Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

406
Photochem
istry
of
Planetary
Atmospheres
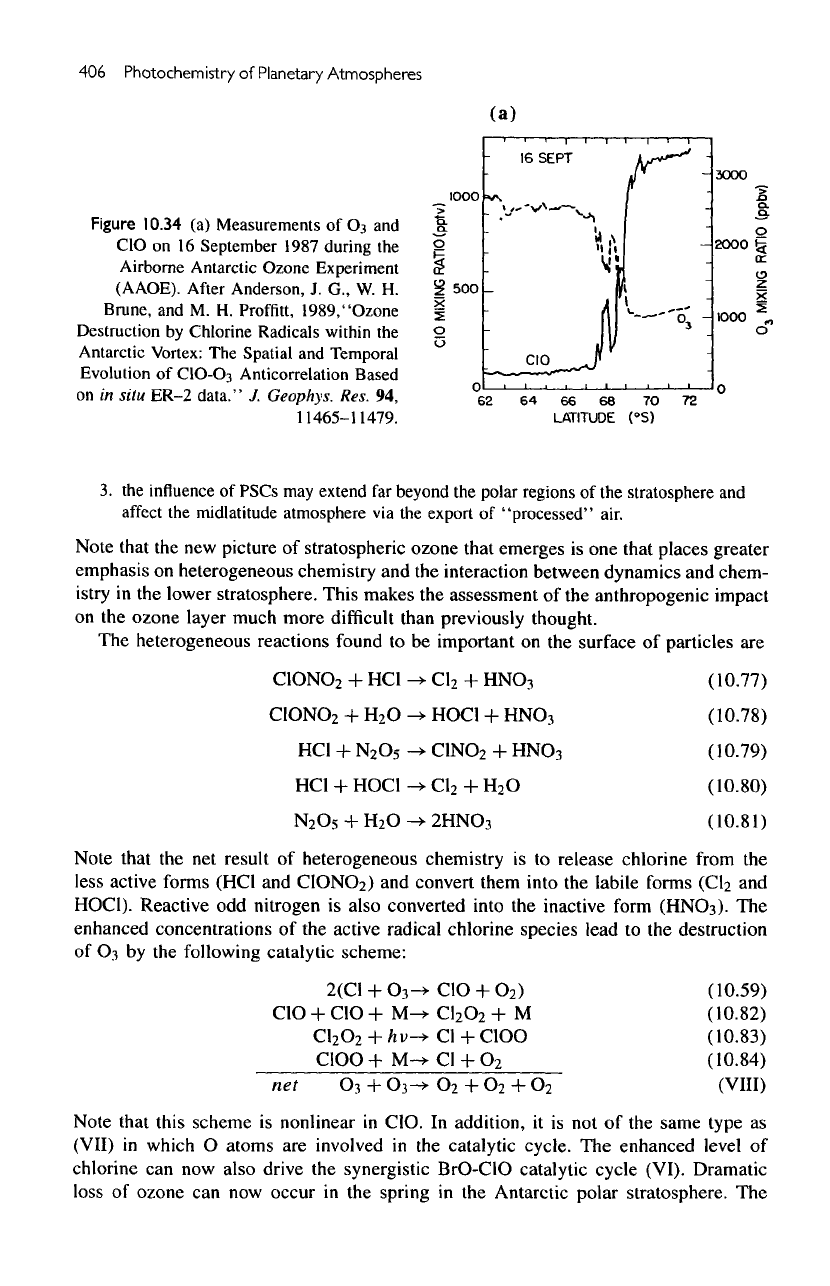
Figure
10.34
(a)
Measurements
of
O
3
and
CIO
on 16
September
1987
during
the
Airborne
Antarctic
Ozone
Experiment
(AAOE).
After
Anderson,
J. G., W. H.
Brune,
and M. H.
Proffitt,
1989,
"Ozone
Destruction
by
Chlorine
Radicals
within
the
Antarctic
Vortex:
The
Spatial
and
Temporal
Evolution
of
ClO-Os
Anticorrelation
Based
on
in
situ
ER-2
data."
J.
Geophys.
Res.
94,
11465-11479.
(a)
3. the
influence
of
PSCs
may
extend
far
beyond
the
polar
regions
of the
stratosphere
and
affect
the
midlatitude
atmosphere
via the
export
of
"processed"
air.
Note
that
the new
picture
of
stratospheric ozone that
emerges
is one
that places greater
emphasis
on
heterogeneous chemistry
and the
interaction between dynamics
and
chem-
istry
in the
lower
stratosphere.
This
makes
the
assessment
of the
anthropogenic
impact
on
the
ozone layer much
more
difficult
than previously thought.
The
heterogeneous reactions found
to be
important
on the
surface
of
particles
are
Note that
the net
result
of
heterogeneous chemistry
is to
release chlorine from
the
less active forms (HC1
and
C1ONO
2
)
and
convert them into
the
labile forms
(C1
2
and
HOC1). Reactive
odd
nitrogen
is
also converted into
the
inactive form
(HNO
3
).
The
enhanced
concentrations
of the
active radical chlorine
species
lead
to the
destruction
of
O
3
by the
following catalytic
scheme:
Note that
this
scheme
is
nonlinear
in
CIO.
In
addition,
it is not of the
same type
as
(VII)
in
which
O
atoms
are
involved
in the
catalytic cycle.
The
enhanced level
of
chlorine
can now
also drive
the
synergistic
BrO-CIO
catalytic cycle
(VI).
Dramatic
loss
of
ozone
can now
occur
in the
spring
in the
Antarctic polar stratosphere.
The
Earth: Human Impact
407
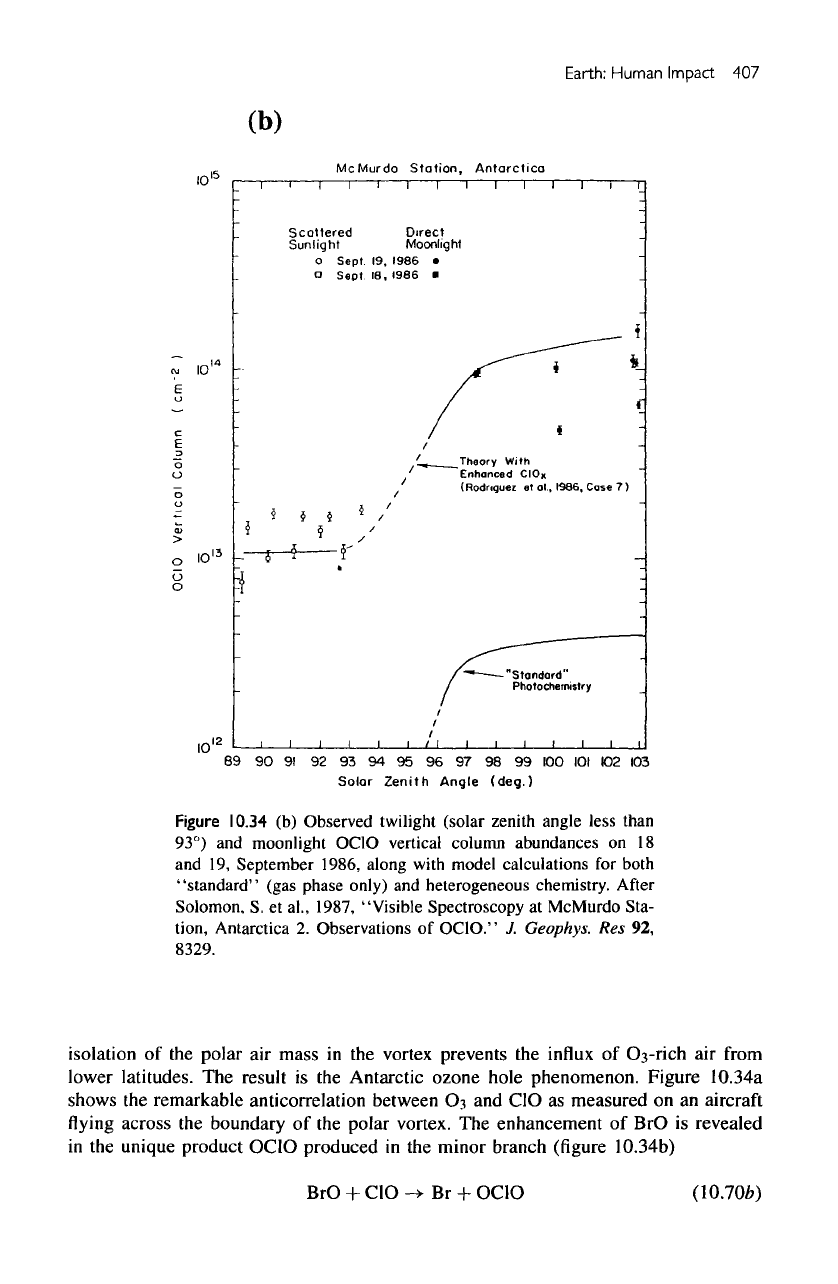
(b)
Figure
10.34
(b)
Observed
twilight
(solar
zenith angle
less
than
93°)
and
moonlight OC1O vertical column abundances
on 18
and 19,
September 1986, along with model calculations
for
both
"standard"
(gas phase only)
and
heterogeneous chemistry. After
Solomon,
S. et
al.,
1987,
"Visible
Spectroscopy
at
McMurdo Sta-
tion, Antarctica
2.
Observations
of
OC1O."
J.
Geophys.
Res 92,
8329.
isolation
of the
polar
air
mass
in the
vortex prevents
the
influx
of
CDs-rich
air
from
lower latitudes.
The
result
is the
Antarctic ozone hole phenomenon. Figure
10.34a
shows
the
remarkable anticorrelation between
03 and
CIO
as
measured
on an
aircraft
flying
across
the
boundary
of the
polar vortex.
The
enhancement
of BrO is
revealed
in
the
unique
product OC1O produced
in the
minor branch
(figure
10.34b)
408
Photochem
istry
of
Planetary
Atmospheres
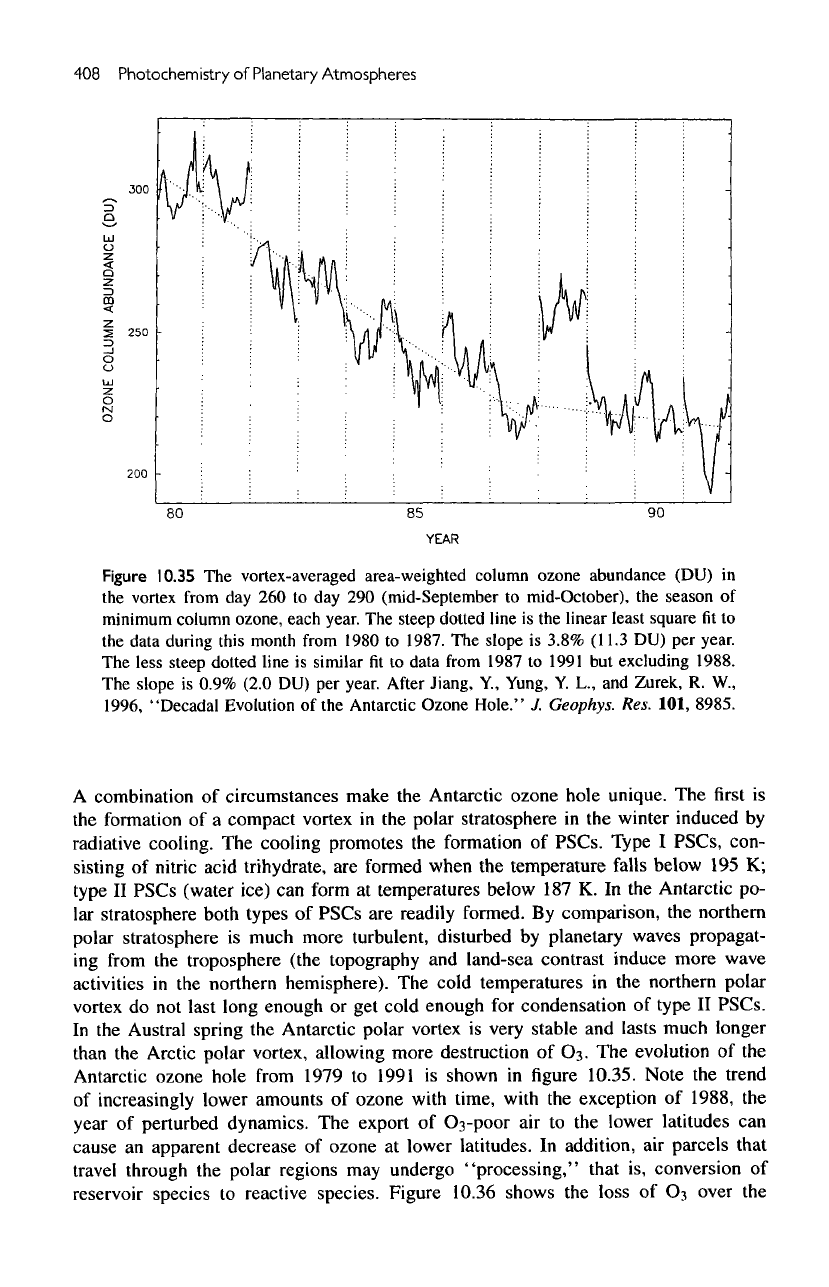
Figure
10.35
The
vortex-averaged area-weighted column ozone abundance (DU)
in
the
vortex
from
day 260 to day 290
(mid-September
to
mid-October),
the
season
of
minimum
column ozone, each year.
The
steep dotted
line
is the
linear least square
fit to
the
data during
this
month
from
1980
to
1987.
The
slope
is
3.8%
(11.3
DU) per
year.
The
less steep dotted line
is
similar
fit
to
data
from
1987
to
1991
but
excluding 1988.
The
slope
is
0.9% (2.0
DU) per
year.
After
Jiang,
Y,
Yung,
Y L., and
Zurek,
R. W.,
1996,
"Decadal
Evolution
of the
Antarctic Ozone
Hole."
J.
Geophys.
Res. 101,
8985.
A
combination
of
circumstances make
the
Antarctic ozone hole
unique.
The first is
the
formation
of a
compact vortex
in the
polar stratosphere
in the
winter induced
by
radiative
cooling.
The
cooling promotes
the
formation
of
PSCs.
Type
I
PSCs,
con-
sisting
of
nitric acid
trihydrate,
are
formed when
the
temperature
falls
below
195 K;
type
II
PSCs
(water ice)
can
form
at
temperatures below
187 K. In the
Antarctic
po-
lar
stratosphere both types
of
PSCs
are
readily formed.
By
comparison,
the
northern
polar stratosphere
is
much more
turbulent,
disturbed
by
planetary waves propagat-
ing
from
the
troposphere (the topography
and
land-sea contrast induce more wave
activities
in the
northern hemisphere).
The
cold temperatures
in the
northern polar
vortex
do not
last long enough
or get
cold enough
for
condensation
of
type
II
PSCs.
In
the
Austral spring
the
Antarctic polar vortex
is
very stable
and
lasts much longer
than
the
Arctic
polar
vortex, allowing
more
destruction
of 03. The
evolution
of the
Antarctic
ozone hole
from
1979
to
1991
is
shown
in figure
10.35.
Note
the
trend
of
increasingly lower amounts
of
ozone
with
time,
with
the
exception
of
1988,
the
year
of
perturbed dynamics.
The
export
of
Os-poor
air to the
lower latitudes
can
cause
an
apparent decrease
of
ozone
at
lower latitudes.
In
addition,
air
parcels that
travel
through
the
polar regions
may
undergo
"processing,"
that
is,
conversion
of
reservoir
species
to
reactive species. Figure 10.36 shows
the
loss
of
Oj
over
the
Earth:
Human
Impact
409
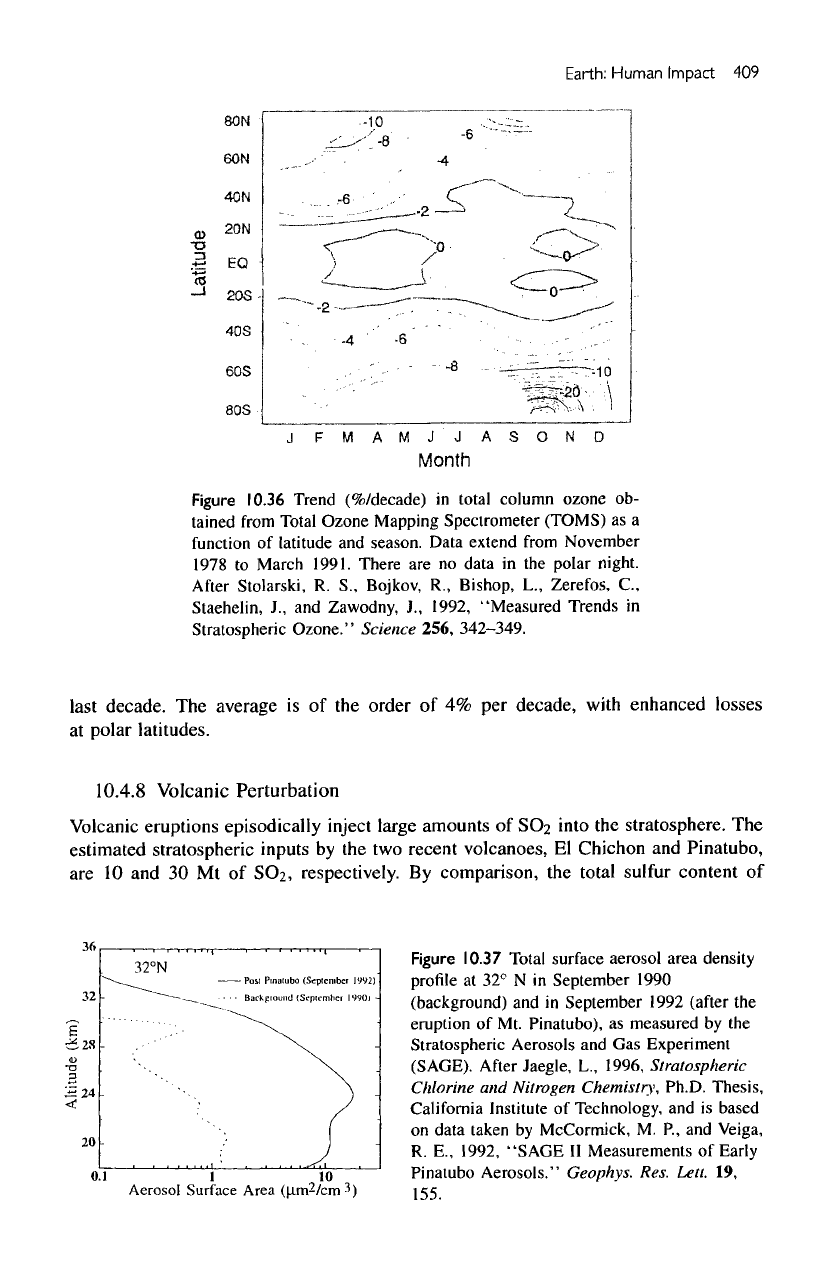
Figure
10.36
Trend
(%/decade)
in
total
column ozone
ob-
tained
from
Total Ozone Mapping Spectrometer (TOMS)
as a
function
of
latitude
and
season. Data
extend
from
November
1978
to
March
1991. There
are no
data
in the
polar
night.
After
Stolarski,
R. S.,
Bojkov,
R.,
Bishop,
L.,
Zerefos,
C,
Staehelin,
J.,
and
Zawodny,
J.,
1992, "Measured Trends
in
Stratospheric
Ozone."
Science 256,
342-349.
last
decade.
The
average
is of the
order
of 4% per
decade,
with
enhanced
losses
at
polar
latitudes.
10.4.8
Volcanic
Perturbation
Volcanic eruptions
episodically
inject
large
amounts
of
SC>2
into
the
stratosphere.
The
estimated
stratospheric
inputs
by the two
recent
volcanoes,
El
Chichon
and
Pinatubo,
are 10 and 30 Mt of
862,
respectively.
By
comparison,
the
total sulfur
content
of
Figure
10.37
Total
surface
aerosol area
density
profile
at 32° N in
September 1990
(background)
and in
September 1992
(after
the
eruption
of Mt.
Pinatubo),
as
measured
by the
Stratospheric
Aerosols
and Gas
Experiment
(SAGE).
After
Jaegle,
L.,
1996, Stratospheric
Chlorine
and
Nitrogen Chemistry, Ph.D. Thesis,
California
Institute
of
Technology,
and is
based
on
data
taken
by
McCormick,
M. P., and
Veiga,
R.
E.,
1992,
"SAGE
II
Measurements
of
Early
Pinatubo
Aerosols."
Geophys.
Res. Lett.
19,
155.
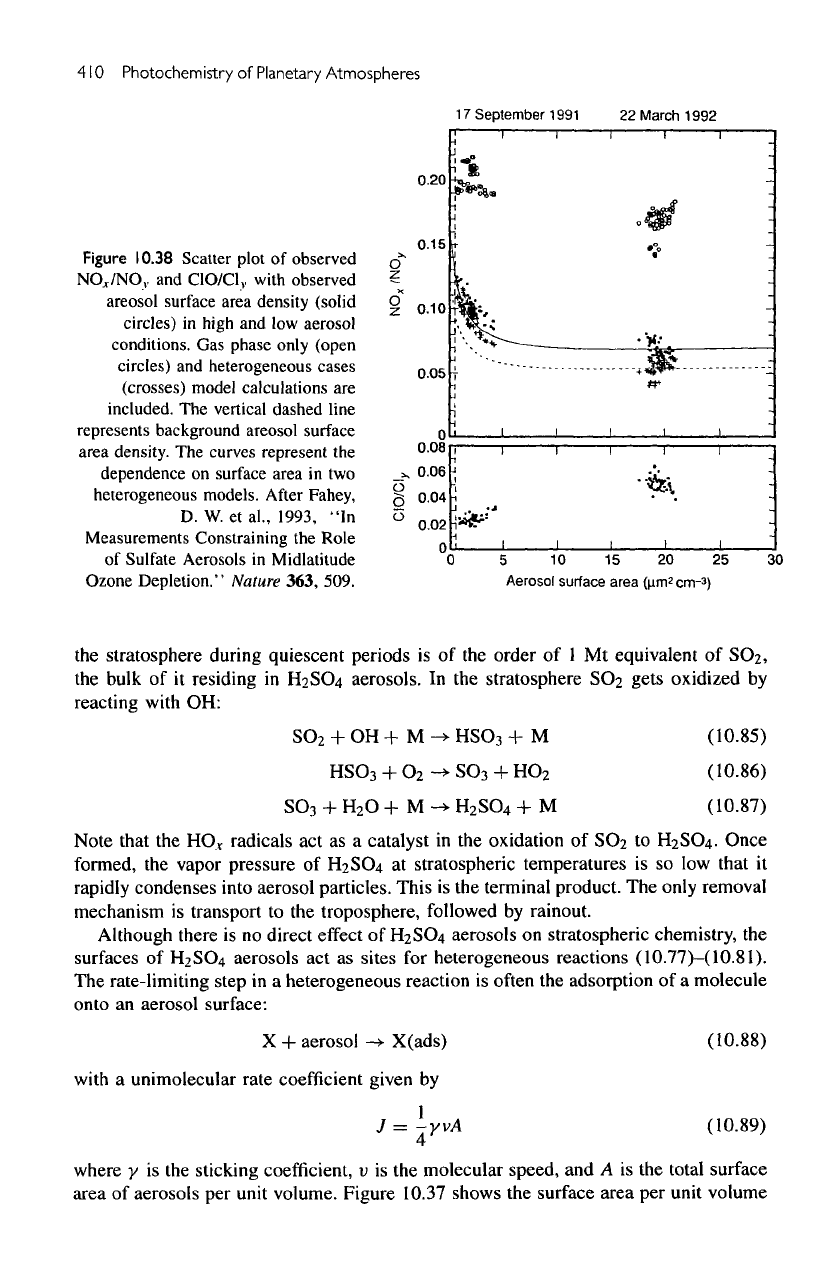
410
Photochemistry
of
Planetary
Atmospheres
Figure
10.38
Scatter plot
of
observed
NOr/NO,,
and
ClO/Cl,,
with
observed
areosol surface area density (solid
circles)
in
high
and low
aerosol
conditions.
Gas
phase only (open
circles)
and
heterogeneous cases
(crosses) model calculations
are
included.
The
vertical dashed line
represents background areosol surface
area density.
The
curves represent
the
dependence
on
surface area
in two
heterogeneous models.
After
Fahey,
D. W. et
al.,
1993,
"In
Measurements
Constraining
the
Role
of
Sulfate
Aerosols
in
Midlatitude
Ozone Depletion." Nature 363, 509.
the
stratosphere during quiescent periods
is of the
order
of 1 Mt
equivalent
of
SC>2,
the
bulk
of it
residing
in
H2SO4 aerosols.
In the
stratosphere
SO
2
gets
oxidized
by
reacting
with
OH:
Note that
the
HO
V
radicals
act as a
catalyst
in the
oxidation
of
SO
2
to
H
2
SC>4.
Once
formed,
the
vapor pressure
of
H
2
SO4
at
stratospheric temperatures
is so low
that
it
rapidly
condenses into aerosol particles. This
is the
terminal product.
The
only
removal
mechanism
is
transport
to the
troposphere, followed
by
rainout.
Although
there
is no
direct
effect
of
H
2
SO4
aerosols
on
stratospheric chemistry,
the
surfaces
of
H
2
SO4
aerosols
act as
sites
for
heterogeneous reactions
(10.77)-(10.81).
The
rate-limiting step
in a
heterogeneous reaction
is
often
the
adsorption
of a
molecule
onto
an
aerosol surface:
with
a
unimolecular rate
coefficient
given
by
where
y is the
sticking
coefficient,
v is the
molecular
speed,
and A is the
total surface
area
of
aerosols
per
unit
volume. Figure 10.37 shows
the
surface area
per
unit
volume

Earth: Human Impact
41
I
(A)
before
and
after
the
Pinatubo eruption. Note
that
there
was an
order
of
magnitude
increase
in the
values
of A due to the
effect
of
volcanic aerosols.
The
impact
of
this
on one
aspect
of
stratospheric chemistry,
the
partitioning between
NC>2
and
HNC>3,
is
shown
in figure
10.38.
The
effect
of the
aerosols
is to
decrease
the
NO
X
/NO
V
ratio,
while increasing
the
ClO/Cl_y
ratio.
The net
result
is an
enhancement
of the
effectiveness
of
chlorine
for
destroying ozone.
10.5
Unsolved Problems
We
list
a
number
of
outstanding unresolved problems related
to the
problems
of the
global environment:
1.
Is
there
a
missing
sink
of
CC>2,
and if so,
what
is it ?
2. Is the
biosphere
responding
to the
increase
of
COa
and
surface
temperature?
3.
What
are the
causes
for the
increase
in
atmospheric
CHU?
4.
What
are the
causes
for the
increase
in
atmospheric
NjO?
5.
What
is an
"acceptable"
level
of
ozone
depletion?
6.
What
fraction
of the
long
term
secular
changes
in
stratospheric
ozone
is
caused
by
natural
fluctuations
and
what
fraction
may be
attributed
to
anthropogenic
influence?
7. Is it
possible
(or
wise)
to
alter
the
global
planetary
environment
for the
welfare
of
man by
environmental
engineering?
There
is an
ultimate unsolved problem challenging Homo sapiens, posed
by
Professor
Bruce Murray
of the
California Institute
of
Technology.
For the first
time
in
human
history,
humanity
will
have
to
manage
the
planetary environment
and our
interaction
with
it. The
frontier
days
of
mining
the
forests
and
seas,
and of
dumping waste
products
into politically unrepresented environments,
are
rapidly
approaching
an
end.
The
inexorable growth
of
human
civilization
since
the
onset
of
agriculture
has
been
financed
by
consumption
and
degradation
of
much
of the
biospheric potential
of
soils
and
forests. Well before
the end of the
next century,
the
world
will
have consumed
nearly
all the
easily extracted
and
transported
oil
that
has
fueled
the
rapid global
economic growth since
the
middle
of
this century. Well before
the end of the
next
century,
much
of the
natural
capital
Man the
Toolmaker inherited
will
be
gone forever.
There
will
be no
chance
to
start over.
All
that
is
uncertain
is
whether
we
will have
used
our
collective intelligence
to
transform that inherited natural capital
into
a
self-
sustaining
social
and
economic system
no
longer dependent
on
exploitation
of
natural
resources.
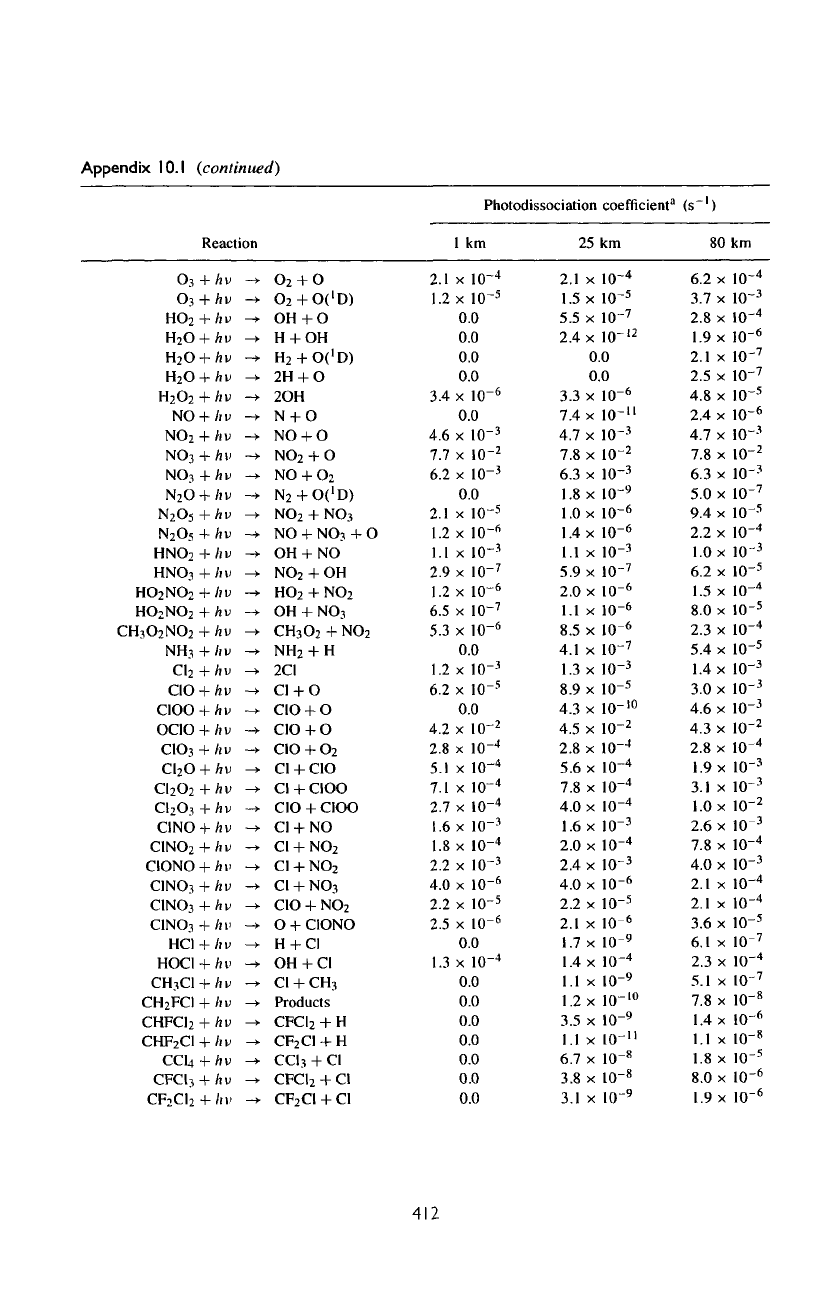
Appendix
Appendix
10.
1
Photodissociation
reactions
in
Earth's
atmosphere
at the
equator
in
January.
Photodissociation
coefficient"
(s~')
Reaction
O-i+hv
->
O2
+
hv
->
2O
O
+
O('D)
1
km
0.0
0.0
25 km
1.2
x
10-'
2
0.0
80 km
3.0
x
10-
9
4.7
x
10-'°
(continued)
Appendix
10.1
(continued)
Photodissociation
Reaction
0
3
0
3
H0
2
H
2
O
H
2
O
H
2
O
H
2
O
2
+
hv
+ hv
+ hv
+ hv
+ hv
+ hv
+ hv
_„
->.
_»
_>
_»
_y
—
>
NO
+
/IV
-»
NO
2
NO
3
NO
3
N
2
O
N
2
0
5
N
2
0
5
HNO
2
HNO
3
HO
2
NO
2
HO
2
NO
2
CH
3
0
2
N0
2
NH
3
C1
2
CIO
CIOO
+
hv
+
hv
+ hv
+
hv
+
hv
+ hv
+ hv
+
hv
+
hv
+ hv
+
hv
+ hv
+ hv
+
hv
+
hv
_>
_,.
-,.
_»
-»
-J.
_»
^
_»
_».
_>.
_>.
—
>
_>
__j.
OCIO
+
/ii>
-»•
C1O
3
+
hv
_,.
C(
2
O
+
hv
-+
CI
2
0
2
C1
2
0
3
CINO
C1NO
2
+ hv
+ hv
+ hv
+ hv
_>.
_*
_»
—
>
CIONO
+
Ai>
-^
C1NO
3
ClNOi
C1NO
3
HC1
HOC1
CH
3
C1
CH
2
FCI
CHFC1
2
CHF
2
C1
ecu
+ hv
+ hv
+ hv
+
hv
+ hv
+ hv
+ hv
+ hv
+ hv
+
hv
_>
_>.
_»
_,.
_,.
_,.
_>
->
_,.
_>.
CFCI
3
+
/ii>
->
CF
2
C1
2
+
hv
-*
0
2
+ 0
0
2
+
0('D)
OH+O
H
+ OH
H
2
+
0('D)
2H
+ O
2OH
N
+ O
NO
+ O
NO
2
+ O
NO
+
O
2
N
2
+O('D)
N0
2
+
NO
3
NO
+
NO
3
+ O
OH
+ NO
NO
2
+ OH
HO
2
+
NO
2
OH
+
NO
3
CH
3
O
2
+
NO
2
NH
2
+ H
2C1
Cl
+ O
CIO
+ O
CIO
+ O
CIO
+
O
2
Cl
+
CIO
Cl
+
CIOO
CIO
+
CIOO
CI
+ NO
Cl
+
NO
2
Cl
+
NO
2
Cl
+
NO
3
CIO
+
NO
2
O
+
CIONO
H
+
CI
OH
+
CI
Cl
+
CH
3
Products
CFCI
2
+ H
CF
2
CI
+ H
CCI
3
+ Cl
CFCI
2
+ Cl
CF
2
C1
+ Cl
2.1
1.2
3.4
4.6
7.7
6.2
2.1
1.2
1.1
2.9
1.2
6.5
5.3
1.2
6.2
4.2
2.8
5.1
7.1
2.7
1.6
1.8
2.2
4.0
2.2
2.5
1.3
1
km
x
IO-
4
x
IO-
5
0.0
0.0
0.0
0.0
x
10~
6
0.0
x
10~
3
x
IO-
2
x
IO-
3
0.0
x
IO--
5
x
ID"
6
x
IO-
3
x
10~
7
x
IO-
6
x
IO-
7
x
IO-
6
0.0
x
IO-
3
x
IO-
5
0.0
x
ID"
2
x
IO-
4
x
IO-
4
x
10~
4
x
IO-
4
x
IO-
3
x
IO-
4
x
1Q-
3
x
IO-
6
x
10~
5
x
10~
6
0.0
x
IO-
4
0.0
0.0
0.0
0.0
0.0
0.0
0.0
25
2.1
1.5
5.5
2.4
x
x
X
X
coefficient"
(s"
1
)
km
io-
4
io-
5
io-
7
,0-12
0.0
0.0
3.3
7.4
4.7
7.8
6.3
1.8
1.0
1.4
1.1
5.9
2.0
1.1
8.5
4.1
1.3
8.9
4.3
4.5
2.8
5.6
7.8
4.0
1.6
2.0
2.4
4.0
2.2
2.1
1.7
1.4
1.1
1.2
3.5
1.1
6.7
3.8
3.1
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
io-
6
10-"
io-
3
io-
2
io-
3
io-
9
io-
6
io-
6
io-
3
io-
7
10~
6
10~
6
10~
6
io-
7
io-
3
io-
5
,0-w
io-
2
io-
4
io-
4
io-
4
io-
4
io-
1
io-
4
10~
3
io-
6
io-
5
io-
6
10~
9
io-
4
io-
9
,0-10
io-
9
io-"
io-
8
io-
8
io-
9
I
80
6.2
3.7
2.8
1.9
2.1
2.5
4.8
2.4
4.7
7.8
6.3
5.0
9.4
2.2
1.0
6.2
1.5
8.0
2.3
5.4
1.4
3.0
4.6
4.3
2.8
1.9
3.1
1.0
2.6
7.8
4.0
2.1
2.1
3.6
6.1
2.3
5.1
7.8
1.4
1.1
1.8
8.0
1.9
x
x
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
km
io-
4
io-
3
io-
4
io-
6
io-
7
io-
7
io-
5
io-
6
io-
3
io-
2
io-
3
io-
7
io-
5
io-
4
io-
1
io-
5
io-
4
io-
5
io-
4
io-
5
io-
3
io-
3
io-
1
io-
2
io-
4
io-
3
io-
3
io-
2
io-
3
io-
4
io-
3
io-
4
io-
4
io-
5
io-
7
io-
4
io-
7
io-
8
10'
6
1Q-
8
io-
5
io-
6
io-
6
412
Appendix
I
O.I
(continued)
Photodissociation
coefficient"
(s
'
)
Reaction
CH
3
CC1
3
+hv
CH
3
CFC1
2
+
hv
CH
3
CF
2
CI
+
Av
C
2
C1
3
F
3
+ hv
C
2
C1
2
F
4
+
hv
CF
3
CHCl
2
+
Ai>
CF
3
CHFC1
+
hv
CF}CCl
3
+hv
CF
3
CFC1
2
+ hv
CF
3
CF
2
CHCI
2
+ hv
CF
2
C1CF
2
CHFCI
+ hv
CHCIO
+
Au
COC\
2
+hv
COFCI
+
Av
HF
+
Au
CF
4
+ hv
C
2
F6
+
hv
COF
2
+hv
SF
6
-|_/,
v
Br
2
+ hv
BrO
+
hv
BrNO
2
+hv
BrNO
3
+ hv
HBr
+
Au
HOBr
+
hv
CH
3
Br
+
Av
CHBr
3
+ hv
BrCl
+
Av
CF
2
ClBr
+ hv
CF
2
Br
2
+hv
CF
3
Br
+ hv
C
2
F.iBr
2
+hv
CHt
+ hv
CO
2
+hv
H
2
CO
+ hv
H
2
CO
+ hv
CH
3
OOH
+ ftv
HCN
+
Av
CH
3
CN
+
Ai>
SO
2
+
hv
H
2
S
+ hv
OCS
+ hv
_
>
_>
_>
-*
_>
_+
_>
_>
->
-*
_>
_»
_»
—
>
_,.
_>
_»
-s.
->.
_>
->
-»
_>
->
—
>•
_,.
->
->.
_>
->
^.
^.
_>
—
>•
^.
^.
->.
->
-^.
-*
-»
—
y
Products
Products
Products
Products
Products
Products
Products
Products
Products
Products
Products
HCO
+
Cl
2CI
+ CO
Products
H
+ F
CF
3
+ F
2CF
3
Products
Products
2Br
Br
+ O
Br
+
NO
2
Br
+
NO
3
H
+ Br
OH
+ Br
CH
3
+ Br
Products
Br
+
CI
CF
2
CI
+ Br
Products
CF
3
+ Br
Products
Products
CO
+ O
HCO
+ H
H
2
+ CO
CH
3
O
+ OH
H
+ CN
CH
3
+ CN
so + o
H
+ SH
CO
+ S
2.8
8.4
1.7
2.1
5.2
3.7
1.6
4.8
1.5
6.4
1.5
2.6
2.6
5.4
1
km
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
0.0
x
10~
4
x
10~
19
0.0
0.0
0.0
0.0
0.0
0.0
x
10~
2
x
10~
2
0.0
x
10-
4
0.0
x
10-
4
0.0
x
10-
7
x
10'
3
x
lO^
1
^
x
10-'°
0.0
0.0
0.0
0.0
x
10~
5
x
I0~
5
x
10-
6
0.0
0.0
0.0
0.0
x
10-"
25 km
5.7
9.8
8.6
6.3
3.5
7.5
7.9
7.2
4.9
2.8
2.5
1.3
9.9
1.6
2.3
5.5
1.1
3.8
8.9
8.8
4.8
1.4
1.2
1.4
1.4
5.3
1.8
3.8
3.0
8.3
6.6
5.9
9.0
x
10-
8
x
10-
9
x
10-"
x
I0~
9
x
10-'°
x
10-"
x
10-"
x
10-
9
x
10-'°
0.0
0.0
x
10~
4
x
10-
8
x
10~
8
0.0
0.0
0.0
x
10-'°
0.0
x
10~
2
x
10~
2
0.0
x
10~
4
x
10~
7
x
lO"
4
x
10-
8
x
10-
7
x
10~
3
x
10~
7
x
10-
7
x
10"
8
x
10-
7
0.0
x
10-'
4
x
10-
5
x
lO"
5
x
10~
6
x
10'
13
0.0
x
I0~
7
x
10~
7
x
10-
9
80
9.2
.4
.3
.6
2.1
.1
.2
2.6
2.1
x
X
X
X
X
X
X
X
X
km
10~
6
10~
6
io-
8
to-
6
io-
7
io-
6
io-
8
io-
6
io-
7
0.0
0.0
2.8
2.5
3.2
1.1
2.0
4.6
1.6
X
X
X
X
X
X
X
io-
4
io-
5
io-
6
io-
7
io-'°
]0
-20
io-
7
0.0
1.6
2.3
X
X
io-
2
io-
2
0.0
1.1
2.2
6.3
2.6
6.2
4.7
5.9
2.3
4.8
5.0
6.6
3.2
3.8
5.6
2.7
1.8
7.0
9.4
1.6
1.3
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
io-
3
io-
s
io-
4
io-
5
io-
4
io-
3
io-
5
io-
4
10~
6
IO-
5
io-
7
io-
9
io-
5
io-
5
io-
5
10~
6
io-
7
io-
5
io-
4
io-
5
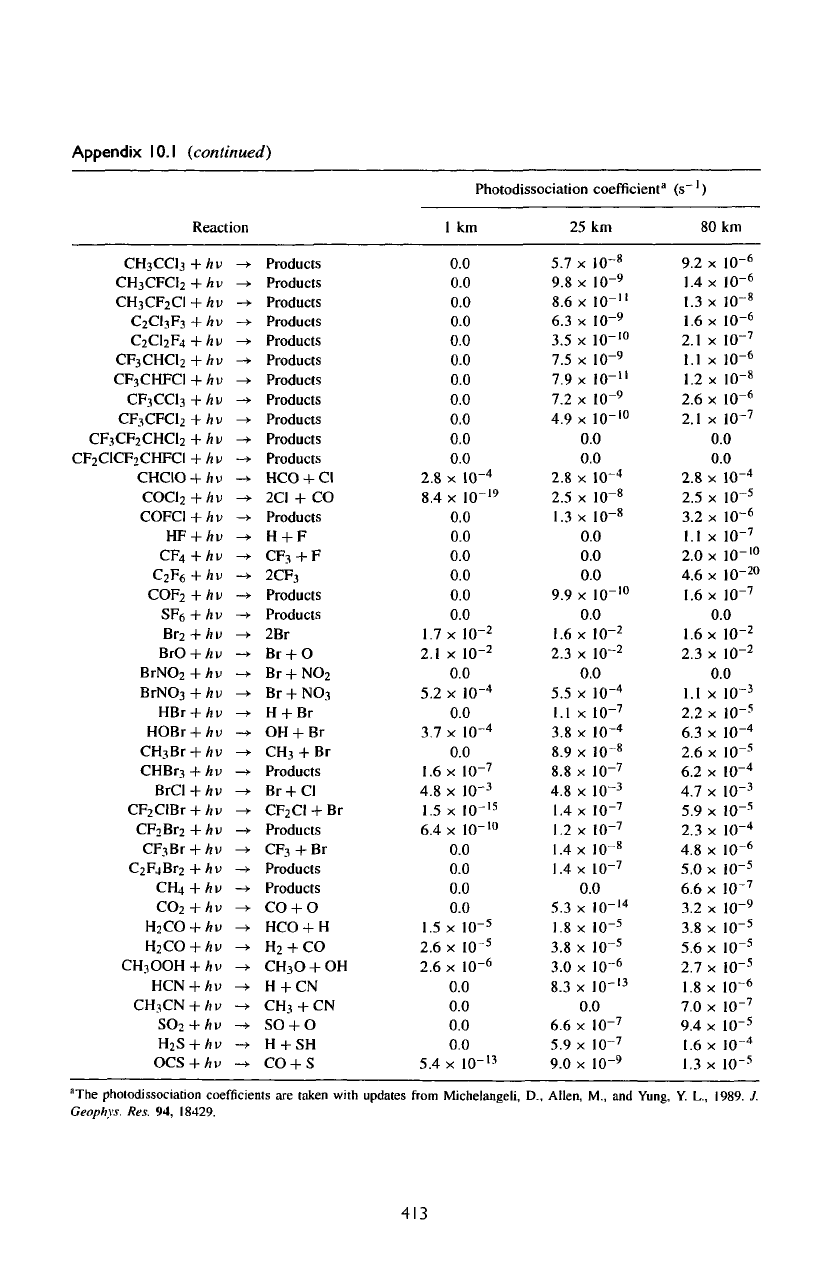
"The
pholodissociation
coefficients
are
taken with updates
from
Mjchelangeli,
D.,
Allen,
M.,
and
Yung,
Y.
L.,
1989.
J.
Geophys.
Res.
94,
18429.
413
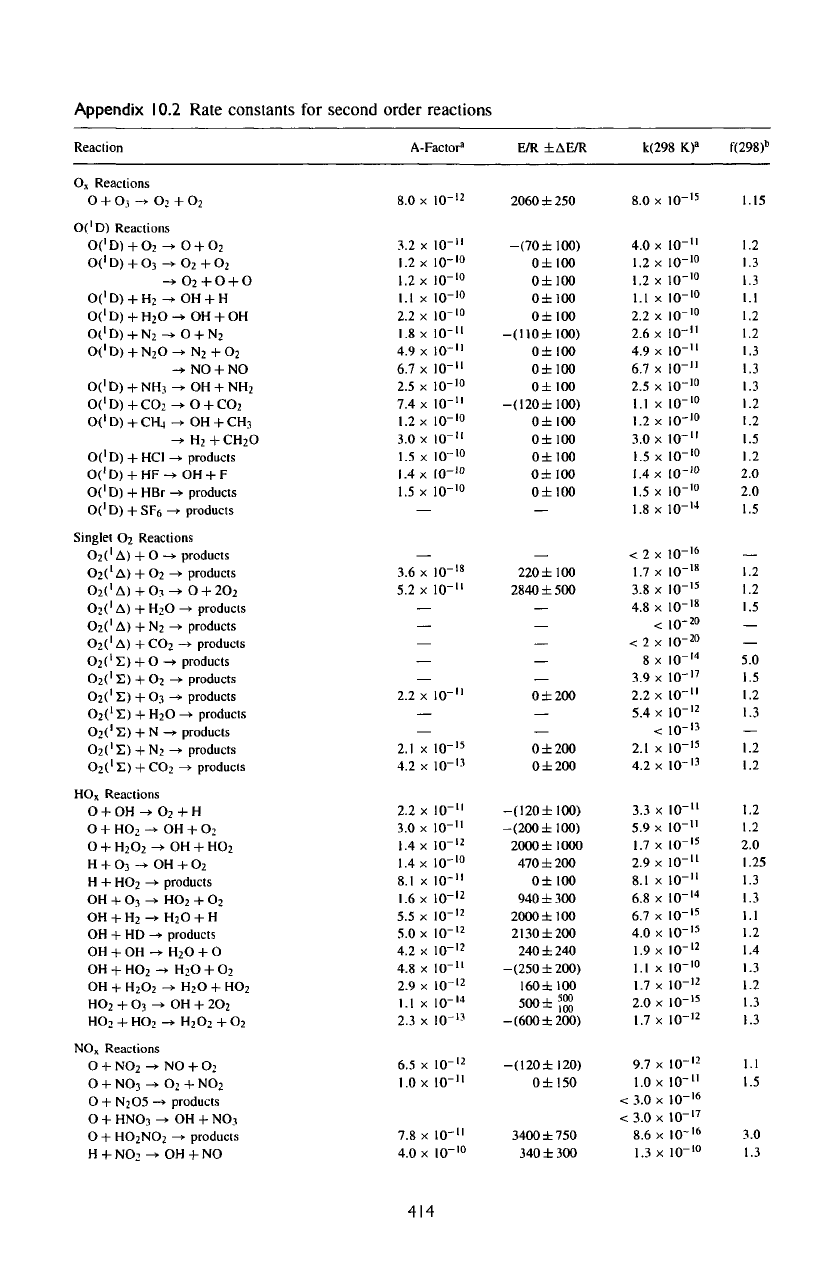
Appendix
10.2
Rate constants
for
second order reactions
Reaction
Oj
Reactions
O
+
O
3
-»
O
2
+
O
2
O('D)
Reactions
0('D)
+
0
2
->
0 +
0
2
0('D)
+
0
3
-»0
2
+0
2
-»
O
2
+ O + O
O('D)
+
H
2
-»
OH + H
O('D)
+
H
2
O->
OH +
OH
0('D)
+
N
2
->
0 +
N
2
O('D)
+
N
2
O->-
N
2
+O
2
->•
NO + NO
0('D)
+
NH
3
->-
OH +
NH
2
O('D)+CO
2
->
O +
CO
2
O('
D) +
CH4
->
OH +
CH.1
->
H
2
+CH
2
O
O('D)
+
HC1
->
products
O('D)
+
HF-+
OH + F
O('D)
+ HBr
-»
products
O('D)
+
SF
6
-»
products
Singlet
O
2
Reactions
O
2
('
A)
+
O->
products
O
2
('A)
+
O
2
->
products
O
2
('A)
+
Oj
->
O +
2O
2
O
2
('
A) +
H
2
O
-»
products
O
2
('
A) +
N
2
->
products
O
2
('
A) +
CO
2
-»
products
Ojf'EJ
+ O
-»
products
O
2
('S)
+
O
2
->
products
O
2
('
E)
+
OT,
->
products
O
2
('S:)
+
H
2
O->
products
O
2
('S)
+ N
->
products
O
2
('£)
+
N
2
->
products
O
2
('£)
+
CO
2
->
products
HO
X
Reactions
0 +
OH
->
O
2
+ H
O
+
HO
2
-»
OH +
O
2
O
+
H
2
O
2
->
OH +
HO
2
H
+
O
3
->
OH +
O
2
H
+
HO
2
->
products
OH
+
0,
->
H0
2
+
0
2
OH
+
H
2
->
H
2
0
+ H
OH
+ HD
->
products
OH
+ OH
->
H
2
O
+ O
OH
+
HO
2
-»
H
2
O
+
O
2
OH
+
H
2
O
2
->
H
2
O
+
HO
2
HO
2
+
O
3
->
OH +
2O
2
HO
2
+HO
2
-»
H
2
O
2
+O
2
NO
X
Reactions
O
+
NO
2
->
NO +
O
2
O
+
NO
3
->
O
2
+
NO
2
O
+
N
2
O5
-»
products
O
+
HNO
3
->
OH +
NO.,
O +
HO
2
NO
2
->
products
H
+
NO
2
->
OH + NO
A-Factor
a
8.0 x
10-'
2
3.2
x
10-"
1.2
x
10~'°
1.2
x
10-'°
1.1
x
10-'°
2.2
x
10-
10
1.8
x
10-"
4.9
x
10-"
6.7
x
10-"
2.5
x
10-'°
7.4
x
10-"
1.2
x
10-'°
3.0
x
10-
"
1.5
x
10"'°
1.4x
10-'°
1.5
x
10-'°
—
—
3.6
x
10-'
8
5.2
x
10-"
—
—
—
—
—
2.2
x
10""
—
—
2.1
x
1Q-'
5
4.2
x
10-'-
1
2.2
x
10-"
3.0
x
10-"
1.4
x
1Q-'
2
1.4
x
10-'°
8.1
x
10-"
1.6
x
10~
12
5.5
x
10-'
2
5.0
x
10-
n
4.2
x
10-'
2
4.8
x
10-"
2.9 x
10^
12
1.1
x
10-'
4
2.3 x
ID"
13
6.5 x
10-
12
1.0
x
10-"
7.8
x
10-"
4.0 x
1(T
10
E/R
±AE/R
2060
±250
-(70
±100)
OilOO
0±100
0±100
0±100
-(110±100)
0±IOO
0±100
0±100
-(I20±100)
0±100
0±100
0±100
0±100
0±100
—
—
220±100
2840
±500
—
—
—
—
—
0±200
—
—
0±200
0±200
-(120±100)
-(200
±100)
2000±1000
470
±200
0±100
940
±300
2000±100
2130
±200
240
± 240
-(250
±200)
160±100
«nn+
50
°
auui
100
-(600
±200)
-(120±120)
0±150
3400
±750
340
±300
k(298
K)
a
8.0 x
1Q-'
5
4.0
x
10-"
1.2x
10-'°
1.2
x
10-'°
1.1
x
10-'°
2.2
x
10-'°
2.6
x
10-"
4.9
x
10-"
6.7 x
1Q-"
2.5
x
10-'°
.1
x
10-'°
.2
x
10-'°
3.0
x
10""
.5
x
10-'°
.4x10-'°
.5
x
10-'°
.8
x
10-'
4
<2 x
1Q-'
6
1.7
x
10-'
8
3.8 x
1Q-'
5
4.8 x
1Q-'
8
<
io-
20
< 2 x
IO-
20
8 x
1Q-'
4
3.9 x
ID'
17
2.2x
10-"
5.4 x
1Q-'
2
<
io-
13
2.1
x
10-'
5
4.2
x
10-
l3
3.3
x
10-"
5.9
x
10-"
1.7
x
IO-'
5
2.9
x
10-"
8.1
x
10-"
6.8 x
IO-'
4
6.7
x
10-'
5
4.0 x
IQ-'
5
1.9
x
IO-'
2
1.1
x
10-'°
1.7
x
10-'
2
2.0 x
IO-'
5
1.7 x
10~
12
9.7 x
10~
12
1.0
x
10-"
< 3.0 x
10~
16
< 3.0 x
1Q-'
7
8.6 x
IO-'
6
1.3
x
10-'°
f(298)
b
1.15
.2
.3
.3
.1
.2
.2
.3
.3
.3
.2
.2
.5
.2
2.0
2.0
1.5
—
1.2
1.2
1.5
—
—
5.0
1.5
1.2
1.3
—
1.2
1.2
1.2
1.2
2.0
1.25
1.3
1.3
1.1
1.2
1.4
1.3
1.2
1.3
1.3
1.1
1.5
3.0
1.3
414
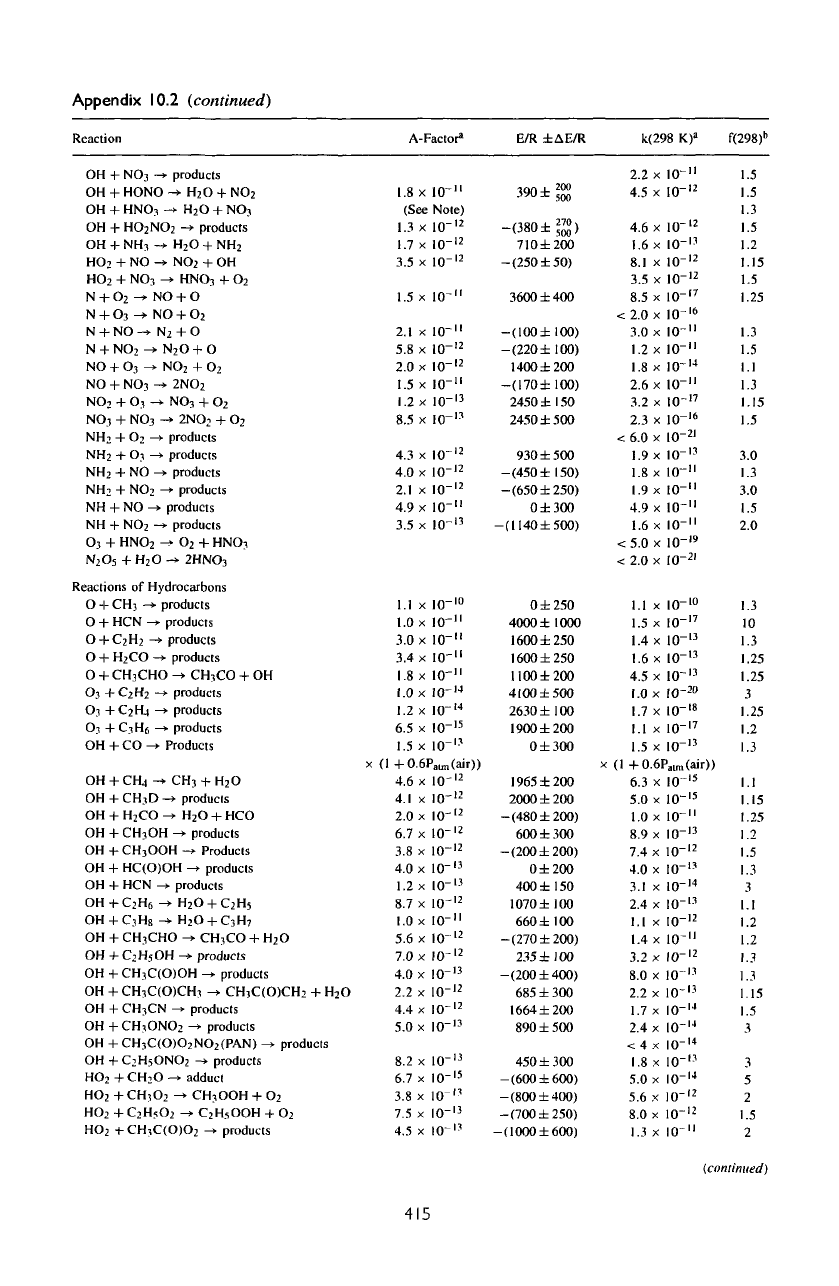
Appendix
10.2
(continued)
Reaction
OH
+ NO]
-»
products
OH
+
HONO-i-
H
2
0
+
N0
2
OH
+
HN0
3
->
H
2
0
+
NOj
OH
+
HO2NO
2
-*
products
OH
+
NH
3
->
H
2
O
+
NH
2
HO
2
+ NO
-»
NO
2
+ OH
HO
2
+
NO
3
->
HNO
3
+
O
2
N
+
O
2
-i-
NO + O
N
+
O
3
->
NO +
O
2
N
+ NO
->
N
2
+ O
N
+
NO
2
->
N
2
O
+ O
NO
+
O
3
->
NO
2
+
O
2
NO
+
N0
3
-*
2N0
2
NO
2
+
Oj
-»
NO
3
+
O
2
NO
3
+
NO
3
->
2NO
2
+
O
2
NH
2
+
O
2
->
products
NH
2
+
Oj
—
»
products
NH
2
+ NO
->
products
NH
2
+
NO
2
->
products
NH
+ NO
->
products
NH
+
NO
2
->
products
O
3
+
HNO
2
->•
O
2
+
HNO
3
N
2
O
5
+
H
2
O
->
2HNO
3
Reactions
of
Hydrocarbons
O +
CH
3
->
products
O
+ HCN
->
products
O
+
C
2
H
2
-»
products
O
+
H
2
CO
->
products
0 +
CHjCHO
->
CH
3
CO
+
OH
O.i
+C
2
H
2
->
products
O
3
+C
2
Kt
->
products
O
3
+C
3
H
6
->
products
OH
+ CO
->
Products
OH
+
CH4
-»
CH
3
+
H
2
0
OH
+
CH
3
D-»
products
OH
+
H
2
CO
->
H
2
0
+
HCO
OH
+
CH
3
OH
->
products
OH
+
CH
3
OOH
-»
Products
OH
+
HC(O)OH
->
products
OH
+ HCN
->
products
OH+C
2
H
6
->
H
2
0
+
C
2
H
5
OH
+
CjHs
->
H
2
0
+
C
3
H
7
OH
+
CHjCHO
->
CH
3
CO
+
H
2
O
OH
+
C
2
H
5
OH
->
products
OH
+
CH,C(0)OH
->
products
OH
+
CH
3
C(O)CHj
->
CH
3
C(O)CH
2
+
H
2
O
OH
+
CH
3
CN
->
products
OH
+
CH
3
ONO
2
->
products
OH
+
CH
3
C(O)O
2
NO
2
(PAN)
->
products
OH
+C
2
H
5
ONO
2
->
products
HO
2
+
CH
2
O
->
adducl
HO
2
+
CH
3
O
2
->
CHiOOH
+
O
2
HO
2
+
C
2
H
5
O
2
->
C
2
H
5
OOH
+
O
2
HO
2
+•
CH
3
C(O)O
2
->
products
A-Factor
1
1.8
x
10-"
(See
Note)
1.3
x
10~
12
1.7
x
1(T'
2
3.5 x
10~
l2
1.5
x
10-"
2.1
x
10-"
5.8 x
10~
12
2.0
x
10-'
2
1.5
x
10""
1.2
x
10~
13
8.5
x
10""
4.3 x
IQ-'
2
4.0 x
1Q-'
2
2.1
x
1Q-'
2
4.9
x
10""
3.5
x
10~
n
1.1
x
10"'°
1.0
x
10'"
3.0
x
10""
3.4
x
IO-"
1.8
x
10-"
1.0
x
10-'
4
1.2
x
10"
l4
6.5
x
10-'
5
1.5
x
10'
1
-
1
x
(l+0.6P
aun
(air))
4.6 x
10"
12
4.1
x
10'
12
2.0 x
IO"-'
2
6.7
x
10-'
2
3.8 x
10~
12
4.0
x
10"
l3
1.2
x
10-
"
8.7 x
10-'
2
1.0
x
IO-"
5.6 x
10~
12
7.0
x
10-'
2
4.0
x
10-'
3
2.2
x
10-'
2
4.4
x
10-'
2
5.0 x
10-'
3
8.2
x
10-'
3
6.7
x
10""
3.8 x
10~
13
7.5
x
IQ-'
3
4.5 x
IO-'
3
E/R
±AE/R
-ion
-t-
20
°
ivu
±
500
-(380
±
2
™)
710±200
-(250
±50)
3600
±400
-(100±IOO)
-(220
±100)
1400±200
-(170±100)
2450
±
1
50
2450
±500
930
±500
-(450
±150)
-(650
±250)
0±300
-(1I40±500)
0±250
4000±1000
1600
±250
1600
±250
1100±200
4100±500
2630
±100
1900±200
0±300
1965
±200
2000
±200
-(480
±200)
600
±300
-(200
±200)
0±200
400±150
1070±100
660±100
-(270
±200)
235
±100
-(200
±400)
685
± 300
1664
±200
890
± 500
450
±300
-(600
±600)
-(800
±400)
-(700
±2
50)
-(1000
±600)
k(298
K)
a
2.2
x
10-"
4.5 x
IO-'
2
4.6 x
1Q-'
2
1.6
x
IQ-'
3
8.1
x
1Q-'
2
3.5
x
10-'
2
8.5
x
10-'
7
< 2.0 x
1Q-'
6
3.0
x
10""
1.2
x
10-"
1.8
x
10-'
4
2.6
x
10-"
3.2
x
10-"
2.3 x
10~
16
< 6.0 x
1Q-
2
'
1.9
x
10-'
3
1.8
x
10-"
1.9
x
10-"
4.9
x
10-"
1.6
x
10""
<5.0x
10-"
<
2.0 x
I0~
21
.1
x
10-'°
.5 x
1Q-'
7
.4 x
10-'
3
.6 x
IO"
13
4.5 x
IO-
13
.0
x
10"
20
.7
x
IO-'
8
.1
x
10-'
7
.5
x
1Q-'
3
x
(l+0.6P
aun
(air))
6.3 x
10~'
5
5.0 x
ID"
15
1.0
x
10-"
8.9 x
IQ-'
3
7.4
x
10-'
2
4.0 x
IO-
13
3.1
x
10-'
4
2.4
x
10"
13
1.1
x
IQ-'
2
1.4
x
10-"
3.2 x
IQ-'
2
8.0 x
IO"
13
2.2
x
10-'
3
1.7
x
IO-'
4
2.4 x
10~
14
<4 x
IO-'
4
l.8x
10"
13
5.0 x
IO-
14
5.6
x
10-'
2
8.0 x
10~
12
1.3
x
10""
f(298)
b
1.5
1.5
1.3
1.5
1.2
1.15
1.5
1.25
1.3
1.5
I.I
1.3
1.15
1.5
3.0
1.3
3.0
1.5
2.0
1.3
10
1.3
1.25
1.25
3
1.25
1.2
1.3
1.1
1.15
1.25
1.2
1.5
1.3
3
1.1
1.2
1.2
1.3
1.3
1.15
1.5
3
3
5
2
1.5
2
(continued)
415
