Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

346
Photochemistry
of
Planetary
Atmospheres
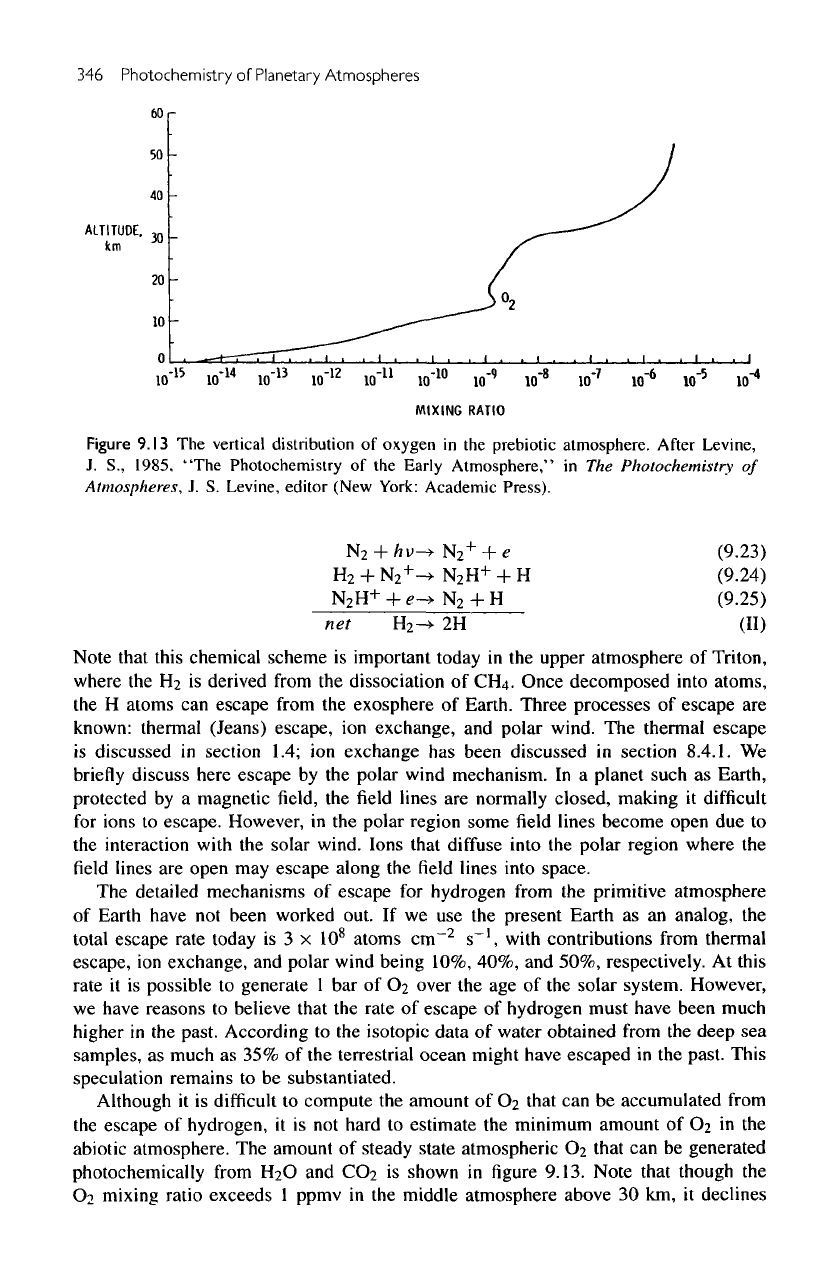
Figure
9.13
The
vertical
distribution
of
oxygen
in the
prebiotic atmosphere.
After
Levine,
J.
S.,
1985, "The Photochemistry
of the
Early
Atmosphere,"
in The
Photochemistry
of
Atmospheres,
J. S.
Levine, editor (New York: Academic Press).
Note that this chemical scheme
is
important today
in the
upper atmosphere
of
Triton,
where
the
H
2
is
derived
from
the
dissociation
of
CH4. Once
decomposed
into atoms,
the
H
atoms
can
escape
from
the
exosphere
of
Earth.
Three
processes
of
escape
are
known:
thermal (Jeans)
escape,
ion
exchange,
and
polar wind.
The
thermal
escape
is
discussed
in
section 1.4;
ion
exchange
has
been discussed
in
section
8.4.1.
We
briefly
discuss here
escape
by the
polar wind mechanism.
In a
planet such
as
Earth,
protected
by a
magnetic
field, the field
lines
are
normally
closed,
making
it
difficult
for
ions
to
escape.
However,
in the
polar region
some
field
lines
become
open
due to
the
interaction
with
the
solar wind. Ions that
diffuse
into
the
polar region where
the
field
lines
are
open
may
escape
along
the field
lines into
space.
The
detailed mechanisms
of
escape
for
hydrogen from
the
primitive atmosphere
of
Earth have
not
been worked out.
If we use the
present Earth
as an
analog,
the
total
escape
rate today
is 3 x
10
8
atoms
cm""
2
s~',
with contributions from thermal
escape,
ion
exchange,
and
polar wind being 10%, 40%,
and
50%, respectively.
At
this
rate
it is
possible
to
generate
1 bar of
O
2
over
the age of the
solar system. However,
we
have reasons
to
believe that
the
rate
of
escape
of
hydrogen must have been much
higher
in the
past. According
to the
isotopic data
of
water obtained from
the
deep
sea
samples,
as
much
as 35% of the
terrestrial ocean might have
escaped
in the
past. This
speculation
remains
to be
substantiated.
Although
it is
difficult
to
compute
the
amount
of
O
2
that
can be
accumulated from
the
escape
of
hydrogen,
it is not
hard
to
estimate
the
minimum amount
of
O
2
in the
abiotic
atmosphere.
The
amount
of
steady state atmospheric
O
2
that
can be
generated
photochemically
from
H
2
O
and
CO
2
is
shown
in figure
9.13. Note that though
the
O
2
mixing
ratio exceeds
1
ppmv
in the
middle atmosphere above
30 km, it
declines

Earth:
Imprint
of
Life
347
rapidly
in the
lower atmosphere.
The
reason
is
that
C>2
is not
stable against reactions
that
restore
it to
CO?
and
H->O.
9.5.2
Oxygen
cycle
The
amount
of
dead organic matter
in the
ocean
is
large
(3000 Gt-C)
compared
with
the
living
marine
biomass
(3
Gt-C). This large reservoir
is
formed
by the
rain
of
organic detritus from
the
biologically productive surface oceans
at the
rate
of 4
Gt-C
yr~'.
This
is
equivalent
to 1.3 x
10
12
QI
molecules
cm~
2
s~',
or
about
10
4
times
the
rate
that
O2 is
generated
by the
escape
of
hydrogen from
the
present atmosphere.
If
the
deep
oceans
were anoxic,
all
this organic carbon would
be
buried
in the
sediments.
At
this rate
all the
O?.
in the
present atmosphere could
be
generated
in 0.3
Myr.
The
actual
rate
of
generation
of O2 is
much less since
the
oceans
are not
anoxic
(at
least
in
the
present epoch)
and is
equal
to 0.2
Gt-C
yr~'.
Even
in
anoxic environments,
not
all the
organics
can be
preserved.
The
bulk
of
organic material
(95%)
is
returned
to the
atmosphere
as
CH^
which
is
then readily oxidized
to CO2
(see
chapter
10).
The net
rate
of
oxygen generation given
the
processes
described above must
be
bal-
anced
by a
similar rate
of
oxygen loss, yielding
a
lifetime
for
atmospheric oxygen
of
6
Myr.
We
argue
that
the
production
of
oxygen
by the
burial
of
photosynthetically derived
organic carbon
is an
inevitable consequence
of the
physical
properties
of
carbon
and
oxygen:
the
enormous
solubility
of CO2 in
water
and the
extreme insolubility
of
©2
in
water. According
to figure
9.4,
the
partitioning
of
CO?
between
the
atmosphere
and
the
ocean
is
1:50;
that
is, 50
times
as
much
CO2
resides
in the
ocean
(mainly
in the
form
of
HCO.-)~)
as in the
atmosphere.
For O2 the
partitioning between
the
atmosphere
and
the
ocean
is
140:1;
that
is, the
atmosphere contains
140
times
as
much
O
2
as the
ocean.
The
difference
is
therefore
a
factor
of
7000
in the
partitioning
of CO2 and
O2
between
the
atmospheric
and
oceanic reservoirs. Since
it is so
difficult
to
deliver
O2
to the
aquatic medium, there
is a
tendency
for it to
become anoxic, thus creating
an
ideal environment
for the
burial
and
preservation
of
organic carbon. Today,
the
bottoms
of the
oceans
are not
anoxic,
but in the
past there have been
periods
of
global
anoxia.
Earth's surface always
has a
substantial fraction
of
area
under marshes
and
wetland
that
are
largely anoxic
and
conducive
to the
preservation
of
organic carbon.
Thus,
we
view
the
rise
of
oxygen
on
this
planet
as the
inevitable consequence
of the
production
of
organic carbon
and the
ease
of
getting
it
buried.
We may
regard
the
enormous atmospheric concentration
of O2 as a way the
system tries
to
make
up for
the
insolubility
of O2 in
water.
In
other words,
if O2
were
7000
times more soluble
in
water
than
CO
2
,
then
its
mixing
ratio would
be
7000
times
less,
making
its
abundance
the
same order
of
magnitude
as
CO2.
But
then
the
bulk
of the
oxygen would
be in the
ocean rather
than
in the
atmosphere.
All
advanced
life
would
be
marine rather than
continental.
The
burial
of
organic carbon
in the
sediments
produces
and
regulates atmospheric
O2,
but its
time constant
is of the
order
of
millions
of
years.
For
shorter time
scales
other regulation mechanisms must
be
important.
The
photosynthetic time constant
for
atmospheric
©2
is
only
1000
yr. It is
believed
that
forest
fires act as
regulator
of
O
2
.
Laboratory experiments have shown
that
spontaneous combustion
of
even living plants
can
start when
the
atmospheric
OT
concentrations exceed about
25%.
Conversely,
348
Photochemistry
of
Planetary
Atmospheres
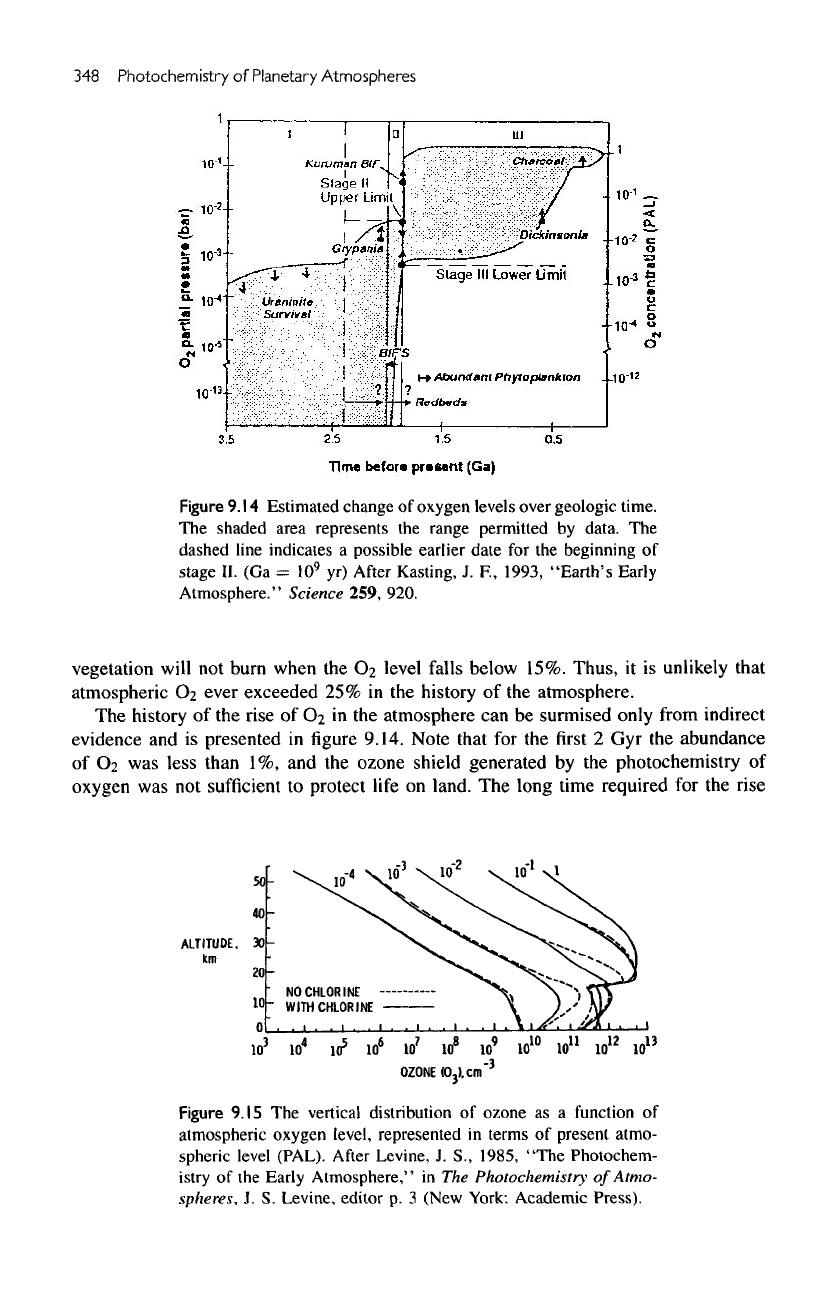
Figure
9.14
Estimated change
of
oxygen levels over
geologic
time.
The
shaded area represents
the
range permitted
by
data.
The
dashed
line
indicates
a
possible earlier date
for the
beginning
of
stage
II. (Ga =
10
9
yr)
After
Kasting,
J. F,
1993,
"Earth's
Early
Atmosphere." Science 259, 920.
vegetation
will
not
burn
when
the
62
level
falls
below
15%.
Thus,
it is
unlikely
that
atmospheric
QI
ever
exceeded
25% in the
history
of the
atmosphere.
The
history
of the
rise
of
C>2
in the
atmosphere
can be
surmised
only
from
indirect
evidence
and is
presented
in
figure 9.14.
Note
that
for the first 2 Gyr the
abundance
of
02 was
less
than
1%, and the
ozone
shield
generated
by the
photochemistry
of
oxygen
was not
sufficient
to
protect
life
on
land.
The
long
time
required
for the
rise
Figure
9.15
The
vertical distribution
of
ozone
as a
function
of
atmospheric
oxygen level, represented
in
terms
of
present atmo-
spheric level (PAL).
After
Levine,
J.
S.,
1985, "The Photochem-
istry
of the
Early Atmosphere,"
in The
Photochemistry
of
Atmo-
spheres,
S. S.
Levine, editor
p. 3
(New York:
Academic
Press).

Earth:
Imprint
of
Life
349
of
atmospheric
O
2
is
attributed
to the
large quantities
of
reduced material present
on
the
surface
of the
early Earth, such
as
ferrous iron.
The
initial
production
of
O
2
was
consumed
by the
oxidation
of
ferrous
to
ferric iron, depositing
the
well-known banded
iron
formations.
It was not
until
the
reduced iron inventories
were
exhausted that there
was a net
accumulation
of
O
2
in the
atmosphere.
The
abundance
and
distribution
of
ozone
at
different
atmospheric oxygen levels
are
shown
in
figure
9.15.
It is a
matter
of
conjecture
how
early
life
on
Earth
coped
with
the
lack
of an
ozone layer.
One
obvious solution
is in the
depth
of the
oceans.
Ocean
water attenuates radiation, such that
by 100
m
depth only
1% of the
incident solar
radiation gets transmitted.
In
addition,
the
filtering
is
more
efficient
for
ultraviolet
and
infrared
radiation. Another possibility
has
been demonstrated
in the
laboratory using
algae:
the
buildup
of a
layer
of
algal mats such that
the
dead
algae above provides
shielding
for the
living
algae
beneath.
9.6
Nitrogen
Cycle
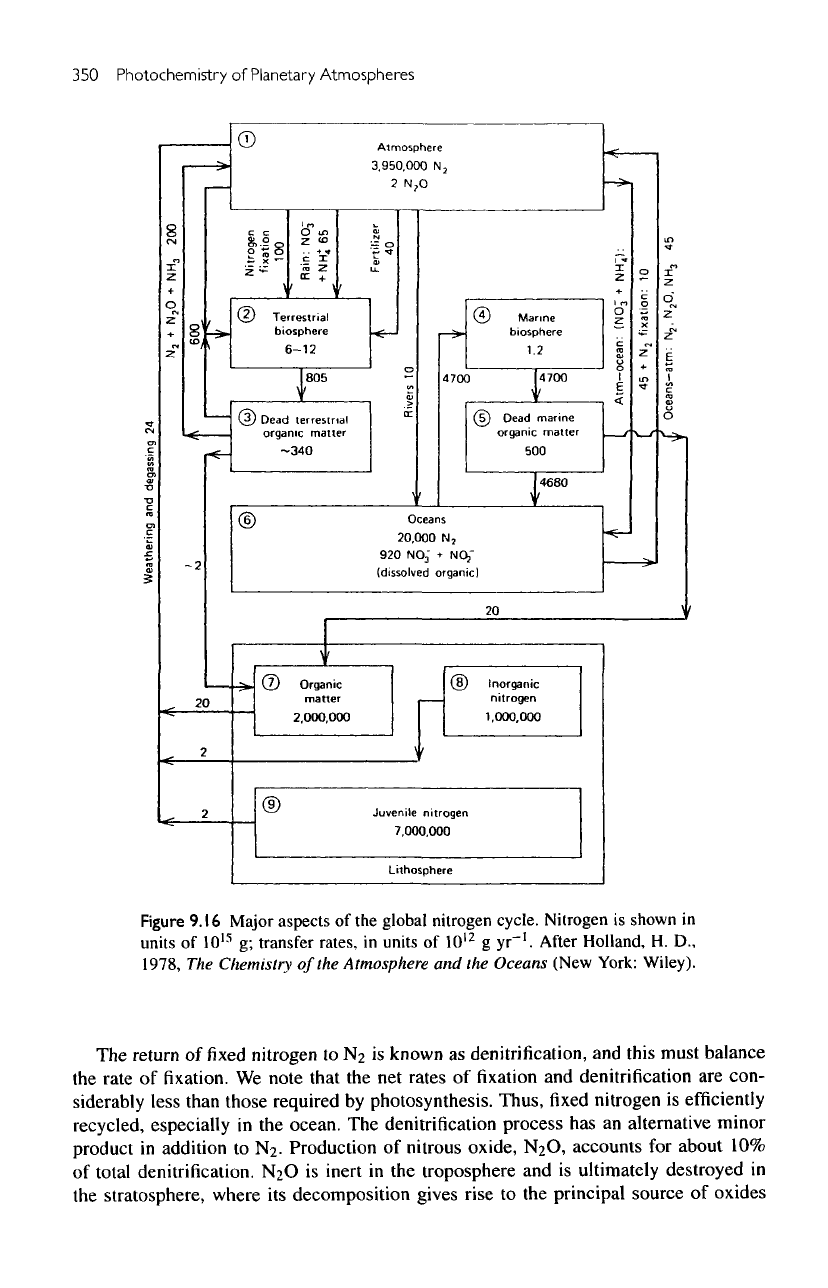
The
nitrogen cycle
of the
biosphere
is
closely related
to the
carbon cycle.
The
C/N
ratios
are 92 and 6.7 for
terrestrial
and
marine plants, respectively. Hence,
the
nitrogen
cycle,
as
shown
in figure
9.16,
is to first
order
a
scaled
down carbon cycle.
To
sustain
the
rate
of
photosynthesis
as
shown
in figure
9.4,
the
demands
for
nitrogen
by the
land
and
ocean
biosphere
are 600 and
4700
Mt-N
yr~',
respectively.
The
bulk
of
nitrogen
in the
surface reservoir
resides
in the
atmosphere
as N2, a
rather inert molecule
chemically.
To be
useful biologically,
the
strong
N—N
bond must
first be
broken,
a
process
known
as
nitrogen
fixation. In the
absence
of
biology, lightning provides
a
background source
of fixed
nitrogen.
In the
lightning
fireball the air
parcel temperature
is
raised
to
10,000
K,
resulting
in the
production
of NO via the
following equilibrium
chemistry:
The first
reaction
is
more
important
in the
present atmosphere with
O
2
as the
oxidant.
The
second reaction
is
more
important
in the
primitive terrestrial atmosphere before
the
rise
of
©2-
This reaction
is
potentially important
also
in the
present atmospheres
of
Mars
and
Venus.
On
cooling
the
equilibrium composition given
by
reactions
(9.26)
and
(9.27)
is
"frozen"
at
about
2000
K and NO is
released into
the
atmosphere.
NO
is
subsequently oxidized
by
atmospheric reactions
to
higher oxides
of
nitrogen
and
eventually
gets
rained
out as
nitrate. This global
source
is
estimated
to be
5-10
Mt-N
yr~'.
The
principal
source
of fixed
nitrogen
is
biological,
via
microbes that live
in
symbiosis with land plants
and
marine organisms.
The
total
input
of fixed
nitrogen
to
the
land
biosphere
is
about
200
Mt-N
yr~',
of
which
100 Mt is fixed
biologically,
65 is via
rain,
and 40 is
from manmade fertilizers
(the
subject
of
anthropogenic
perturbations
is
addressed
in
chapter
10).
The
total
input
into
the
ocean biosphere
is
85
Mt-N
yr~',
of
which
30 Mt is fixed in
situ,
45 is
from rain,
and 10 is
from river
runoffs.
350
Photochemistry
of
Planetary
Atmospheres
Figure
9.16
Major aspects
of the
global nitrogen cycle. Nitrogen
is
shown
in
units
of
10
15
g;
transfer rates,
in
units
of
10
12
g
yr"
1
.
After
Holland,
H. D.,
1978,
The
Chemistry
of the
Atmosphere
and
the
Oceans (New York: Wiley).
The
return
of fixed
nitrogen
to N2 is
known
as
denitrification,
and
this must
balance
the
rate
of fixation. We
note
that
the net
rates
of fixation and
denitrification
are
con-
siderably
less than those required
by
photosynthesis. Thus,
fixed
nitrogen
is
efficiently
recycled,
especially
in the
ocean.
The
denitrification
process
has an
alternative minor
product
in
addition
to
Na-
Production
of
nitrous
oxide, N2O, accounts
for
about
10%
of
total
denitrification.
N2<3
is
inert
in the
troposphere
and is
ultimately
destroyed
in
the
stratosphere, where
its
decomposition gives rise
to the
principal source
of
oxides

Earth:
Imprint
of
Life
351
Table
9.7
Estimates
of
global emission
to the
atmosphere
of
gaseous
sulfur
compounds
Source
Annual
flux
(Tg)
Anthropogenic
(mainly
SC>2
from fossil
fuel
combustion)
80
Biomass
burning
(SC>2)
7
Oceans
(DMS)
40
Soils
and
plants
(H
2
S,
DMS)
10
Volcanoes
(H
2
S,
SO
2
)
10
Total
147
±1.4
From
Intergovernmental
Panel
on
Climate Change (1990).
The
uncertainly
ranges
are
estimated
to
be
about
30% for the
anthropogenic
flux and a
factor
of 2 for the
natural
fluxes.
of
nitrogen. This topic
is
pursued
in
greater detail
in
chapter
10.
There
is a net
loss
of
nitrogen from
the
atmosphere-ocean reservoirs when
the
organic material
is
buried
in
the
sediments,
at a
rate
of 22 Mt
yr~',
which must
be
balanced
by the
recovery
of
nitrogen from
the
weathering
of
sedimentary rocks.
The
lifetime
for
cycling atmo-
spheric
N2 by
this
process
is 2 x
10
8
yr, a
value
that
is
about
two
orders
of
magnitude
larger
than
that
for
02.
9.7
Minor
Elements
In
addition
to the
major elements carbon, oxygen,
and
nitrogen, organic matter also
contains
smaller amounts
of
other elements such
as
sulfur
and
phosphorus.
As
part
of the
biospheric cycle
of
organic matter,
the
trace elements
are
cycled through
the
atmosphere.
The
marine organisms have unusual abilities
to
concentrate large amounts
of
halogens
in
their bodies,
and on
death
some
of the
halogens
are
released
to the
atmosphere
as
methyl
halidcs.
9.7.1 Sulfur
cycle
The
bulk
of
sulfur
in the
surface reservoirs
of the
planet resides
in the
ocean
as
sulfate,
with
mean concentration
of
2.71
g
kg"
1
.
The
total
sulfur
content
of the
oceans
amounts
to 1.3 x
10
21
g, or
about
a
quarter
of the
total mass
of the
entire atmosphere.
The
total
amount
of
sulfur
in the
atmosphere, about
3.6
x
10
12
g, is
utterly
trivial
compared
with
the
oceanic reservoir. Estimates
of the
global emissions
of
gaseous
compounds
to the
atmosphere
are
summarized
in
table
9.7.
The sum
total
of the
emission rates
is 147
Mt-S
yr"
1
,
with
the
largest contribution derived from combustion
of
fossil
fuel.
We
discuss
the
anthropogenic impact
of
sulfur
emission
on the
atmosphere
in
chapter
10.
The
largest
natural
source
of
atmospheric
sulfur
appears
to be
dimethyl
sulfide
(DMS)
emitted
by
marine organisms. Volcanoes
are
sporadic sources; their
influence
is
small
on the
average
but can be
large
in the
years following
an
eruption.
The
ultimate
fate
of all
reduced
sulfur
species (such
as
H
2
S
and
DMS)
is
oxidation
to
SO2,
followed
by the
oxidation
of
SO
2
to
SOj.
The
terminal product
is
H2SO4
aerosol.
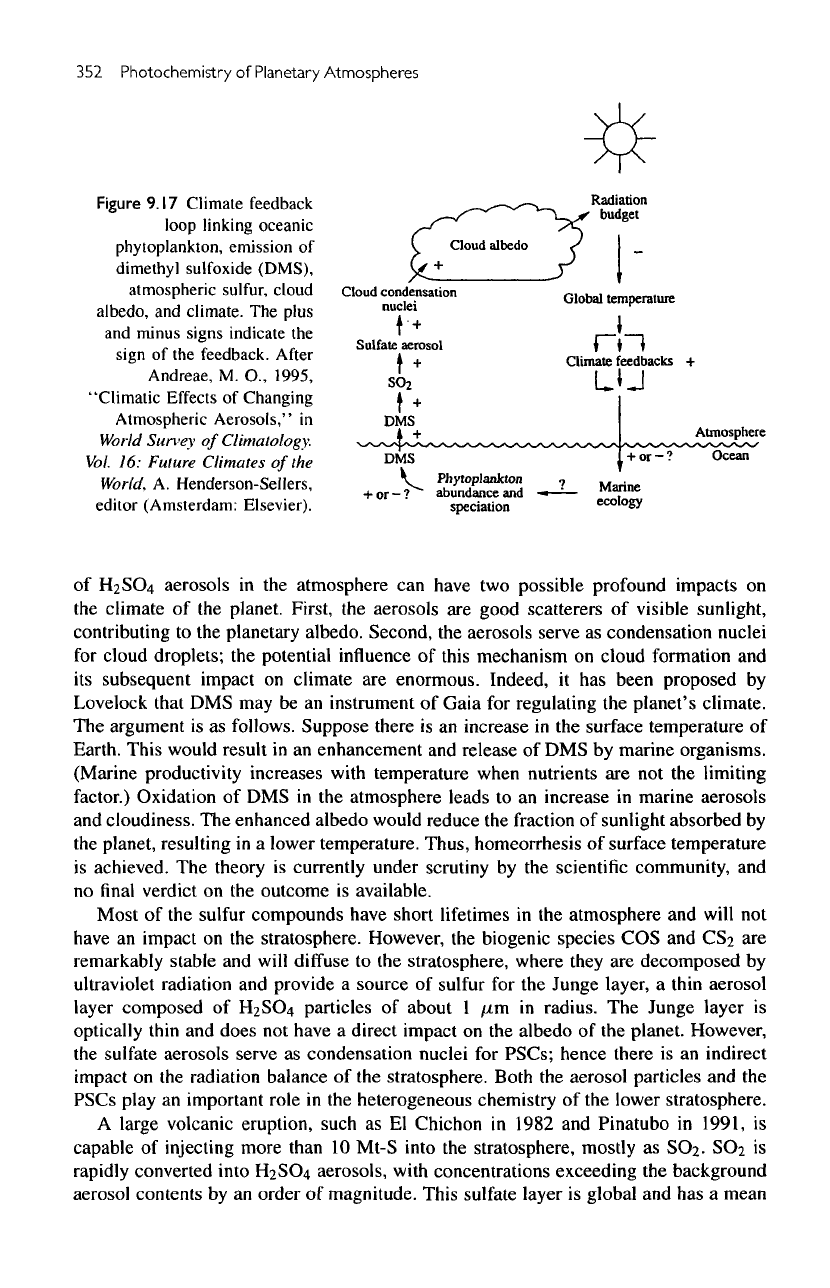
A
schematic
of the
chemical pathways
is
shown
in figure
9.17.
The
presence
352
Photochemistry
of
Planetary
Atmospheres
Figure
9.17
Climate feedback
loop
linking
oceanic
phytoplankton,
emission
of
dimethyl
sulfoxide
(DMS),
atmospheric
sulfur,
cloud
albedo,
and
climate.
The
plus
and
minus signs indicate
the
sign
of the
feedback. After
Andreae,
M.
O.,
1995,
"Climatic
Effects
of
Changing
Atmospheric
Aerosols,"
in
World
Suney
of
Climatology.
Vol.
16:
Future
Climates
of the
World,
A.
Henderson-Sellers,
editor
(Amsterdam: Elsevier).
of
H2SC>4
aerosols
in the
atmosphere
can
have
two
possible profound impacts
on
the
climate
of the
planet. First,
the
aerosols
are
good scatterers
of
visible sunlight,
contributing
to the
planetary albedo. Second,
the
aerosols
serve
as
condensation nuclei
for
cloud droplets;
the
potential influence
of
this mechanism
on
cloud formation
and
its
subsequent impact
on
climate
are
enormous. Indeed,
it has
been
proposed
by
Lovelock that
DMS may be an
instrument
of
Gaia
for
regulating
the
planet's climate.
The
argument
is as
follows. Suppose there
is an
increase
in the
surface temperature
of
Earth. This would result
in an
enhancement
and
release
of DMS by
marine organisms.
(Marine productivity increases
with
temperature when nutrients
are not the
limiting
factor.)
Oxidation
of DMS in the
atmosphere leads
to an
increase
in
marine aerosols
and
cloudiness.
The
enhanced albedo would reduce
the
fraction
of
sunlight absorbed
by
the
planet, resulting
in a
lower temperature. Thus,
homeorrhesis
of
surface temperature
is
achieved.
The
theory
is
currently under scrutiny
by the
scientific community,
and
no final
verdict
on the
outcome
is
available.
Most
of the
sulfur
compounds have short lifetimes
in the
atmosphere
and
will
not
have
an
impact
on the
stratosphere. However,
the
biogenic
species
COS and
€82
are
remarkably
stable
and
will
diffuse
to the
stratosphere, where they
are
decomposed
by
ultraviolet
radiation
and
provide
a
source
of
sulfur
for the
Junge layer,
a
thin
aerosol
layer
composed
of
H2SO4 particles
of
about
1
(J.m
in
radius.
The
Junge layer
is
optically
thin
and
does
not
have
a
direct impact
on the
albedo
of the
planet. However,
the
sulfate
aerosols
serve
as
condensation nuclei
for
PSCs;
hence there
is an
indirect
impact
on the
radiation balance
of the
stratosphere. Both
the
aerosol
particles
and the
PSCs play
an
important role
in the
heterogeneous chemistry
of the
lower stratosphere.
A
large volcanic eruption, such
as El
Chichon
in
1982
and
Pinatubo
in
1991,
is
capable
of
injecting more than
10
Mt-S
into
the
stratosphere, mostly
as
SOa-
SO2 is
rapidly
converted into H2SO4 aerosols, with concentrations exceeding
the
background
aerosol contents
by an
order
of
magnitude. This sulfate layer
is
global
and has a
mean
Earth:
Imprint
of
Life
353
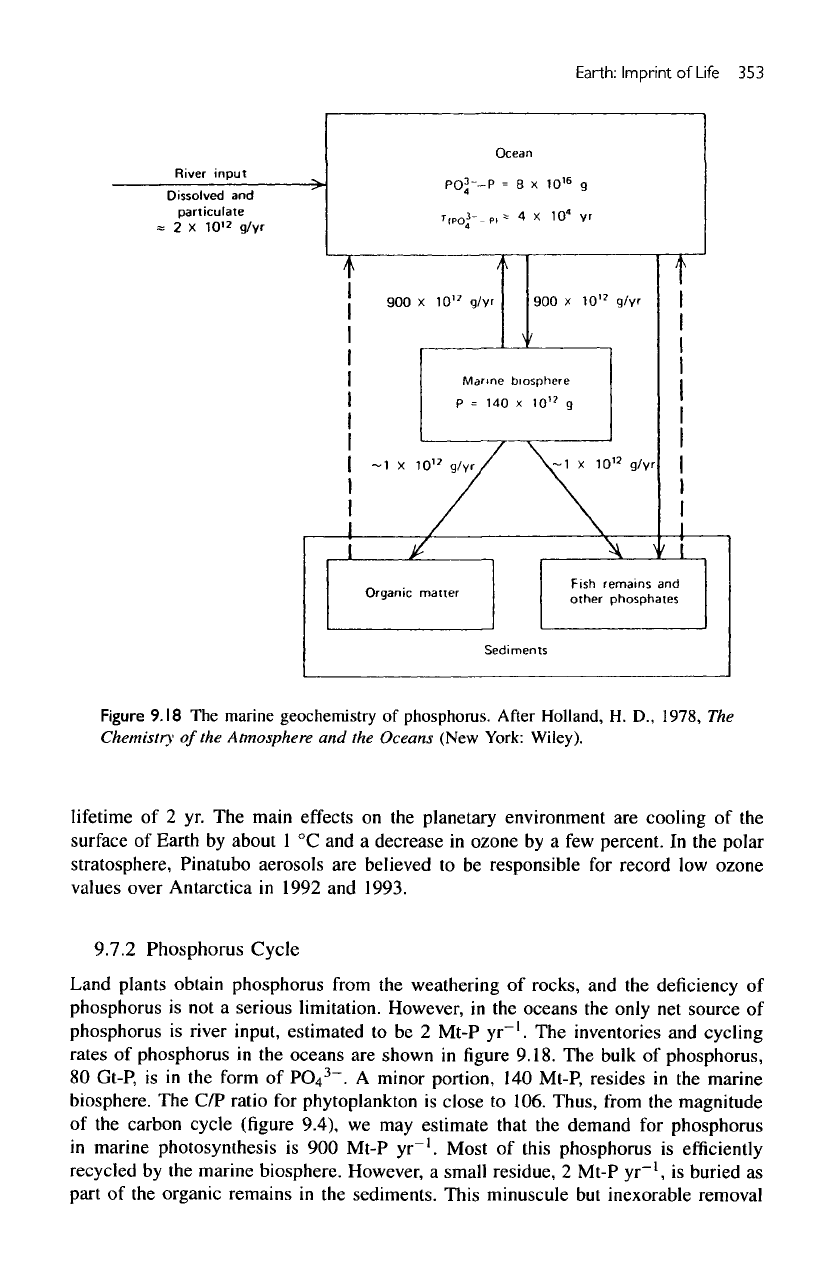
Figure
9.18
The
marine geochemistry
of
phosphorus.
After
Holland,
H. D.,
1978,
The
Chemistry
of the
Atmosphere
and the
Oceans (New York: Wiley).
lifetime
of 2 yr. The
main effects
on the
planetary environment
are
cooling
of the
surface
of
Earth
by
about
1 °C and a
decrease
in
ozone
by a few
percent.
In the
polar
stratosphere,
Pinatubo aerosols
are
believed
to be
responsible
for
record
low
ozone
values
over Antarctica
in
1992
and
1993.
9.7.2 Phosphorus
Cycle
Land
plants obtain phosphorus
from
the
weathering
of
rocks,
and the
deficiency
of
phosphorus
is not a
serious limitation. However,
in the
oceans
the
only
net
source
of
phosphorus
is
river
input,
estimated
to be 2
Mt-P
yr"
1
.
The
inventories
and
cycling
rates
of
phosphorus
in the
oceans
are
shown
in figure
9.18.
The
bulk
of
phosphorus,
80
Gt-P,
is in the
form
of
PO
4
3
~.
A
minor portion,
140
Mt-P, resides
in the
marine
biosphere.
The
C/P
ratio
for
phytoplankton
is
close
to
106. Thus,
from
the
magnitude
of the
carbon
cycle
(figure
9.4),
we may
estimate that
the
demand
for
phosphorus
in
marine photosynthesis
is 900
Mt-P
yr"
1
.
Most
of
this
phosphorus
is
efficiently
recycled
by the
marine biosphere. However,
a
small residue,
2
Mt-P
yr"
1
,
is
buried
as
part
of the
organic remains
in the
sediments. This minuscule
but
inexorable removal

354
Photochemistry
of
Planetary
Atmospheres
rate
would
eventually
deplete
the
oceans
of all
phosphorus
in 40
kyr
were
it not for
the
input
from rivers.
The
phosphorus cycle releases
no
known gaseous compounds,
and
the
atmosphere plays
no
role
in the
phosphorus cycle.
The
short time constant associated
with
the
depletion
of
phosphorus
has
been
the
basis
of an
interesting
theory
of the
ocean's
role
in
causing
the ice
age.
The
time
constant
for
phosphorus
is
intriguingly
close
to the
Milankovitch
time
scales
for
orbital
changes. According
to
Broecker's
idea,
the
oceans periodically receive
a
large
input
of
phosphorus
at the end of an ice age
when warm climate returns
and the
continental
shelves
are
re-submerged under water. This enhances
the
marine productivity, depleting
the
atmosphere
of
CC>2
via the
biological pump
and
sending
the
planet into another
ice
age. Eventually,
the
surplus phosphorus
is
used
up and the
marine productivity
slows down.
The
biological pump
now
becomes
less
effective,
and
CC>2
is
released
back
to the
atmosphere, marking
the end of an ice
age.
The
cycle
now
starts again
with
a new
injection
of
phosphorus
from
the
coastal shelves. This imaginative theory
of
the
biological modulation
of
Earth's
climate
has not
been
verified
by
available data.
On a
planetary scale phosphorus
is the
limiting
nutrient
that intimately couples
the
biospheric
productivity
to the
geochemical cycle
of
sedimentation, subduction,
mountain
building,
and
weathering.
The
former
is
driven
by the
energy
from
the
sun,
the
latter
by
solar energy
and
energy from
the
interior
of
Earth.
Is the
total productivity
of
the
biosphere ultimately limited
by the
geochemical cycle
of
phosphorus?
We can
imagine
a
planet
like
Mars that
initially
had a
biosphere, with
P as the
limiting
nutrient.
As the
planet
cools,
the
rate
of
turnover
of the
sediments
is
greatly reduced, trapping
most
of the
available phosphorus
in the
sediments. Eventually,
the
biosphere
dies
from
the
lack
of
tectonic activities
for
recycling phosphorus.
9.7.3
Organic
Halogen
The
oceans contain abundant supplies
of
halogens.
The
concentrations
of
Cl~,
Br~,
and
I~ are
19.4
g
kg"',
67
mg
kg"
1
and 59
/zg
kg"',
respectively.
The
direct transfer
of
halogens from
the
ocean
to the
atmosphere
is
small
and
includes
for
small amounts
present
in sea
spray. Most
of the
halogen
in the
marine
aerosols
remains immobilized
and
eventually
returns
to the
ocean
by
rainout. However,
a
small
fraction
may be
released
to the
atmosphere
by
Similar
reactions also apply
to
salts
of Br and I. The
release
of
chlorine into
the
atmosphere
as HC1
would
not
initiate
any
active chlorine chemistry because
HC1 is
eventually
removed
by
rainout
to the
oceans.
However,
if
reactive
Cl
atoms were
released
via
(9.29)
followed
by the
photolysis
of
CINCh,
there would
be an
impact
on
tropospheric
chemistry:
Cl
atoms
can
remove hydrocarbons
by
hydrogen abstraction.
The
subject
is
still
at a
somewhat speculative stage
at
present.
The
oceans
are a
source
of
three
important
organic
halides,
CHjCl,
CH.iBr,
and
CH.iI,
with
atmospheric concentrations equal
to
600,
20, and 1
parts
per
trillion
by
volume,
respectively.
CH.il
is
readily removed
by
photolysis
in the
troposphere.
Its
lifetime
is
only
a few
days
and the
molecule
has no
known impact
on the
chemistry
of
Earth:
Imprint
of
Life
355
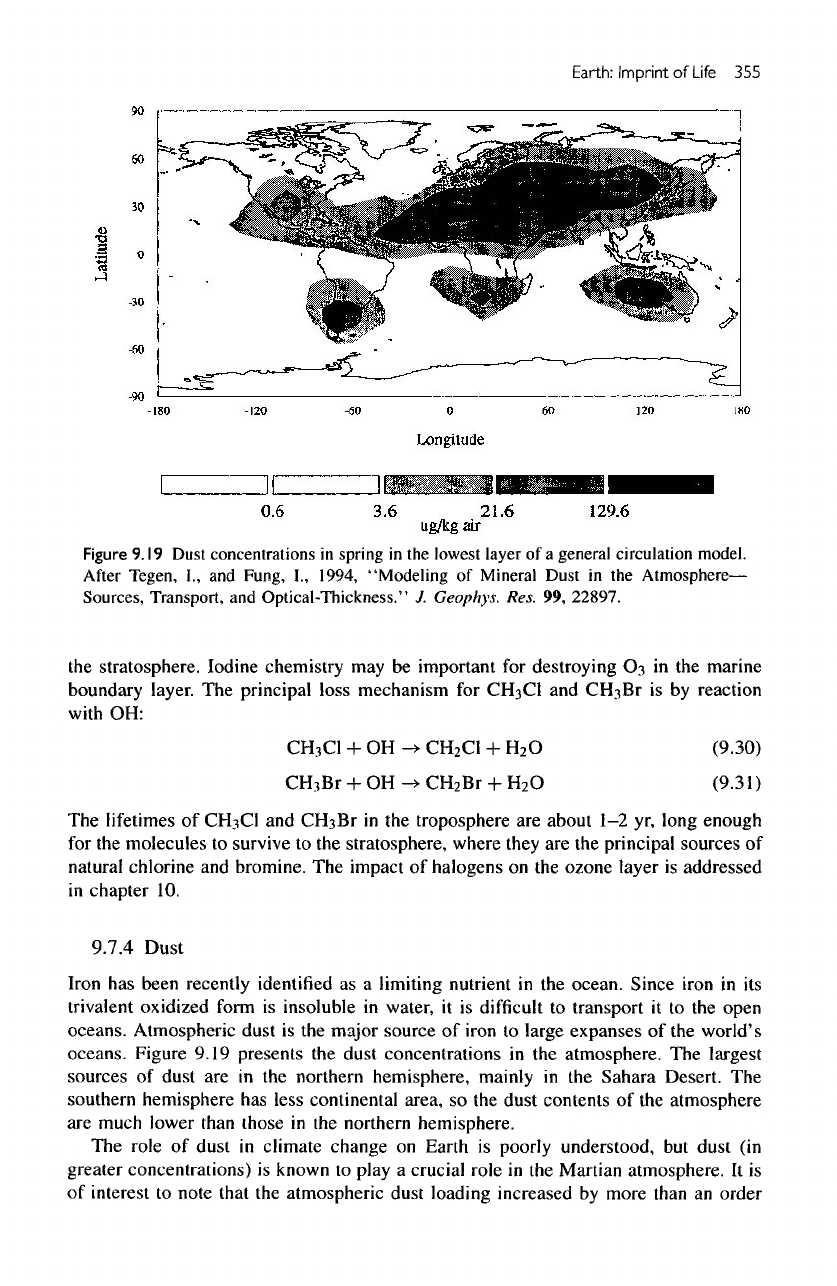
Figure
9.19
Dust
concentrations
in
spring
in the
lowest
layer
of a
general
circulation
model.
After
Tegen,
I.,
and
Fung,
I.,
1994, "Modeling
of
Mineral
Dust
in the
Atmosphere—
Sources,
Transport,
and
Optical-Thickness."
J.
Geophys.
Res.
99,
22897.
the
stratosphere. Iodine chemistry
may be
important
for
destroying
03 in the
marine
boundary
layer.
The
principal
loss
mechanism
for
CH3C1
and
CH^Br
is by
reaction
with
OH:
The
lifetimes
of
CHjCl
and
CH3Br
in the
troposphere
are
about
1-2 yr,
long enough
for
the
molecules
to
survive
to the
stratosphere, where they
are the
principal sources
of
natural
chlorine
and
bromine.
The
impact
of
halogens
on the
ozone layer
is
addressed
in
chapter
10.
9.7.4
Dust
Iron
has
been recently
identified
as a
limiting
nutrient
in the
ocean.
Since iron
in its
trivalent
oxidized form
is
insoluble
in
water,
it is
difficult
to
transport
it to the
open
oceans. Atmospheric dust
is the
major source
of
iron
to
large expanses
of the
world's
oceans. Figure 9.19 presents
the
dust concentrations
in the
atmosphere.
The
largest
sources
of
dust
are in the
northern hemisphere,
mainly
in the
Sahara Desert.
The
southern
hemisphere
has
less
continental
area,
so the
dust contents
of the
atmosphere
are
much lower than those
in the
northern hemisphere.
The
role
of
dust
in
climate change
on
Earth
is
poorly understood,
but
dust
(in
greater concentrations)
is
known
to
play
a
crucial role
in the
Martian atmosphere.
It is
of
interest
to
note that
the
atmospheric
dust
loading increased
by
more
than
an
order
