" buffer solutions at subzero
temperatures // Cryobiology.– 1984.– Vol. 21, N6.– P. 672–
681.
Saleh J.M., Khalil M., Hokmat N.A. Investigation of some
physical properties of glycerol- water mixtures at 298.15 K //
J. Iraqi Chem. Soc.– 1986.– Vol.11, N1.– P. 89–104.
Saluja A., Kalonia D.S. Measurement of fluid viscosity at
microliter volumes using quartz // AAPS Pharm. Sci. Tech.–
2004.– Vol. 5, N3.– P. 1–14.
Schonherr J., Bucovac M.J. Penetration of stomata by liquids.
Dependence on surface tension, wettability, and stomatal
morphology // Plant Physiol.– 1972.– Vol. 49, N5.– P. 813–819.
Sears P.G., Siegfried W.D., Sands D.E. Viscosities, densi-
ties, and related properties of solutions of some sugars in
dimethyl sulfoxide // J. Chem. Eng. Data.– 1964.– Vol. 9, N2.–
P. 261–263.
Sengers J.V., Kamgar-Parsi B. Representative equations for
the viscosity of water substance // J. Phys. Chem. Ref. Data.–
1984.– Vol. 13, N1.– P. 185–205.
Shell Chemical [Electronic resource] // [web-site]
www.shell.com (11.06.2010).
Shih W.Y., Li X., Gu H., Shih W.-H., Aksay I.A. Simultaneous
liquid viscosity and density determination with piezoelectric
unimorph cantilevers // J. Appl. Phys.– 2001.– Vol. 89, N2.–
P. 1497–1505.
Simetric.co.uk [Electronic resource] // [web-site]
www.simetric.co.uk/si_liquids.htm (11.06.2010)
Simmonds C. Alcohol, its production, properties, chemistry,
and industrial applications [Electronic resource] / [web-site]
http://chestofbooks.com/food/beverages/Alcohol-Propeties/
index.html (11.01.2010).
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.

problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
435
Simetric.co.uk [Электронный документ] // [веб-сайт]
www.simetric.co.uk/si_liquids.htm (11.06.2010).
Simmonds C. Alcohol, its production, properties, chemistry,
and industrial applications [Электронный документ] // [веб-
сайт] http://chestofbooks.com/food/beverages/Alcohol-
Propeties/index.html (11.01.2010).
Smadel J.E., Pickels E.G., Shedlovsky T. Ultracentrifugation
studies on the elementary bodies of vaccine virus. II. The
influence of sucrose, glycerol, and urea solutions on the
physical nature of vaccine virus // JEM.– 1938.– Vol. 68, N4.–
P. 607–627.
Solvay Chemicals Worldwide [Электронный документ] //
[веб-сайт] www.solvaychemicals.com.
Specific gravity and viscosity of liquids / CSG Network.com.
Free information [Электронный документ] // [веб-сайт]
www.scgnetwork.com (5.05.2010).
Surface tension values of some common test liquids for
surface energy analysis [Электронный документ] // [веб-
сайт] www.surface-tension.de/index.html (5.05.2010).
Technology lubricant corporation. A comparison of ethylene
glycol and propylene glycol [Электронный документ] // [веб-
сайт] www.technologylubricants.com (5.05.2010).
Tezze A.A., Farina W.M. Trophallaxis in the honeybee, Apis
mellifera: the interaction between viscosity and sucrose
concentration of the transferred solution // Animal Behaviour.–
1999.– Vol. 57, N6.– P. 1319–1326.
The dow chemical company [Electronic resource] / [web-
site] www.dow.com/ (11.01.2010)
The engineering tool box [Electronic resource] / [web-site]
www.engineeringtoolbox.com (7.09.2010)
Toegel R., Luther S., Lohse D. Viscosity destabilizes sono-
luminescing bubles // Phys. Rev. Lett.– 2006.– Vol. 96, N11.–
P. 1143011–1143014.
Tracton A.A. Coatings technology handbook.– Boca Raton:
Taylor & Francis, 2005.– 936 p.
Uedaira H., Uedaira H. Activity coefficients of aqueous xylose
and maltose solutions // Bull. Chem. Soc. Jpn.– 1969.– Vol. 42,
N11.– P. 2137–2140.
Uribe S., Sampedro J.G. Measuring solution viscosity and
its effect on enzyme activity // Biol. Proced. Online.– 2003.–
Vol. 5, N1.– P. 108–115.
Venables D.S., Schmuttenmaer C.A. Spectroscopy and
dynamics of mixtures of water with acetone, acetonitrile,
and methanol // J. Chem. Phys.– 2000.– Vol. 113, N24.– P.
11222–11236.
Wensink E.J.W., Hoffmann A.C. Dynamic properties of wa-
ter/alcohol mixtures studied by computer simulation // J. Chem.
Phys.– 2003.– Vol. 119, N14.– P. 7308–7317.
Williams R.J., Harris D. The distribution of cryoprotective
agents into lipid interface // Cryobiology.– 1977.– Vol.14, N6.–
P. 670–680.
Xie G., Timasheff S.N. Mechanism of the stabilization of
ribonuclease A by sorbitol: preferential hydration is greater
for the denatured than for the native protein // Protein science.–
1997.– Vol. 6, N1.– P. 211–221.
Yang C., Ma P., Tang D., Jin F. Excess molar volume, viscosity
and heat capacity for the mixture of 1,2-propanediol-water
at different temperatures // Chinese J. Chem. Eng.– 2003.–
Vol.11, N2.– P. 175–180.
Поступила 12.10.2010
Рецензент Т.П. Линник
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
Smadel J.E., Pickels E.G., Shedlovsky T. Ultracentrifugation
studies on the elementary bodies of vaccine virus. II. The
influence of sucrose, glycerol, and urea solutions on the
physical nature of vaccine virus // JEM.– 1938.– Vol. 68, N4.–
P. 607–627.
Solvay Chemicals Worldwide [Electronic resource] // [web-
site] www.solvaychemicals.com.
Specific gravity and viscosity of liquids / CSG Network.com.
Free information [Electronic resource] // [web-site]
www.scgnetwork.com (5.05.2010).
Surface tension values of some common test liquids for
surface energy analysis [Electronic resource] // [web-site]
www.surface-tension.de/index.html (5.05.2010).
Technology lubricant corporation. A comparison of ethylene
glycol and propylene glycol [Electronic resource] // [web-
site] www.technologylubricants.com (5.05.2010).
Tezze A.A., Farina W.M. Trophallaxis in the honeybee, Apis
mellifera: the interaction between viscosity and sucrose
concentration of the transferred solution // Animal behaviour.–
1999.– Vol. 57, N6.– P. 1319–1326.
The dow chemical company [Electronic resource] // [web-
site] www.dow.com/ (11.01.2010).
The engineering tool box [Electronic resource] // [web-site]
www.engineeringtoolbox.com (7.09.2010).
Toegel R., Luther S., Lohse D. Viscosity destabilizes sono-
luminescing bubles // Phys. Rev. Lett.– 2006.– Vol. 96, N11.–
P. 1143011–1143014.
Tracton A.A. Coatings technology handbook.– Boca Raton:
Taylor & Francis, 2005.– 936 p.
Uedaira H., Uedaira H. Activity coefficients of aqueous xylose
and maltose solutions // Bull. Chem. Soc. Jpn.– 1969.– Vol. 42,
N11.– P. 2137–2140.
Uribe S., Sampedro J.G. Measuring solution viscosity and
its effect on enzyme activity // Biol. Proced. Online.– 2003.–
Vol. 5, N1.– P. 108–115.
Venables D.S., Schmuttenmaer C.A. Spectroscopy and
dynamics of mixtures of water with acetone, acetonitrile,
and methanol // J. Chem. Phys.– 2000.– Vol. 113, N24.– P.
11222–11236.
Wensink E.J.W., Hoffmann A.C. Dynamic properties of wa-
ter/alcohol mixtures studied by computer simulation // J. Chem.
Phys.– 2003.– Vol. 119, N14.– P. 7308–7317.
Williams R.J., Harris D. The distribution of cryoprotective
agents into lipid interface // Cryobiology.– 1977.– Vol. 14,
N6.– P. 670–680.
Xie G., Timasheff S.N. Mechanism of the stabilization of
ribonuclease A by sorbitol: preferential hydration is greater
for the denatured than for the native protein // Protein science.–
1997.– Vol. 6, N1.– P. 211–221.
Yang C., Ma P., Tang D., Jin F. Excess molar volume, viscosity
and heat capacity for the mixture of 1,2-propanediol-water
at different temperatures // Chinese J. Chem. Eng.– 2003.–
Vol.11, N2.– P. 175–180.
Accepted in 12.10.2010
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.

436
* Автор, которому необходимо направлять корреспонденцию:
ул. Переяславская, 23, г. Харьков, Украина 61015; тел.:+38
(057) 373-30-34, факс: +38 (057) 373-30-84, электронная почта:
yuripetrenko@cryo.org.ua.
* To whom correspondence should be addressed: 23,
Pereyaslavskaya str., Kharkov, Ukraine 61015; tel.:+380 57 373
3034, fax: +380 57 373 3084, e-mail: yuripetrenko@cryo.org.ua.
Institute for Problems of Cryobiology and Cryomedicine of the Na-
tional Academy of Sciences of Ukraine, Kharkov, Ukraine
Институт проблем криобиологии и криомедицины
НАН Украины, г. Харьков
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
УДК 615.014.41:611.018.46.08
Ю.А. ПЕТРЕНКО*, Н.Г. СКОРОБОГАТОВА, Н.А. ВОЛКОВА, А.Ю. ПЕТРЕНКО
Характеристика иммунофенотипа и дифференцировочного
потенциала мезенхимальных стромальных клеток костного мозга
человека после криоконсервирования
UDC 615.014.41:611.018.46.08
YU.A. PETRENKO*, N.G. SKOROBOGATOVA, N.A. VOLKOVA, A.YU. PETRENKO
Characterization of Immunophenotype and Differentiation
Potential of Human Bone Marrow Mesenchymal
Stromal Cells after Cryopreservation
Стромальные клетки, изолированные из костного мозга (КМ) взрослого человека, после экспансии in vitro демонстрируют
специфический иммунофенотип и способность к мультилинейной дифференцировке, свойственные мезенхимальным стволовым
клеткам (МСК). Установлено, что криоконсервирование МСК КМ человека путем медленного двухступенчатого замораживания
под защитой 10% ДМСО позволяет сохранить их иммунофенотип и способность к индуцированным in vitro остеогенной и
адипогенной дифференцировкам.
Ключевые слова: криоконсервирование, мезенхимальные стромальные клетки, костный мозг человека, адипогенная и
остеогенная дифференцировка, иммунофенотип.
Стромальні клітини, які були ізольовані з кісткового мозку (КМ) дорослої людини, після експансії in vitro демонструють
специфічний імунофенотип та здатність до мультилінійного диференціювання, що є характерним для мезенхімальних стовбурових
клітин (МСК). Встановлено, що кріоконсервування МСК КМ людини шляхом повільного двоступінчастого заморожування
під захистом 10% ДМСО дозволяє зберегти їхній імунофенотип та здатність до індукованого in vitro остеогенного та адипогенного
диференціювань.
Ключові слова: кріоконсервування, мезенхімальні стромальні клітини, кістковий мозок людини, адипогенне та остеогенне
диференціювання, імунофенотип.
Stromal cells derived from adult human bone marrow (BM) after in vitro expansion demonstrate a specific immunophenotype and
ability to multilineage differentiation, inherent to mecenchymal stromal cells (MSCs)m. It was established that cryopreservation of
human BM MSCs by two-step freezing under protection of 10% DMSO enables to preserve their immunophenotype and ability to
induced osteogenic and adipogenic differentiation in vitro.
Key words: cryopreservation, mesenchymal stromal cells, human bone marrow, adipogenic and osteogenic differentiation, immuno-
phenotype.
Мезенхимальные стволовые (стромальные)
клетки (МСК) находят все более широкое приме-
нение в различных областях биологии и медицины
благодаря их высокой способности дифференци-
роваться в различные типы клеток и низкой имму-
ногенности [3, 5, 7]. Показано, что МСК присут-
ствуют во многих органах и тканях взрослого орга-
низма – костном мозге (КМ), коже, жировой,
костной, мышечной тканях и др. [3, 4, 6, 9]. Однако
в качестве "золотого стандарта" при оценке и срав-
нении биологического потенциала данного типа
клеток, выделенных из разных источников, рас-
сматривают МСК КМ взрослых доноров. В связи
с этим актуально исследование морфофункцио-
нальных свойств МСК КМ после различных воз-
действий.
Криоконсервирование МСК КМ требует приме-
нения таких методических подходов, которые
Mesenchymal stem (stromal) cells (MSCs) have
found a wide application in different fields of biol-
ogy and medicine due to their high ability of differ-
entiation into various cell types and low immunoge-
nicity [3, 5, 7]. MSCs have been shown to be present
in many organs and tissues of adult organism: bone
marrow (BM), skin, adipose tissue, muscle etc. [3,
4, 6, 9]. However as "a gold standard" when estimat-
ing and comparing biological potential of cells, iso-
lated from different sources, the BM MSCs of adult
donors are considered. The study of morpho-func-
tional properties of BM MSCs after different effects
is an important point. .
BM MSCs cryopreservation needs the application
of methodical approaches allowing the maximum
preservation of not only viability but also unique bio-
logical properties of these cells. Usually the BM
MSCs are cryopreserved after sub-culturing by slow

437
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
позволяют максимально сохранять не только
жизнеспособность, но и уникальные биологические
свойства этих клеток. Обычно криоконсервиро-
вание МСК КМ проводят после предварительного
субкультивирования путем медленного заморажи-
вания с применением криозащитных сред, содер-
жащих диметилсульфоксид (ДМСО) [6, 8]. Необхо-
димым этапом криобиотехнологического процесса
является контроль стабильности иммунофенотипа
и способности к направленной мультилинейной
дифференцировке МСК КМ после криоконсерви-
рования.
Цель работы – исследование иммунофенотипа
и способности к направленным специфическим
дифференцировкам мезенхимальных стромальных
клеток костного мозга человека до и после криокон-
сервирования.
Материалы и методы
Эксперименты проводили на КМ, полученном
от взрослых доноров после их информированного
письменного согласия, в соответствии с рекомен-
дациями Хельсинкской декларации Всемирной
медицинской ассоциации по проведению биомеди-
цинских исследований. Источником клеток были
фрагменты спонгиозной ткани, которые извлекали
в виде цилиндров диаметром 6–8 мм из гребня
подвздошной кости с помощью инструментов для
мозаичной хондропластики, что обеспечивало ма-
лую инвазивность процедуры. Первичную суспен-
зию клеток получали вымыванием изотонической
средой из костных цилиндров, затем ее центрифу-
гировали при 150g в течение 10 мин. Ядросодер-
жащие клетки из полученного осадка подсчиты-
вали в камере Горяева с использованием 3%-й
уксусной кислоты. Культивировали клетки в среде,
дополненной 15% эмбриональной сыворотки (ЭС)
крупного рогатого скота ("Биолот", Россия), 2 мМ
L-глутамина, 50 ед/мл пенициллина и 50 мг/мл
стрептомицина при 37°С, 5% СО
2
и абсолютной
влажности. Первую замену среды осуществляли
через 48 ч культивирования и далее – через каждые
3-е суток.
Культивированные МСК КМ 3–4-го пассажей
криоконсервировали в культуральной среде, содер-
жащей 20% ЭС и 10% ДМСО, со скоростью
1 градус/мин до –80°С по протоколу фирмы-произ-
водителя Cryo 1°С Freezing Container (“Nalgene”,
США) с последующим погружением в жидкий
азот.
Образцы хранили при –196°С в течение 4–6
месяцев. Криоконсервированные образцы отогре-
вали на водяной бане при 37°С. Для удаления
криозащитной среды клеточные суспензии разбав-
freezing with the application of cryoprotective me-
dia, containing dimethyl sulfoxide (DMSO) [6, 8]. The
essential stage of cryobiotechnological process is the
post-thaw control of immunophenotype stability and
ability to directed multi-lineage differentiation of BM
MSCs.
The aim of this research was to investigate the
immunophenotype and specific differentiations abili-
ties of human bone marrow mesenchymal stromal
cells prior to and after cryopreservation.
Materials and methods
The experiments were carried-out using BM,
obtained from adult donors after their informed writ-
ten consent in the accordance with the WMA dec-
laration of Helsinki for medical research. As the
source of the cells spongious tissue fragments were
used. These fragments (6-8 mm cylinders) were iso-
lated from iliac crest by the application of instruments
for mosaic chondroplastics, which provided low in-
vasiveness of the procedure. Primary cell suspension
was obtained by washing-out from bone cylinders
with isotonic medium, followed by centrifugation at
150g for 10 min. Nucleated cells from the obtained
pellets were counted in Goryaev's chamber using 3%
acetic acid. The cells were cultured in the medium
supplemented with 15% fetal bovine serum (FBS)
(Biolot, Russia), 2 mM L-glutamine, 50 units/ml peni-
cillin and 50 mg/ml streptomycin at 37°C, 5% CO
2
and absolute humidity. The first change of the me-
dium was performed after 48 hrs of culturing and later
every 3 days.
Cultured BM MSCs of the 3
rd
–4
th
passages were
cryopreserved in the culture medium, supplemented
by 20% FS and 10% DMSO with the cooling rate
of 1 degree/min down to –80°C using Cryo 1°C
Freezing Container (Nalgene, USA) according to the
manufacturing protocol with following plunging into
liquid nitrogen.
The samples were stored at –196°C for 4–6
months. Cryopreserved samples were thawed on wa-
ter bath at 37°C. To remove cryoprotective medium
the cell suspensions were diluted with the culture
medium, containing 10% FS in 1:10 ratio with follow-
ing centrifugation (150g, 10 min). Cell pellets were
re-suspended in a new portion of culture medium.
The cell integrity was assessed by vital staining with
trypan blue.
Freshly isolated and cryopreserved stromal cells
were cultured for 3–4 passages, afterwards immuno-
phenotyping and induction of osteo- and adipogenesis
in vitro were performed. Vital control of the state
of cell cultures was prepared daily using inverted mi-
croscope CETI (Belgium).

438
problems
of cryobiology
Vol. 20, 2010, №4
проблемы
криобиологии
Т. 20, 2010, №4
ляли средой культивирования, содержащей 10% ЭС,
в соотношении 1:10 с последующим центрифугиро-
ванием (150g, 10 мин). Клеточный осадок ресус-
пендировали в новой порции культуральной среды.
Сохранность клеток оценивали с помощью прижиз-
ненного окрашивания трипановым синим.
Свежевыделенные и криоконсервированные
стромальные клетки культивировали в течение 3–
4-х пассажей, после чего проводили иммунофеноти-
пирование и индукцию остео- и адипогенеза in vitro.
Прижизненный контроль состояния клеточных
культур осуществляли ежедневно с помощью ин-
вертированного микроскопа “CETI” (Бельгия).
Для иммунофенотипического анализа культиви-
рованные клетки окрашивали моноклональными
антителами CD29-РЕ, CD45-PE, CD105-FITC
(“Serotec”, Великобритания), CD34 Class II-FITC,
CD38-RPE (“DAKO”, Голландия), CD44-FITC,
CD73-PE (“BD Biosciences”, США) согласно инст-
рукции производителей, дважды отмывали центри-
фугированием при 200 g в течение 10 мин в раст-
воре Хэнкса и анализировали на проточном цито-
метре “FACS Calibur” (“BD Biosciences”, США).
Дифференцировочный потенциал МСК опреде-
ляли in vitro при культивировании в специфичных
индуктивных средах. Для индукции остеогенеза
применяли среду alpha-МЕМ, содержащую 10%
ЭС; 0,1 мкМ дексаметазона; 0,05 мМ аскорбиновой
кислоты; 10 мМ глицерол-фосфата. Адипогенез ин-
дуцировали в среде alpha-МЕМ, дополненной 10%
Adipogenic Stimulatory Supplements (“Stem Cell
Technologies Inc.”, Канада). Клетки культивировали
в течение 3-х недель, среду меняли через каждые
3-е суток.
Остеогенез выявляли по экспрессии щелочной
фосфатазы и наличию минерализации внеклеточ-
ного матрикса (окрашивание по Ван Коссу) [10].
Адипогенную дифференцировку клеток определяли
по накоплению нейтральных жиров (окрашивание
Oil Red O) [10]. Контролем на спонтанную диффе-
ренцировку служили клетки, культивированные в
ростовой среде без специальных индукторов.
Результаты и обсуждение
Полученные первичные культуры свежеизоли-
рованных стромальных клеток КМ были гетеро-
генными по составу и содержали незначительную
примесь адгезивных гемопоэтических клеток, а
также макрофагов, которые постепенно элимини-
ровались в ходе культивирования. В первичной
культуре стромальных клеток КМ были выявлены
колониеобразующие единицы фибробластов
(КОЕф) с частотой 1–2 на 100 000 посеянных
ядросодержащих клеток [2]. Согласно современ-
For immunophenotypic analysis the cultured cells
were stained with monoclonal antibodies CD29-PE,
CD-45PE, CD-05-FITC (Serotec, UK), CD34 Class
II-FITC, CD38-RPE (Dako, Holland), CD44-FITC,
CD73-PE (BD Biosciences, USA) according to the
manufacturing instructions. Cells were washed-out
twice by centrifugation at 200g for 10 min in Hanks
solution and analyzed using flow cytometer FACS
Calibur (BD Biosciences, USA).
Differentiation potential of MSCs was examined
in vitro when culturing in specific inductive media.
To induce osteogenesis there was applied alpha-
MEM medium, containing 10% FS; 0.1 mM dexame-
tazone; 0.05 mM ascorbic acid; 10 mM glycerol-
phosphate. Adipogenesis was induced in alpha-MEM
medium supplemented with 10% Adipogenic Stimu-
latory Supplements (Stem Cell Technologies Inc.,
Canada). The cells were cultured for 3 weeks, the
medium was changed every 3 days.
Osteogenesis was revealed by the expression of
alkaline phosphatase and presence of mineralization
of extracellular matrix (von Kossa staining) [10]. Adi-
pogenic differentiation of cells was assessed by Oil
Red O staining of accumulated intracellular neutral
lipids [10]. The cells cultured in growth medium with
no special inducers served as the control.
Results and discussion
The obtained primary cultures of freshly isolated
BM stromal cells were heterogenous on the compo-
sition and contained a slight amounts of adhesive
hematopoietic cells, as well as macrophages, gradu-
ally eliminated during following culture. In primary
culture of BM stromal cells the colony-forming units
of fibroblasts (CFU
f
) were revealed with the fre-
quency of 1–2 per 100,000 plated nucleated cells [2].
According to the current notions this pool of stromal
progenitor cells comprises multipotent MSCs [6].
During following sub-culture there was noted the
formation of new colonies in the culture, testifying
to the proliferation of early MSCs. After 3-4 passa-
ges the subcultures mainly consisted of predominantly
spindle-shaped fibroblast-like cells with small oval
nucleus, which proliferated and formed characteris-
tic flows.
One of the main criteria of characterization of cell
population is the investigation of the specific surface
markers’ expression. The studied human BM fibro-
blast-like cells, subcultured during 4 passages were
characterized by immunophenotype of CD29
+
,
CD44
+
, CD73
+
, CD105
+
(Fig. 1).
Herewith the content of the cells expressed these
markers in the studied cultures comprised more than
90%. Simultaneously they did not express the mark-
