Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

386
Photochemistry
of
Planetary
Atmospheres
where
in
(
10.20)
we
have listed only
the
principal branch
(60%)
of
CH
3
O
2
dispro-
portionation.
The
methyl hydroperoxy radical
is
removed
by
photolysis,
or by
rainout,
CH
CH
3
OH
will
most likely
be
rained
out
from
the
atmosphere.
The
primary fate
of the
methoxy
radical
is to
react with
02,
yielding formaldehyde:
H2CO
is
removed
by
photolysis:
Additional
removal mechanisms include reaction
with
OH and
rainout:
The
formyl
radical
is
removed
by
reaction with
O
2
:
The
terminal product
of the
oxidation chain
is
CO
2
,
formed
by
reaction
(10.4).
There
is
a
crucial question
in the
oxidation chain
of
CH
4
on
whether
the
destruction
of CH4
is
a
source
or
sink
of
HO^.
This
may be
more easily seen
if we
carefully examine
one
possible chemical path
of
oxidation:
This chemical scheme implies
that
each molecule
of
CH
4
consumes
2HO
V
and
pro-
duces
O
3
and
4HO
X
.
The net
gain
of odd
hydrogen
is
2HO
X
.
In
scheme
(Ila),
we
have
chosen branch
(10.24a)
for the
photolysis
of
H
2
CO.
If we
choose
the
other branch
(10.24b)
for the
photolysis
of
H
2
CO,
the net
result would
be
Earth:
Human
Impact
387
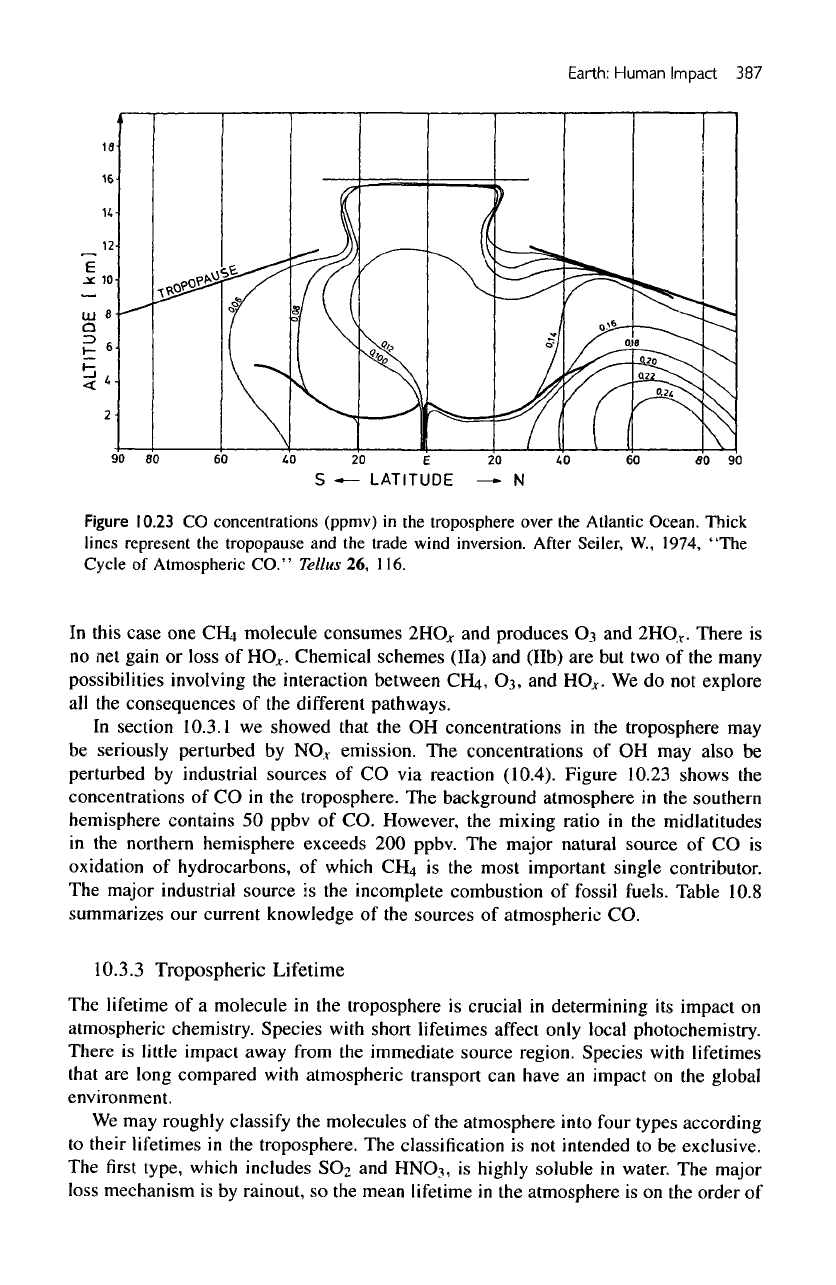
Figure
10.23
CO
concentrations
(ppmv)
in the
troposphere over
the
Atlantic Ocean. Thick
lines
represent
the
tropopause
and the
trade wind inversion.
After
Seiler,
W.,
1974, "The
Cycle
of
Atmospheric
CO."
Tellus
26,
116.
In
this
case
one
Crii
molecule consumes
2HOjr
and
produces
63
and
2HO
V
.
There
is
no
net
gain
or
loss
of
HO^.
Chemical schemes (Ha)
and
(lib)
are but two of the
many
possibilities
involving
the
interaction between
CUt,
03,
and
HO^.
We do not
explore
all
the
consequences
of the
different
pathways.
In
section 10.3.1
we
showed that
the OH
concentrations
in the
troposphere
may
be
seriously perturbed
by
NO,
emission.
The
concentrations
of OH may
also
be
perturbed
by
industrial
sources
of CO via
reaction
(10.4).
Figure
10.23
shows
the
concentrations
of CO in the
troposphere.
The
background atmosphere
in the
southern
hemisphere contains
50
ppbv
of CO.
However,
the
mixing
ratio
in the
midlatitudes
in
the
northern hemisphere exceeds
200
ppbv.
The
major natural source
of CO is
oxidation
of
hydrocarbons,
of
which
CH
4
is the
most important single contributor.
The
major
industrial
source
is the
incomplete combustion
of
fossil fuels. Table
10.8
summarizes
our
current knowledge
of the
sources
of
atmospheric
CO.
10.3.3
Tropospheric
Lifetime
The
lifetime
of a
molecule
in the
troposphere
is
crucial
in
determining
its
impact
on
atmospheric chemistry. Species
with
short lifetimes
affect
only local photochemistry.
There
is
little
impact away from
the
immediate source region.
Species
with
lifetimes
that
are
long compared
with
atmospheric transport
can
have
an
impact
on the
global
environment.
We may
roughly
classify
the
molecules
of the
atmosphere into
four
types according
to
their
lifetimes
in the
troposphere.
The
classification
is not
intended
to be
exclusive.
The first
type,
which
includes
SO2 and
HNOi,
is
highly
soluble
in
water.
The
major
loss mechanism
is by
rainout,
so the
mean
lifetime
in the
atmosphere
is on the
order
of
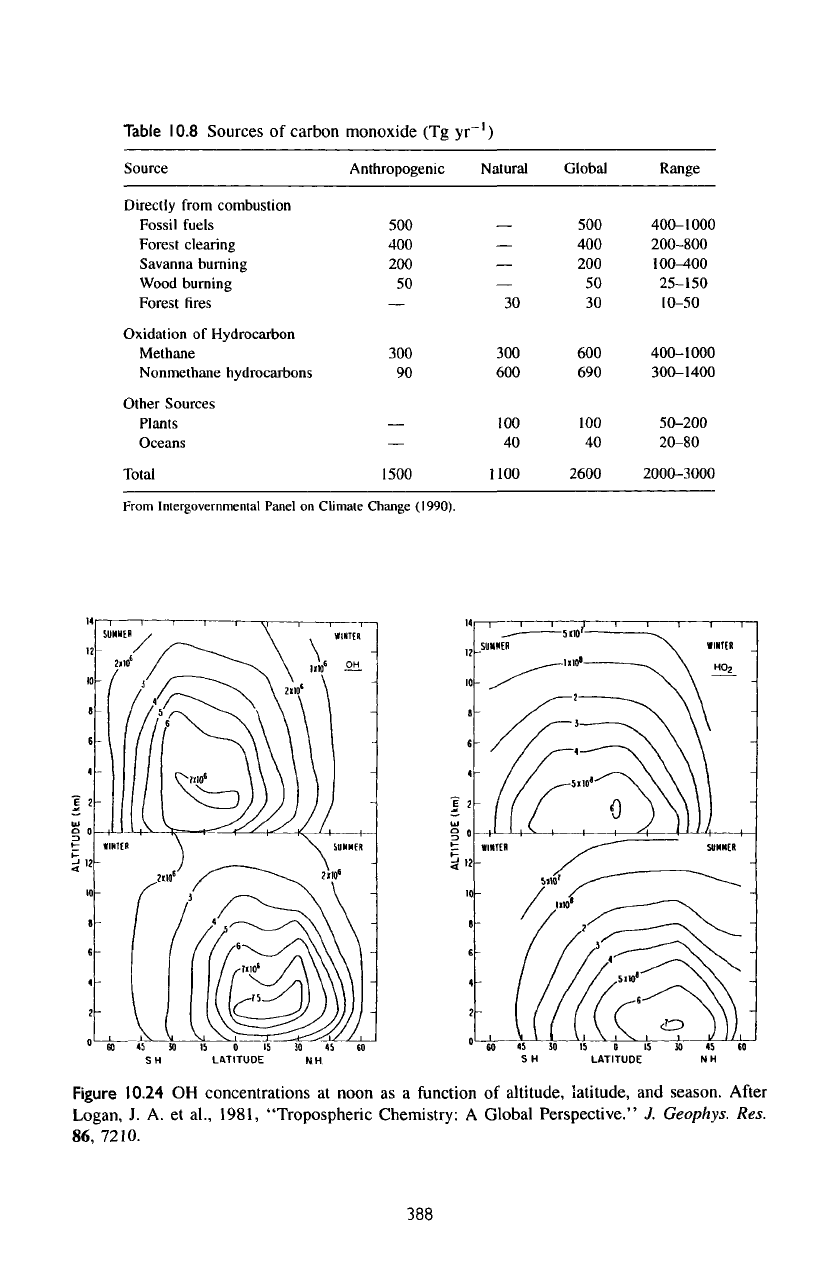
Table
10.8
Sources
of
carbon monoxide
(Tg yr
')
Source
Directly
from
combustion
Fossil
fuels
Forest clearing
Savanna
burning
Wood burning
Forest
fires
Oxidation
of
Hydrocarbon
Methane
Nonmethane
hydrocarbons
Other Sources
Plants
Oceans
Total
Anthropogenic
500
400
200
50
—
300
90
—
—
1500
Natural
—
—
—
—
30
300
600
100
40
1100
Global
500
400
200
50
30
600
690
100
40
2600
Range
400-1000
200-800
100-400
25-150
10-50
400-1000
300-1400
50-200
20-80
2000-3000
From
Intergovernmental
Panel
on
Climate Change (1990).
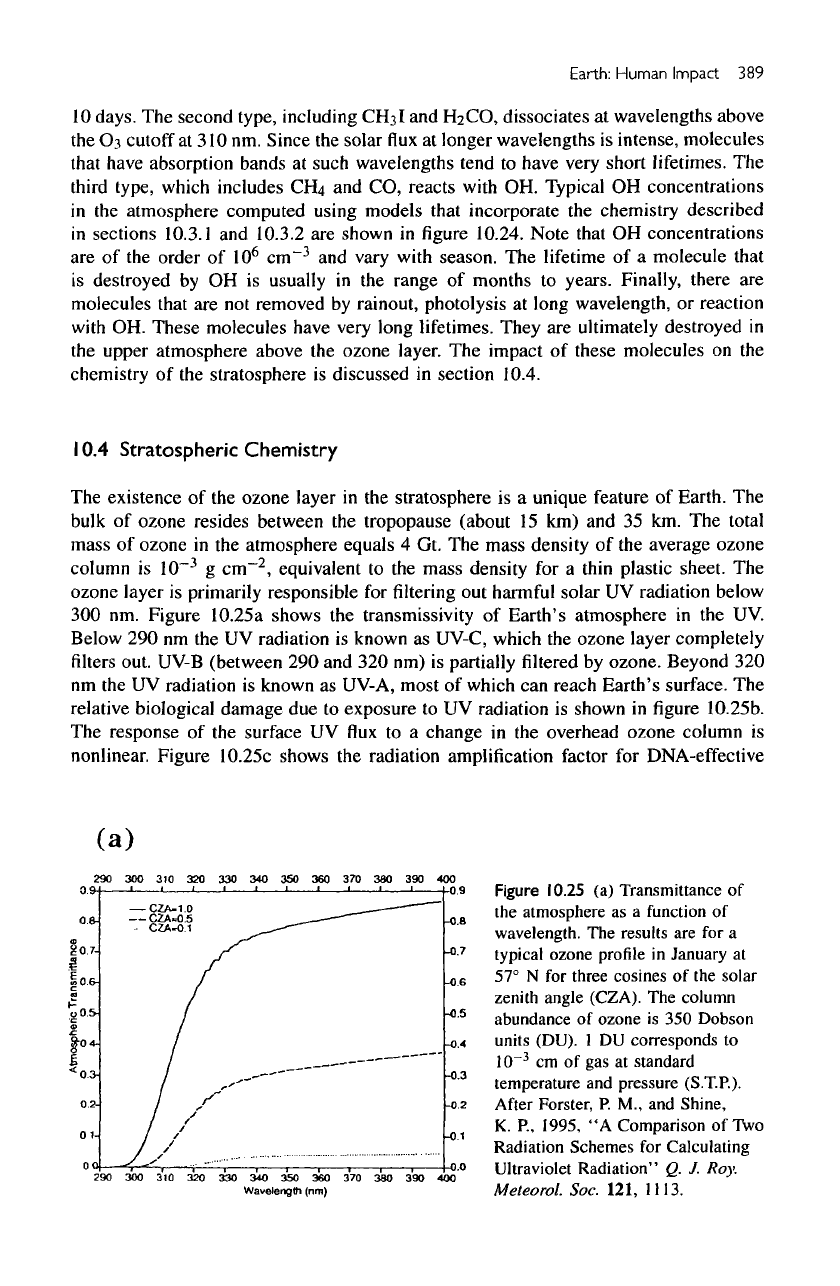
Figure
10.24
OH
concentrations
at
noon
as a
function
of
altitude,
latitude,
and
season.
After
Logan,
J. A. et
al.,
1981,
"Tropospheric
Chemistry:
A
Global Perspective."
J.
Geophys. Res.
86,
7210.
388
Earth:
Human
Impact
389
10
days.
The
second type,
including
CHsI
and
FhCO,
dissociates
at
wavelengths above
the
O.i
cutoff
at
310
nm.
Since
the
solar
flux at
longer wavelengths
is
intense, molecules
that
have absorption bands
at
such wavelengths tend
to
have very short lifetimes.
The
third
type,
which
includes
CH4 and CO,
reacts
with
OH.
Typical
OH
concentrations
in
the
atmosphere computed using models
that
incorporate
the
chemistry described
in
sections 10.3.1
and
10.3.2
are
shown
in
figure
10.24.
Note
that
OH
concentrations
are of the
order
of
10
6
cm~
3
and
vary
with
season.
The
lifetime
of a
molecule
that
is
destroyed
by OH is
usually
in the
range
of
months
to
years. Finally, there
are
molecules
that
are not
removed
by
rainout, photolysis
at
long wavelength,
or
reaction
with
OH.
These
molecules have very long lifetimes. They
are
ultimately
destroyed
in
the
upper atmosphere above
the
ozone layer.
The
impact
of
these molecules
on the
chemistry
of the
stratosphere
is
discussed
in
section 10.4.
10.4
Stratospheric
Chemistry
The
existence
of the
ozone layer
in the
stratosphere
is a
unique feature
of
Earth.
The
bulk
of
ozone resides between
the
tropopause (about
15 km) and 35 km. The
total
mass
of
ozone
in the
atmosphere equals
4 Gt. The
mass density
of the
average ozone
column
is
10~
3
g
cm""
2
,
equivalent
to the
mass density
for a
thin
plastic sheet.
The
ozone layer
is
primarily
responsible
for
filtering
out
harmful
solar
UV
radiation below
300 nm.
Figure 10.25a shows
the
transmissivity
of
Earth's atmosphere
in the UV.
Below
290 nm the UV
radiation
is
known
as
UV-C, which
the
ozone layer completely
filters
out.
UV-B (between
290 and 320 nm) is
partially
filtered by
ozone. Beyond
320
nm
the UV
radiation
is
known
as
UV-A, most
of
which
can
reach Earth's surface.
The
relative
biological
damage
due to
exposure
to UV
radiation
is
shown
in figure
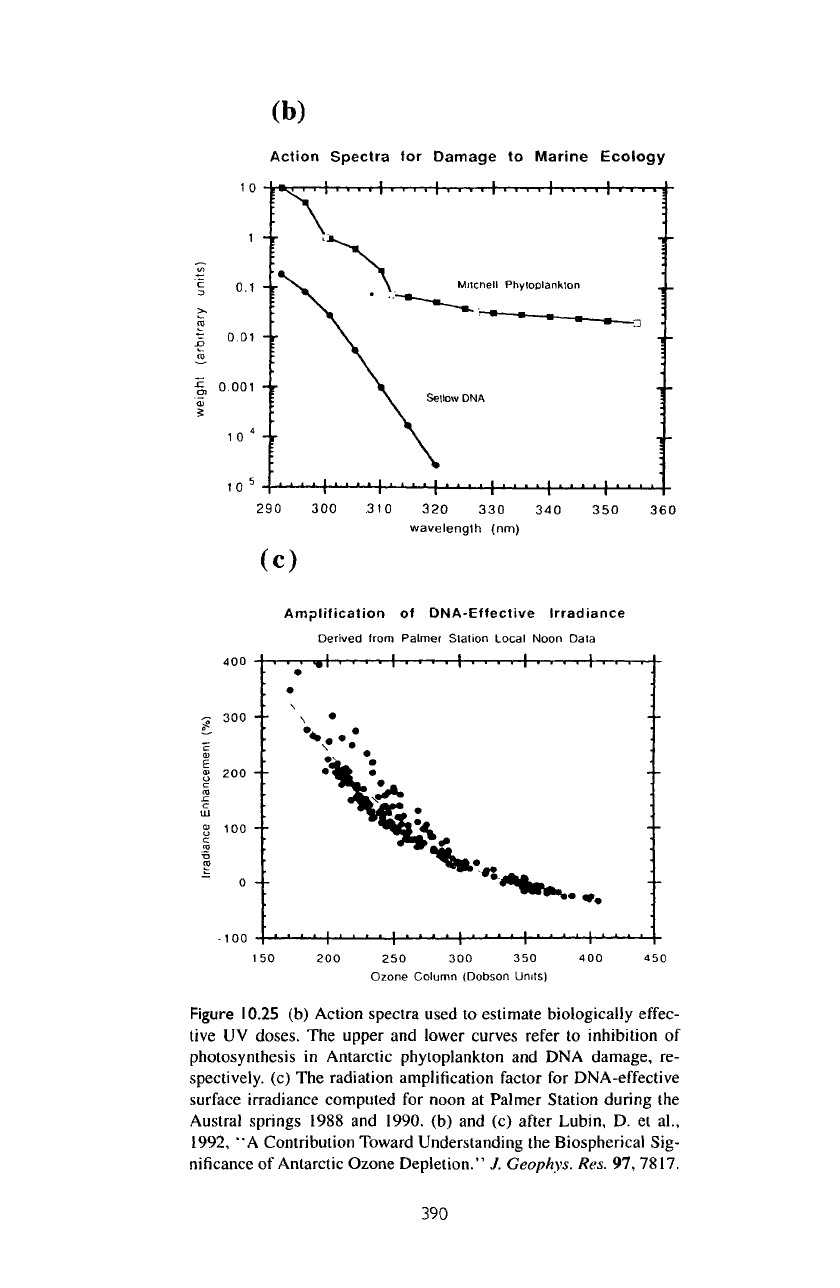
10.25b.
The
response
of the
surface
UV flux to a
change
in the
overhead ozone column
is
nonlinear.
Figure
10.25c
shows
the
radiation
amplification
factor
for
DNA-effective
(a)
Figure
10.25
(a)
Transmittance
of
the
atmosphere
as a
function
of
wavelength.
The
results
are for a
typical
ozone
profile
in
January
at
57° N for
three cosines
of the
solar
zenith
angle (CZA).
The
column
abundance
of
ozone
is 350
Dobson
units
(DU).
1 DU
corresponds
to
10~
3
cm of gas at
standard
temperature
and
pressure
(S.T.P.).
After
Forster,
P.
M.,
and
Shine,
K.
P.,
1995,
"A
Comparison
of Two
Radiation
Schemes
for
Calculating
Ultraviolet
Radiation"
Q.
J.
Roy.
Meteorol.
Sac.
121,
1113.
(b)
(c)
Figure
10.25
(b)
Action
spectra
used
to
estimate
biologically
effec-
tive
UV
doses.
The
upper
and
lower
curves
refer
to
inhibition
of
photosynthesis
in
Antarctic phytoplankton
and DNA
damage,
re-
spectively,
(c) The
radiation amplification factor
for
DNA-effective
surface
irradiance
computed
for
noon
at
Palmer Station
during
the
Austral
springs 1988
and
1990.
(b) and (c)
after
Lubin,
D. et
al.,
1992,
"A
Contribution
Toward Understanding
the
Biospherical Sig-
nificance
of
Antarctic Ozone
Depletion."
J.
Geophys.
Res.
97,
7817.
390

Earth:
Human
Impact
391
surface
irradiance
computed
for
noon
at
Palmer Station during
the
Austral springs
1988
and
1990. Note
that
a 50%
reduction
in the
ozone column results
in a
300%
enhancement
in
DNA-effective irradiance
at the
surface.
The
absorption
of UV
energy
by
ozone heats
the
atmosphere
in the
ozone layer,
creating
a
large temperature gradient.
The
thermal
stratification
of the
stratosphere
is
in
fact
caused
by
ozone
itself.
One
consequence
of
this
stratification
is the
inhibition
of
vertical
motion,
thereby creating
a
stable
and
quiescent environment
for the
storage
of
ozone.
A
fundamental
understanding
of the
distribution
of
stratospheric ozone requires
an
understanding
of
photochemistry, radiation,
and
dynamics
and
their
interactions. This
is
a
problem
of
considerable
intricacy
and
complexity,
but due to
recent
intensive
research
efforts,
considerable progress
has
been made. Despite
the
great advance
of
current
knowledge,
we
believe
that
the
future
may
continue
to
hold surprises.
It is
perhaps
a
sobering
fact
that
the
Antarctic ozone hole
was
never predicted before
its
discovery
in
1985.
The
crux
of
stratospheric chemistry
is
concerned
with
the
sources
and
sinks
of 03.
The
core
of the
photochemistry
of
Oj
is the
Chapman chemistry
involving
pure oxygen
species,
O, O2, and 03. The
importance
of the
catalytic chemistry
of
HO*,
NO
r
,
and
halogens
for
destroying
03 is now
recognized.
The
problem acquires
a
deeper
sense
of
urgency when some
of
these catalytic agents
are
known
to be
derived from
our
industrial
activities. Heterogeneous chemistry plays
an
important role
in the
chemistry
of the
polar stratosphere.
The
recognition
of our
adverse impact
on the
ozone layer
prompted
an
intensive
effort
in the
photochemistry
and
chemical kinetics
of
simple
molecules
of
importance
to the
stratosphere. This extensive database
is
summarized
in
four
tables
in the
appendix
of
this
chapter. Photolysis reactions
in
Earth's atmosphere
are
presented
in
table 10.A1. Binary
and
ternary reactions
are
given
in
tables 10.A2
and
10.A3,
respectively.
Equilibrium
constants
for
selected reactions
are
listed
in
table
10.
A4.
10.4.1
Chapman Chemistry
First
proposed
by
Chapman
in
1930,
this theory
gives
the
correct
first-order
explanation
of
the
ozone layer,
which
was
discovered
at the
time.
The
basic concepts
of the
Chapman
theory
are as
beautiful
as
they
are
simple. Consider
an
atmosphere containing
O2-
Absorption
of
solar
UV
radiation below
240
nm
leads
to
photolysis:
The O
atom combines
with
C«2
in a
three body reaction forming
03:
Ozone
is
removed
by
photolysis:
This
is the
reaction that
is
responsible
for
absorbing
the
bulk
of UV
solar radiation
in
the
stratosphere. Note
that
reaction (10.29)
is not a
permanent
sink
for
ozone, because
392
Photochemistry
of
Planetary
Atmospheres
the
most
likely
fate
of the O
atom
it
produces
is to
react with
02 via
reaction (10.16)
and
restore
03. It is
convenient
to
define
odd
oxygen,
O
x
,
as the sum of O and 03.
Reactions such
as
(10.16)
and
(10.29)
turn
one odd
oxygen into another
odd
oxygen.
There
is no net
production
or
destruction
of
O*.
We may
consider
the
(chemically)
net
nothing cycle
as a
"catalytic
cycle"
for
converting solar
UV
energy into thermal
energy:
The
beauty
of the
ozone layer
is
that
the
absorber
(63)
is not
efficiently
destroyed
by
the
UV
radiation,
and
this accounts
for the
extraordinary effectiveness
of
ozone
for
blocking
UV
radiation. Ultimately ozone
(odd
oxygen)
is
removed
by the
reactions
The set of five
reactions listed above,
(10.16)
and
(10.28)-(
10.31),
is
known
as
Chap-
man
chemistry.
It can be
shown
that
reaction (10.31)
is not
important
in the
part
of
the
stratosphere where ozone concentrations
are
maximum.
In
this
case
there
are
approximate analytic solutions
for the
concentrations
of O and
O^
as
defined
by the
Chapman chemistry:
Note
that
the
expression
for
63
concentration
in
(10.33)
implies
the
existence
of an
ozone layer.
At
high
altitudes,
the
values
of M and
[O
2
]
are
small, implying that
[03]
will
be
small.
In the
deep atmosphere
J\ is
small
(all
the
photons
are
absorbed
by
the
overlying
O
2
column),
and
[03]
will also
be
small. Maximum values
of
[03]
are
attained
in the
stratosphere.
Chapman chemistry provides
a
generally accurate model
of
ozone concentrations
(to
within
a
factor
of 2)
above
the
ozone peak. However, there
are at
least
two
weaknesses
of
the
theory. First,
in the
lower stratosphere below
the
ozone peak
the
lifetime
of 03
becomes long compared
with
transport. Dynamics play
a
crucial role
in
determining
the
seasonal
and
latitudinal distributions
of
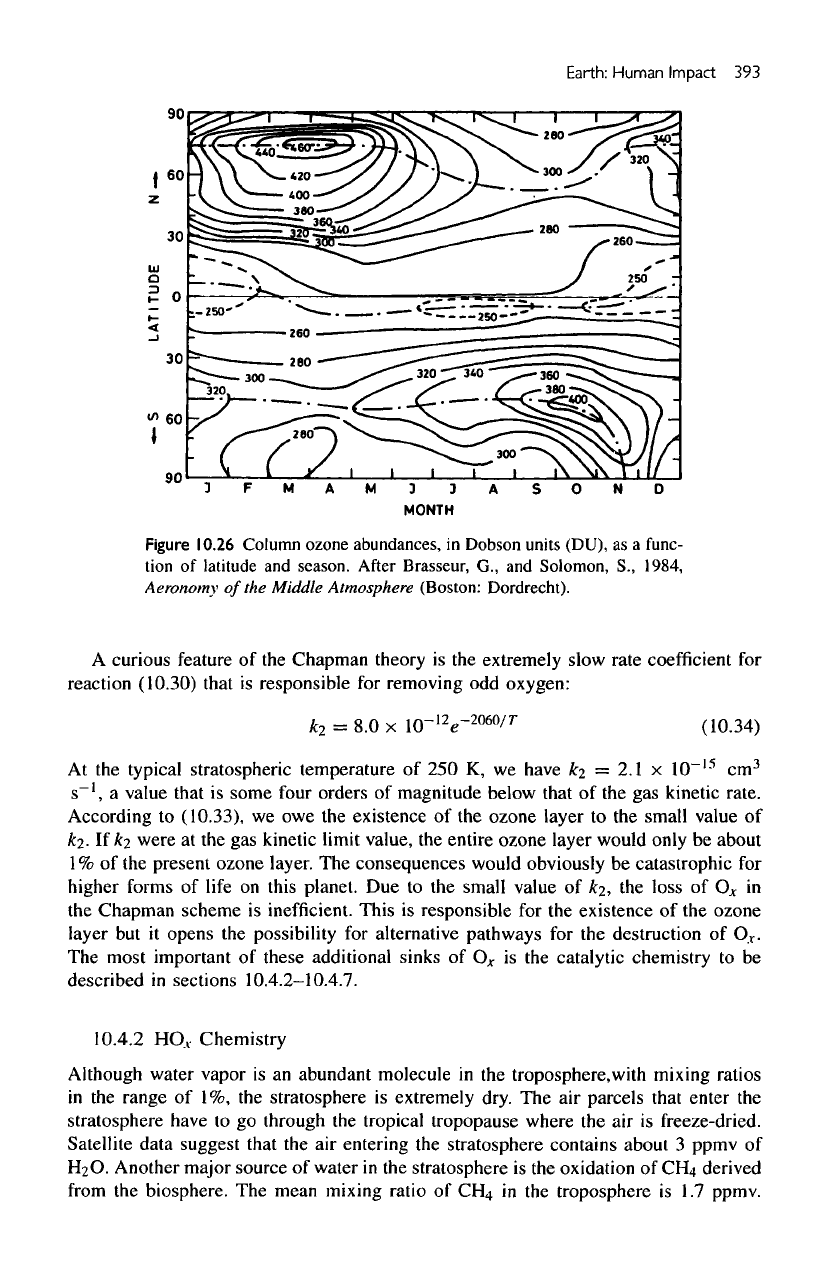
ozone. Figure
10.26
presents
the
zonally
averaged observed column abundance
of
atmospheric ozone
as a
function
of
latitude
and
time
of the
year. Note
the low
column densities
in the
tropics (where
03 is
produced
by
Chapman chemistry)
and the
higher column densities
in the
polar regions.
This
distribution
is the
opposite
of
what
a
photochemical theory
of
ozone would
predict.
The
spatial
and
temporal variations
of
ozone,
as
displayed
in figure
10.26,
are
the
result
of the
dynamical motion
of the
lower atmosphere.
Earth:
Human
Impact
393
Figure
10.26
Column ozone abundances,
in
Dobson units (DU),
as a
func-
tion
of
latitude
and
season.
After
Brasseur,
G., and
Solomon,
S.,
1984,
Aeronomy
of the
Middle
Atmosphere
(Boston: Dordrecht).
A
curious feature
of the
Chapman theory
is the
extremely slow rate
coefficient
for
reaction
(10.30)
that
is
responsible
for
removing
odd
oxygen:
At
the
typical stratospheric temperature
of 250 K, we
have
#2
= 2.1 x
10~
15
cm
3
s~',
a
value that
is
some
four orders
of
magnitude below that
of the gas
kinetic rate.
According
to
(10.33),
we owe the
existence
of the
ozone
layer
to the
small value
of
#2-
If
&2
were
at the gas
kinetic
limit
value,
the
entire
ozone
layer would only
be
about
1
% of the
present
ozone
layer.
The
consequences would obviously
be
catastrophic
for
higher forms
of
life
on
this planet.
Due to the
small value
of
k
2
,
the
loss
of
O^
in
the
Chapman scheme
is
inefficient.
This
is
responsible
for the
existence
of the
ozone
layer
but it
opens
the
possibility
for
alternative pathways
for the
destruction
of
O
r
.
The
most important
of
these additional sinks
of
O^
is the
catalytic chemistry
to be
described
in
sections
10.4.2-10.4.7.
10.4.2
HO
A
.
Chemistry
Although
water vapor
is an
abundant molecule
in the
troposphere,with
mixing
ratios
in
the
range
of 1%, the
stratosphere
is
extremely dry.
The air
parcels
that
enter
the
stratosphere have
to go
through
the
tropical tropopause where
the air is
freeze-dried.
Satellite
data suggest that
the air
entering
the
stratosphere contains about
3
ppmv
of
H2O.
Another major source
of
water
in the
stratosphere
is the
oxidation
of
CH
4
derived
from
the
biosphere.
The
mean
mixing
ratio
of
CH
4
in the
troposphere
is
1.7
ppmv.
394
Photochemistry
of
Planetary
Atmospheres
Since
CH4 is
long-lived
and
does
not
condense
at the
tropopause,
it
readily enters
the
stratosphere, where
on
oxidation
[the
chemistry
is
similar
to
schemes
(Ha)
and
(lib)]
it
is
converted into
3.4
ppmv
of
H
2
O.
Thus,
the
concentration
of H2O
increases
with
altitude
in the
stratosphere.
HO*
radicals
are
generated
by
reaction (10.3) between
O('D)
and
H
2
O,
where
the
O('D)
is
derived from
the
photolysis
of
O
3
(10.2).
OH
reacts
with
O and
O
3
:
The H
atom produced
in
(10.35) readily combines
with
O
2
via
(10.6)
to
form
HO
2
,
or it may
react
with
O
3
:
HO
2
may
also react
with
NO
(10.8)
or
with
O or 03:
The
reaction
provides
a
major
sink
for
HO,-
in the
stratosphere.
In the
lower stratosphere,
HO*
is
also removed (catalytically)
by
reactions
with
oxides
of
nitrogen
(see
section
10.4.3
on
the
chemistry
of odd
nitrogen compounds),
including
(10.13)
and
The
catalytic destruction
of
ozone
(O
v
)
by
HO
V
radicals
may be
summarized
by
three
cycles:
HO.v
catalysis,
(Ilia)
and
(Illb),
is
most important
in the
upper stratosphere. Scheme
(IIIc)
is
more important
in the
lower stratosphere.
A
schematic diagram summarizing
the
chemical pathways connecting
the
major
HO.
V
species
is
given
in
figure
10.27a.
The
concentrations
of the
major
HO^
species
(OH,
HO
2
)
are
shown
in figure
10.27b.
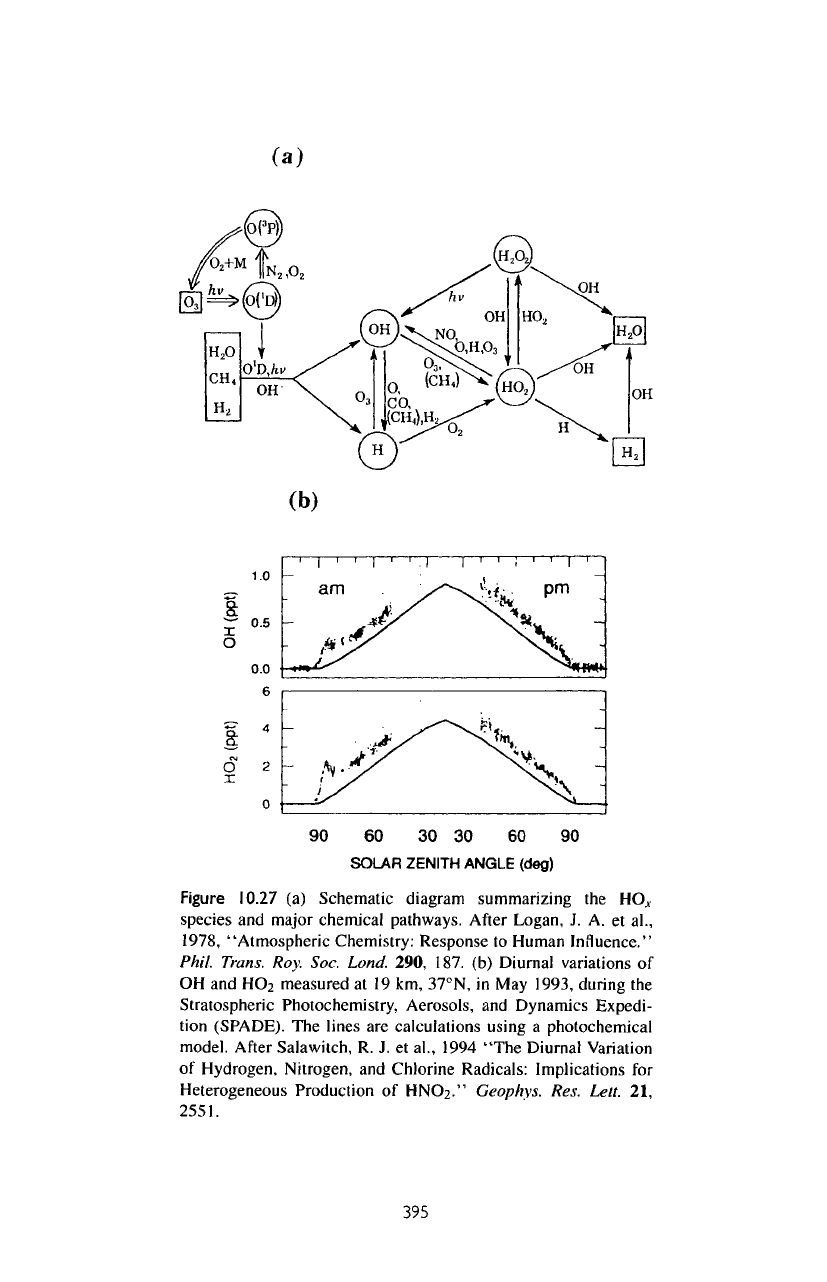
(a)
(b)
Figure
10.27
(a)
Schematic diagram summarizing
the
HO.,
species
and
major chemical pathways. After Logan,
J. A. et
al.,
1978, "Atmospheric Chemistry: Response
to
Human Influence."
Phil.
Trans. Roy. Soc.
Land.
290, 187.
(b)
Diurnal variations
of
OH and
HO
2
measured
at 19 km,
37°N,
in May
1993, during
the
Stratospheric Photochemistry, Aerosols,
and
Dynamics Expedi-
tion
(SPADE).
The
lines
are
calculations using
a
photochemical
model.
After
Salawitch,
R. J. et
al.,
1994 "The Diurnal Variation
of
Hydrogen, Nitrogen,
and
Chlorine Radicals: Implications
for
Heterogeneous Production
of
HNOz."
Geophvs.
Res.
Lett.
21,
2551.
395
