Weinan E. Principles of Multiscale Modeling
Подождите немного. Документ загружается.

4.1. CONTINUUM MECHANICS 157
Assume that h is a function of the form:
h(x, t) = h
0
(F, T, x), (4.1.53)
then
∂
t
h = ∇
F
h
0
: ∂
t
F +
∂h
0
∂T
∂
t
T (4.1.54)
From (4.1.52), we also have
∂
t
h = ∂
t
e − s∂
t
T − T ∂
t
s.
In terms of F, the energy conservation law (4.1.49) can be expressed as
ρ
0
∂
t
e = ∂
t
F : σ
0
− ∇
x
· q (4.1.55)
Using the Clausius-Duhem inequality
ρ
0
∂
t
s ≥ −
1
T
∇
x
· q +
1
T
2
q · ∇
x
T (4.1.56)
we get
ρ
0
∇
F
h
0
: ∂
t
F +
∂h
0
∂T
∂
t
T
= ρ
0
∂
t
h ≤ −ρ
0
s∂
t
T − q ·
∇
x
T
T
+ ∂
t
F : σ
0
, (4.1.57)
or equivalently,
∂
t
F : (ρ
0
∇
F
h
0
− σ
0
) + ∂
t
T
ρ
0
∂h
0
∂T
+ ρ
0
s
+ q ·
∇
x
T
T
≤ 0. (4.1.58)
Since F, T can take arbitrary values, the inequality (4.1.58) implies [23]
∂W
∂F
= σ
0
, (4.1.59)
s = −
∂h
∂T
,
q ·
∇
x
T
T
≤ 0.
where W = ρ
0
h
0
. These are constraints on the possible constitutive relations.
In the absence of additional information, the simplest thing to do is to assume a linear
relation between the remaining flux q and the corresponding driving force ∇
x
T/T :
q = −k
∇
x
T
T
. (4.1.60)
Here k, the thermal conductivity, must be positive. The other constitutive information
needed is an expression for W , the stored energy density, which was discussed earlier.

158 CHAPTER 4. THE HIERARCHY OF PHYSICAL MODELS
4.1.5 Dynamics of fluids
Next we turn to fluids. As we said earlier, it is much more convenient to use the
Eulerian coordinates to describe fluids. We will focus on dynamic models of fluids. For
convenience, we now use u to denote the velocity field (instead of the displacement field
as was done earlier). In Eulerian coordinates, the basic principles take the form
• Conservation of mass:
∂
t
ρ + ∇ · J
ρ
= 0 (4.1.61)
• Conservation of momentum:
∂
t
(ρu) + ∇ · J
m
= 0 (4.1.62)
• Conservation of energy:
∂
t
E + ∇ · J
E
= 0 (4.1.63)
• The Clausius-Duhem principle, namely, the second law of thermodynamics:
ρ (∂
t
s + u · ∇s) + ∇ ·
q
T
≥ 0 (4.1.64)
where J
ρ
, J
m
, J
E
are the mass, momentum and energy fluxes, respectively, given by
J
ρ
= ρu, (4.1.65)
J
m
= ρu ⊗ u − σ,
J
E
= Eu − σ · u + q
It is convenient to introduce the material derivative. For any function ϕ, its material
derivative is defined as
˙ϕ = D
t
ϕ =
∂
∂t
ϕ + u · ∇ϕ. (4.1.66)
Using material derivative, the conservation laws can be written as
˙ρ + ρ∇ · u = 0, (4.1.67)
ρ
˙
u = ∇ · σ, (4.1.68)
ρ ˙e + ∇ · q = Tr
σ
T
∇u
, (4.1.69)
ρ ˙s + ∇ · (
q
T
) ≥ 0. (4.1.70)
4.1. CONTINUUM MECHANICS 159
where Tr(A) denotes the trace of a matrix A. To obtain (4.1.69), multiply both sides of
(4.1.68) by u to get,
∂
t
ρ
2
|u|
2
+ ∇ ·
ρ
2
|u|
2
u − u · σ
= −Tr
σ
T
∇u
(4.1.71)
Combining this equation and (4.1.63) gives (4.1.69).
Again, we define the Helmholtz free energy per unit mass as
h = e − T s, (4.1.72)
where T is the temperature of the system. We assume that h is only a function of T and
ρ, h = h
0
(T, ρ), then
ρ
˙
h = ρ
˙e −
˙
T s − T ˙s
(4.1.73)
Using (4.1.69), we get
ρ
˙
h = Tr
σ
T
∇u
− ∇ · q − ρs
˙
T − ρT ˙s. (4.1.74)
On the other hand, we also have
ρ
˙
h = ρ
∂h
0
∂T
˙
T + ρ
∂h
0
∂ρ
˙ρ = ρ
∂h
0
∂T
˙
T − ρ
2
∂h
0
∂ρ
(∇ · u) . (4.1.75)
Combining (4.1.73),(4.1.75), and (4.1.64), we get
Tr
σ +
∂h
0
∂ρ
ρ
2
I
T
∇u
!
− q ·
∇T
T
− ρ
˙
T
s +
∂h
0
∂T
≥ 0. (4.1.76)
Since (4.1.76) should hold for any value of
˙
T , we must have
s = −
∂h
0
∂T
(4.1.77)
which is a well-known result in thermodynamics. Now (4.1.76) becomes
Tr
σ +
∂h
0
∂ρ
ρ
2
I
T
∇u
!
− q ·
∇T
T
≥ 0 (4.1.78)
Let p =
∂h
0
∂ρ
ρ
2
be the thermodynamic pressure, and write
σ = −˜pI + σ
d
(4.1.79)

160 CHAPTER 4. THE HIERARCHY OF PHYSICAL MODELS
where ˜p =
1
3
Tr(σ), and Tr(σ
d
) = 0. (4.1.78) becomes
(p − ˜p)(∇ · u) + Tr
(σ
d
)
T
∇u
− q ·
∇T
T
≥ 0. (4.1.80)
This is the constraint on the constitutive relation from thermodynamic consideration.
In the absence of additional information, we again resort to a linear relation between
the fluxes and the driving forces. This means that p − ˜p, σ
d
and q should all be linear
functions of ∇·u, ∇u and ∇T /T . It is easy to see that for isotropic fl uids, the constitutive
relation must be of the form:
p − ˜p = κ∇ · u, (4.1.81)
σ
d
= µ
∇u + ∇u
T
2
− (∇ · u)I
, (4.1.82)
q = −k
∇T
T
, (4.1.83)
Presence of other cross terms at the right hand side would violate isotropy or invariance
under reflection. Here κ, µ, k are constants, representing respectively the bulk viscos-
ity, shear viscosity and heat conductivity. Together with the conservation laws (4.1.67)-
(4.1.69), we obtain the familiar compressible Navier-Stokes equation in fluid dynamics.
So far we have only discussed the simplest example of a flu id system. For more
complicated systems, the principles are the same as described above. As an example, let
us consider a two-component fluid system. Let C be the mass concentration of one of
the two components, then conservation of mass reads
ρ
˙
C + ∇ · J
C
= 0, (4.1.84)
where J
C
is the diffusive mass current for the corresponding component. Now besides
T and ρ, the free energy also depends on C, i.e. h = h
0
(T, ρ, C). The Clausius-Duhem
principle gives
(p − ˜p)∇ · u + Tr
(σ
d
)
T
∇u
− q ·
∇T
T
− J
C
· ∇˜µ ≥ 0. (4.1.85)
4.2. MOLECULAR DYNAMICS 161
where ˜µ is the chemical potential ˜µ =
∂h
∂C
. In the linear resp onse regime, the constitutive
relation in this case is
p − ˜p = κ∇ · u, (4.1.86)
σ
d
= µ
∇u + ∇u
T
2
− (∇ · u)I
, (4.1.87)
q = −A
1
∇˜µ − A
2
∇T
T
, (4.1.88)
J
C
= −B
1
∇˜µ − B
2
∇T
T
. (4.1.89)
This suggests that temperature gradient may drive mass diffusion, a phenomenon that
is commonly referred to as thermo-diffusion or the Soret effect [12]. The coefficients
A
1
, B
1
, A
2
, B
2
have to satisfy the constraint that the left hand side of (4.1.85) as a
quadratic form of (∇u, ∇˜µ, ∇T/T ) is semi-positive definite. Further constraints may
come from Onsager’s reciprocity relation [12], which in this case states that A
2
= B
1
.
To summarize, the basic steps for developing (dynamic) continuum models are as
follows:
1. Write down the conservation laws.
2. Find the constraints on the constitutive relations from the second law of thermo-
dynamics.
3. Find the constraints on the constitutive relations from symmetry considerations. In
most cases, as a first approximation, one can postulate linear constitutive relations.
Such a general approach has its merits: It gives a framework that allows us to explore
all the possible leading order physical effects, particularly cross effects such as thermo-
diffusion.
4.2 Molecular dynamics
Next we turn to the atomistic models in the form of classical molecular dynamics. In
this section, we discuss the basic ideas of molecular dynamics, including the empirical
inter-atomic potentials, the different ensembles, the continuum limits and the linear
response theory for computing transport coefficients using atomistic modeling.

162 CHAPTER 4. THE HIERARCHY OF PHYSICAL MODELS
Consider a system of N atoms and denote by m
j
and y
j
the mass and position of the
j-th atom, respectively. Molecular dynamics models the evolution of this system using
Newton’s second law
m
j
¨
y
j
= F
j
= −∇
y
j
V (y
1
, . . . , y
N
), j = 1, ··· , N (4.2.1)
where V is the potential energy function of the system. This system of equations can
also be written in Hamilton’s form (p
j
= m
j
˙
y
j
):
dy
j
dt
= ∇
p
j
H,
dp
j
dt
= −∇
y
j
H, j = 1, ··· , N (4.2.2)
where the Hamiltonian H is given by
H =
X
j
1
2m
j
|p
j
|
2
+ V (y
1
, . . . , y
N
). (4.2.3)
4.2.1 Empirical potentials
The first issue we have to address is: What is the function V ? As we will see later when
discussing quantum mechanics, in principle V can be obtained from electronic structure
models. In practice, however, this is a rather costly procedure, and most models of
molecular dynamics still use empirical potentials that are carefully designed for specific
purposes.
It should be emphasized that at this point, there are no systematic ways of finding
empirical inter-atomic potentials. Current practice is a combination of coming up with
a good guess for the functional form of the potential and calibrating the parameters by
experimental data as well as data from first principle-based calculations.
Empirical potentials for molecules
Potentials for molecules are usually in the following form:
V = V
bonded
+ V
non-bonded
= V
I
+ V
II
(4.2.4)
where V
I
is the contribution due to the covalently bonded-interactions, and V
II
is the
contribution due to the non-bonded interactions. Bonded interactions include contribu-
tions from the changes in bond length, bond angle and torsion ang le (also called dihedral
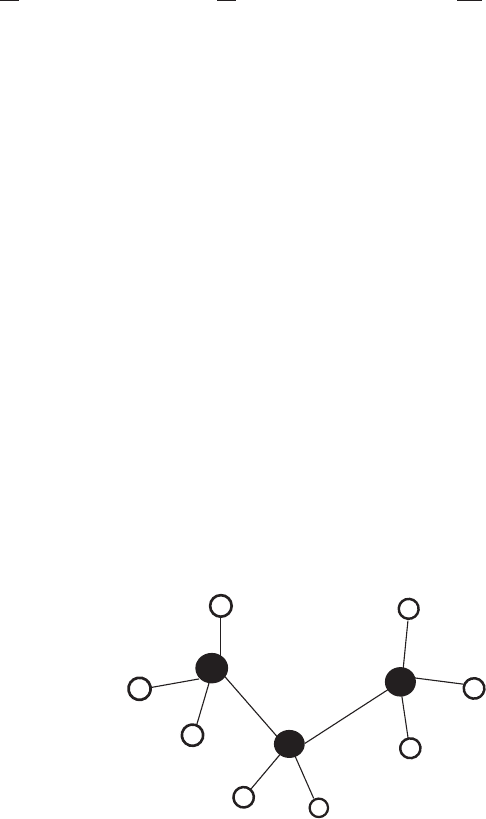
angle). In the example of propane (CH
3
− CH
2
− CH
3
, see Figure 4.2), there are 10
4.2. MOLECULAR DYNAMICS 163
bonds (2 C-C bonds, 8 C-H bonds), 18 angles (1 C-C-C angle, 10 C-C-H angle and 7
H-C-H angle), and 18 torsion angles (12 H-C-C-H angles and 6 H-C-C-C angles).
A typical form of V
I
is
V
I
=
X
bonds
k
i
2
(l
i
− l
i,0
)
2
+
X
angles
ξ
j
2
(θ
j
− θ
j,0
)
2
+
X
torsion
angles
V
n
2
(cos ω
n
− cos ω
n,0
)
2
(4.2.5)
The first term is the energy due to bond stretching, l is the bond length and l
0
is the
equilibrium bond length. The second term is due to the change in bond angles which
causes bending, θ
j
and θ
j,0
are respectively the j-th bond angle and its equilibrium
value. The third term is t he contribution from the change in torsion angles which causes
twisting. ω
n
is a torsion angle between three consecutive bonds, ω
n,0
is its equilibrium
value. Note that the functional form of the terms are rather simplistic, either quadratic
or simple cosines. The motivation is the same as before when we considered constitutive
relations for solids and fluids: We always choose the simplest form that is consistent
with what we already know. Secondly, the parameters in (4.2.5) can be computed from
quantum mechanical calculations. This has become a standard practice when developing
force fields [5].
In principle, one might also consider mixed terms such as bond angle and torsion
angle interactions. But these are only included when it is absolutely necessary to do so.
Figure 4.2: An illustration of Propane.
better pics needed
Next we discuss the non-b onded interaction. There are two main contributions: elec-

164 CHAPTER 4. THE HIERARCHY OF PHYSICAL MODELS
Figure 4.3: An illustration of basic variables associated with covalent bonds.
trostatic and van der Waals interaction:
V
II
=
1
2
X
i6=j
4ε
ij
(
σ
ij
r
ij
12
−
σ
ij
r
ij
6
)
+
1
2
X
i6=j
q
i
q
j
4πε
0
r
ij
(4.2.6)
Here r
i,j
= |y
i
− y
j
|, q
i
is the partial charge of the ith atom, ε
0
is the permitivity
of vaccum. Their values need to be changed when solvent effects, which is often an
important factor when studying biomolecules, are taken into account.
Van der Waals interaction accounts for the effect of quantum fluctuations on the
dipole moments. Consider two atoms, of distance r apart, where r is much larger than
the size of the atoms. Even though the atoms may not have permanent dipoles, quantum
and thermal fluctuations will cause them to have temporary dipole moments. If one atom
acquires some temporary dipole moment, it induces a polarization on the other atom.
This induced polarization is of the order O(r
−3
) (see for example problem 3 in Chapter
11 of [32]). The energy for the dipole-dipole interaction is of the form
V (r) = −
α
1
α
2
r
6
(4.2.7)
where α
1
and α
2
are the polarizability constants for the two atoms.
(4.2.7) is always negative, implying t hat the van der Waals interaction is attractive. At
short distance, it has to be compensated by a repulsive force that prevents the two atoms
from collapsing into each other. The repulsive force comes either from charge-charge
interaction, or Pauli’s exclusion principle which prevents two electrons from occupying
the same quantum state. Unfortunately, there is no analogous derivation for the form of
the repulsive force. As a matter of convenience, it is often mo deled by a r
−12
term. This
gives the Lennard-Jones form of the van der Waals interaction:
V (r) = 4ε
σ
r
12
−
σ
r
6
(4.2.8)

4.2. MOLECULAR DYNAMICS 165
The two parameters in the Lennard-Jones potential are: ε, which is a scale of interaction
energy, and σ, which is a scale of the length. When multiple species of atoms are
encountered, a mixing rule is often used for the parameter values in the Lennard-Jones
potential, e.g.
σ
AB
=
1
2
(σ
AA
+ σ
BB
), ε
AB
=
√
ε
AA
ε
BB
(4.2.9)
Empirical potentials for solids
Solids can be classified into several d ifferent types according to the nature of the
bonding between the atoms in the solid. These are: Covalently bonded, ionic, metallic,
molecular and hydrogen-bonded [4]. The last type occurs mostly in biological systems.
We will not discuss it here.
1. Molecular crystals. These occur mainly for inert gas elements in the last column of
the periodic table, for which the atoms interact mainly through the van der Waals
effect, modeled by the Lennard-Jones potential.
2. Metallic systems. An important ingredient for modeling met allic systems is the
charge density, which is often defined through
ρ
i
=
X
j
ρ
∗
(r
ij
) (4.2.10)
where ρ
∗
is a fitting function. The most popular inter-atomic potential for metals
is the embedded atom model (EAM) [11]
V =
1
2
X
i6=j
Φ(r
ij
) +
X
i
F (ρ
i
) (4.2.11)
Here the functions Φ, F are also fitting functions. One special case is the Finnis-
Sinclair potential where F (ρ) = A
√
ρ.
3. Covalently-bonded systems. These are somewhat similar to the molecules discussed
earlier. Examples include the diamond structure of silicon and the graphene struc-
ture of carbon . Two most p opular potentials are the Stillinger-Weber potential and
Tersoff potential. The Stillinger-Weber potential takes the form:
V =
1
2
X
f(r
ij
) +
X
(h(r
ij
, r
ik
, θ
jik
) + permutations) (4.2.12)

166 CHAPTER 4. THE HIERARCHY OF PHYSICAL MODELS
where
h(r, r
′
, θ) = λe
γ
r−a
+
γ
r
′
−a
cos θ +
1
3
2
(4.2.13)
This potential is similar to the potentials we discussed above for molecules. The first
term is the pair-wise interaction. The second three-body term is the contribution
of the bond angles.
Tersoff potential, on the other hand, is quite different. It takes the form:
V =
X
i6=j
Ae
−αr
ij
− b
ij
Be
−βr
ij
(4.2.14)
The interesting parameter here is b
ij
, the local bond-order, which depends on the
coordination of the atoms and the bond strength [35].
4. Ionic crystals, such as rock salt. In these systems, the atoms interact mainly
through the ion-ion electrostatic interaction and the short range repulsion. Born
proposed the following inter-atomic potential [4]
V =
1
2
X
i6=j
q
i
q
j
4πε
0
r
ij
+
X
A
r
n
ij
(4.2.15)
where q
i
is the partial charge of the i-th ion, A and n are empirical parameters.
The difficulty with this potential and with modeling ionic systems in general is the
long range nature of the electrostatic interaction.
Empirical potentials for fluids
Fluids are rarely modeled at the level of atomic detail. They are often coarse-grained.
The choice of the empirical potential depend s on how we coarse-grain the system. If
the coarse-grained particles are treated as spherically-shaped point particles, then the
Lennard-Jones potential is often used . If they are treated as ellipsoids or rods, then one
may use some anisotropic forms of the Lennard-Jones potential such as the Gay-Berne
potential [1]. If the coarse-grained particles are chains, i.e. beads connected by springs,
then bead-spring models are used. S uch a model typically contains two components:
1. The beads interact through a Lennard-Jones potential.
2. Neighboring beads on the same chain interact via a spring force.
An example of this type is treated in Chapter 6. Coarse-grained models of fluids are
discussed more systematically in [40].
