Water and Wastewater Engineering
Подождите немного. Документ загружается.

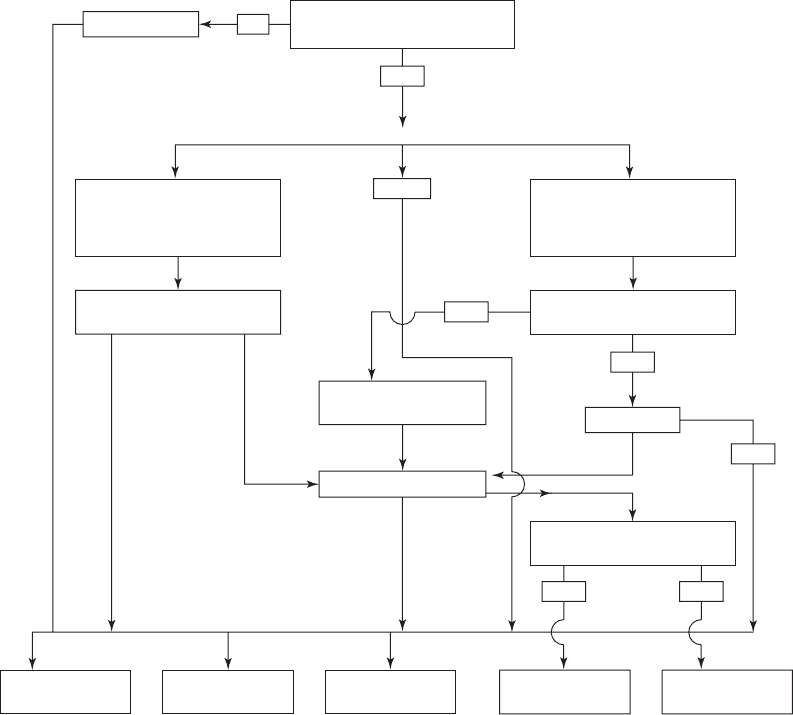
WATER PLANT RESIDUALS MANAGEMENT 15-47
No pretreatment
Does liquid residual arsenic level
prohibit discharge or recycle/reuse?
– Disposable AA
– Fe-based media adsorption
– IX
– Conventional treatment
– Lime softening
– Mn greensand filtration
– CMF
– Coagulant and clarification
– Adsorption
Polymer and clarification
for arsenic removal
Liquid
decant
Treatment alternatives
Removal arsenic from SFBW or
blowdown without chemical?
Solids pass TCLP or CALWET
for arsenic?
POTW discharge*
(0.05 – 1 mg/L)
Direct discharge**
(< 0.05 mg/L)
Recycle/reuse Nonhazardous
landfill
Hazardous waste
landfill
Thickening/dewatering
Clarification
Solids
Solids
Liquid
dec
ant
Yes
Yes
Yes No
No
No
RO/NF
No
FIGURE 15-14
Arsenic residuals handling and disposal decision tree.
Notes: Spent media disposed of in nonhazardous waste landfill. AA and Iron-based media adsorption backwash waters are
expected to meet POTW direct discharge or recycle arsenic criteria.
*Depends on bac
kwash analysis
**Depends on state regulations
AA—Activated Alumina
CALWET—California Waste Extraction Test
CMF—Coagulation Microfiltration
IX—Ion Exchange
TCLP—Toxicity Characteristic Leaching Procedure
POTW—Publicly Owned Treatment Works
15-48 WATER AND WASTEWATER ENGINEERING
Fluoride Residuals
The liquid streams from activated alumina (AA) regeneration or reverse osmosis (RO) treatment
to remove fluoride may be disposed of in the same manner as other AA and RO residuals sum-
marized in Figures 15-12 and 15-13.
Iron and Manganese Residuals
Waste filter wash water can be disposed of by dewatering the solids on sand drying beds and
landfilling the solids. GLUMRB recommends that the sand be 30 cm deep with an effective size
of 0.3 to 0.5 mm and a uniformity coefficient of less than 3.5. The sand should have a support-
ing layer consisting of 10 cm of torpedo sand and 20 cm of gravel. The area of the sand drying
bed should be sufficient to allow the entire volume of wash water for one d ay to be placed at a
depth of less than 0.60 m, unless production filters are washed on a rotating schedule (GLUMRB,
2003). The sand drying beds need to be located
at some distance from drinking water wells so
they do not become “under the influence of a surface water source.”
Nitrate Residuals
The liquid streams from ion exchange treatment to remove nitrate may be disposed of in the same
manner as other ion exchange residuals summarized in Table 15-10 . See “Perchlorate Residuals”
for a recycling option.
Perchlorate Residuals
The destruction of perchlorate and nitrate in regenerant brines can be accomplished by either mi-
crobial or chemical processes. Destruction by chemical means generally requires high tempera-
ture (50C–60 C) and pressure. If polystyrene or polyvinylpyridine resin is used, the heate
d brine
can be used directly in regeneration of the resin. Although large amounts of NaCl are required
to strip perchlorate from the exhausted resin, because of the relatively small fraction of sites oc-
cupied by perchlorate, only a small fraction of the chloride applied is actually used to replace
perchlorate. By
destroying the perchlorate and nitrate in the spent brine, the available chloride
salt can be recycled for many regeneration cycles (Tripp and Clifford, 2006).
Radioactive Residuals
Although other radioisotopes are of concern, radium is the radionuclide of primary concern.
The U.S. Environmental Protection Agency s uggested alternatives for disposal of water treat-
ment plant liquid and solid wastes are illustrated in Figures 15-15 and 15-16 on pages 15-49
and 15-
50.
Synthetic Organic Chemical (SOC) Residuals
Granular activated carbon (GAC) that becomes saturated is usually regenerated. Thermal regen-
eration destroys the SOC. Regeneration may be on-site for large facilities or off-site for smaller
plants.
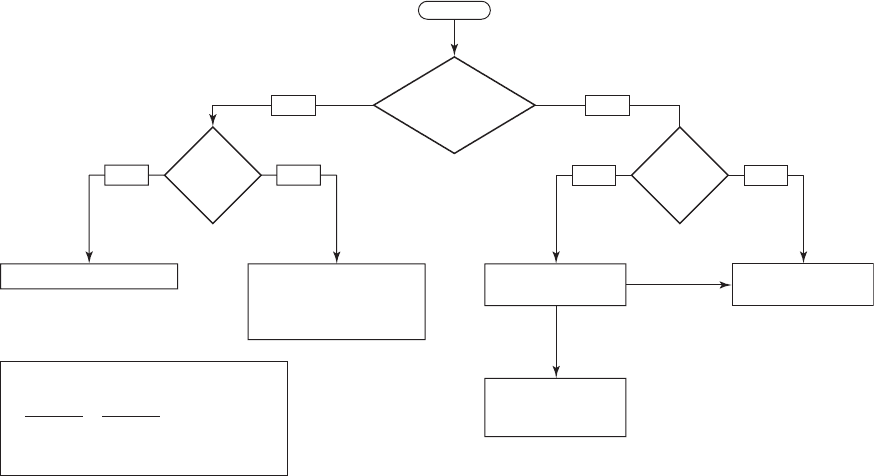
WATER PLANT RESIDUALS MANAGEMENT 15-49
15-10 ULTIMATE DISPOSAL
After all possible sludge treatment has been accomplished, a residual sludge remains, which must
be sent to ultimate disposal or used in a beneficial manner. Of the many theoretical alternatives
for ultimate disposal, only four are of practical interest:
• Land spreading.
• Other beneficial use.
• Codisposal with sewage sludge.
• Landfilling.
Land Spreading
Land application of water treatment plant residuals is regulated in the United States under the
Resource Conservation and Recovery Act (RCRA) as well as state and local agencies. RCRA
rules require that the residuals pass the TCLP test.
Residuals that have been land applied include coagulant slu
dges, lime softening sludges,
nanofiltration concentrate, and slow sand filter washings (Novak, 1993). Depending on local soil
conditions, application of lime sludges may have beneficial effects on the soil and crop yields.
This is especially true when nitrogen fertilizers are used becaus
e they typically lower the soil pH,
Box A
Radio active
by criteria in
40 CFR 144.3
1. Ra 226 14.8 Bq/L?
2. Ra 228 29.6 Bq/L?
4. Yearly total 3.7 10
10
Bq
5. No accumulation in sewer
Discharge to sanitary
sewer (10 CFR 20)
Treat to remove
radionuclides
StartStart
Meets
criteria in
Box A?
YesNo
Liquid
Solids
Direct discharge to river
Inject below formations
containing drinking water
[40 CFR 144.6 (a) (2)
and 40 CFR 144.12 (c)]
No
NPDES
permit?
Yes No
Residual solids to
disposal (see figure
15-16)
Yes
Ra 226
14.8
Ra 228
29.6
U 3.7 10
4
Bq
3.
FIGURE 15-15
U.S. EPA suggested disposal alternatives for water treatment plant liquid wastes containing natural radioactivity. (NPDES National
Pollution Discharge Elimination System)
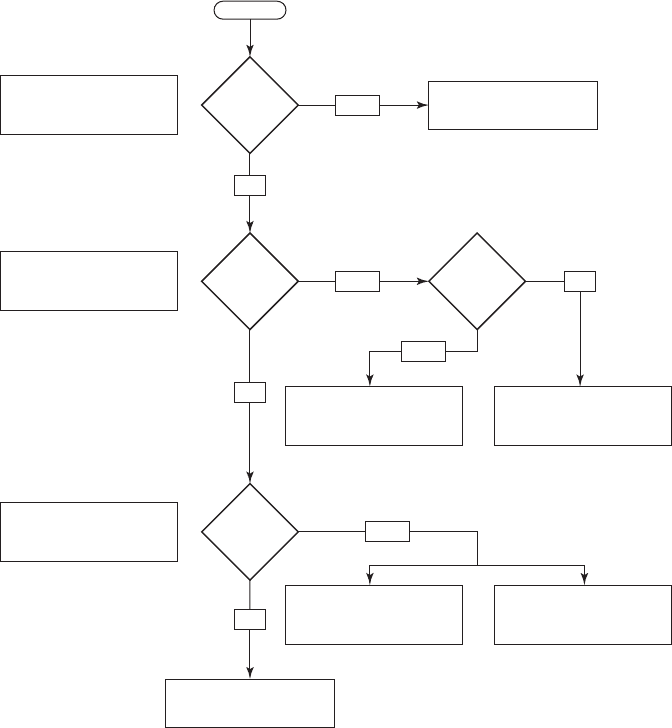
15-50 WATER AND WASTEWATER ENGINEERING
which results in a decrease in calcium availability. The addition of lime sludge raises the soil pH
to a level comparable to commercially available agricultural limestone. In contrast, the addition
of aluminum or iron coagulant sludge may have a negative impact by reducing the availability of
phosphorus
and increasing compaction. Iron application to grazing land may result in a negative
effect on copper metabolism in sheep (Marshall, 2002).
Other Beneficial Uses
T urf grass has a relatively low nutrient demand, but requires significant moisture levels. Dewa-
tered sludge applied to a turf farm at the beginning of the seeding process can improve water
retention (Cornwell, 1999).
Box A
Box B
Meets
criteria in
Box A?
Box C
Disposal at low – level
radioactive waste facility
Case by case determination,
may be disposed as
uranium mill tailings
Naturally occuring
radioactive material disposal
where appropriate
If U is > 0.05% waste is
regulated as a
source
(10 CFR 40)
Stabilized landfill with
> 3 m cover; long = term
control
Ra: < 0.15 Bq/g
Pb-210: < 0.15 Bq/g
U: < 1.1 Bq/g
Ra: 0.15 to 1.85 Bq/g
Pb-210: 0.15 to 1.85 Bq/g
U: 1.1 to 1.85 Bq/g
Ra: 1.85 to 74 Bq/g
Pb-210: 1.85 to 74 Bq/g
U: 1.85 to 74 Bq/g
Dewatered sludge to
landfill
Yes
No
Meets
criteria in
Box B?
Is
U > 0.05%?
StartStart
Yes
Yes
Meets
criteria in
Box C?
Yes
No
No
No
FIGURE 15-16
U.S. EPA suggested disposal alternatives for water treatment plant solid wastes containing natural radio-
activity.

WATER PLANT RESIDUALS MANAGEMENT 15-51
Commercial producers of topsoil use a raw soil blended with some organic material to form
their product. Sludge can be blended during the production process to increase nutrient value
and water retention. The quality of the sludge must meet metal limits of the topsoil producer
(Vandermeyden and Cornwell, 199
3).
Many other beneficial uses such as cement manufacturing, brick manufacturing, road sub-
grade, landfill cover, and flue gas desulfurization are dis cussed in Water Treatment Residuals
Engineering (Cornwell, 2006).
Codisposal with Sewage Sludge
Water treatment plant sludge can be mixed with biosolids from wastewater treatment prior to
disposal. F or a utility that operates both the water and wastewater facilities, this option allows
permitting and monitoring for “one” solid waste. In addition, the water plant residuals will often
lower the metal concentrations in the biosolids product because the water treatment res idu
als
dilute the residuals from the wastewater treatment plant.
Landfilling
When the beneficial options cannot be exercised, landfilling becomes the alternative of choice.
The landfill can be either a monofill, with only the water treatment resid uals, or it can be co-
disposed with municipal solid waste.
The design of a monofill is m ore appropriately covered in other tex ts such as Water Treat-
ment Residuals Engineeri
ng (Cornwell, 2006). However, it should be noted that a major require-
ment is that the sludge be capable of supporting excavating equipment that will be working at the
landfill site.
Codisposal with municipal solid waste is generally limited by the permit limits of the landfill
site, and the requirement that the sludge pass the TCLP test so that it is not consid
ered to be a
hazardous waste. Typically, there is also a requirement that the sludge release no free water dur-
ing transport and placement. A typical requirement is 30 percent solids (Cornwell, 2006).
Visit the text website at www.mhprofessional.com/wwe for supplementary materials
and a gallery of photos.
15-11 CHAPTER REVIEW
When you have completed studying this chapter, you should be able to do the following without
the aid of your textbook or notes:
1 . Outline the logical steps in implementing a residual management program.
2. On a process flow diagram, label the sources of residuals.
3. Explain the difference between coagulation sludges and lime softening sludges with
respe
ct to their specific gravity and solids concentration.
4. State one method of minimization of residuals generation for each of the following: co-
agulation, lime-soda softening, and spent backwash water.
15-52 WATER AND WASTEWATER ENGINEERING
5. Given the characteristics of a sludge, select the appropriate thickening process.
6. Explain the climatic factors that favor lagoons and sand drying beds for dewatering sludges.
7. Given the characteristics of a sludge, select an appropriate mechanical dewatering process.
8. Identif
y three alternatives for management of liquid residuals.
9. Explain the major regulatory limitation on the disposal of arsenic residuals.
10. Compare the four generic categories of ultimate disposal of water treatment plant re-
siduals with respect to constraints, ease of implementation, and sus
tainability.
W ith the aid of this text, you should be able to do the following:
11. Calculate the specific gravity of sludge given the specific gravity of the solids and the
fraction that is solids.
12. Calculate the volume of sludge given the mass of solids, specific gravity of the solids,
and the percent solids or the mass of s
olids given the specific gravity and percent
solids.
13. Estimate the volume of dewatered sludge by either the approximate or rigorous
method.
14. Estimate the mass of dry solids produced by either coagulation or lime softening.
15. Compute a mass balance for solids for a
sedimentation basin.
16. Design a gravity thickening tank using the batch flux method given the appropriate
batch settling data.
17. Design a dewatering lagoon given the general climate scenario, that is, wet or dry.
18. Design a sand drying bed given the appropriate precipitation, pan evaporation, and pilot
data or similar data.
19. Select a centrifuge using manufactu
rers’ data and anticipated operating conditions.
20. Select a plate filter press using manufacturers’ data and anticipated operating conditions.
21. Select a liquid residuals management technique given the appropriate data.
22. Select an arsenic residuals management technique given the appropriate data.
23.
Select a radium residuals management technique given the appropriate data.
15-12 PROBLEMS
15-1. Estimate the specific gravity of a lime softening sludge with a solids specific gravity
of 1.9 and a solids concentration of 10%.
15-2. If the sludge in Problem 15-1 is dewatered to achieve 35% solids, what is the specific
gravity of the filter cake?
15-3. If the volume of 1.0% sludge is 15
1.4 m
3
, what is the mass of dry solids? Assume the
specific gravity of the sludge is 1.0.
WATER PLANT RESIDUALS MANAGEMENT 15-53
15-4. If the mass of dry solids in 171.2 m
3
of a sludge is 4,280 kg, what is the percent solids?
Assume the specific gravity of the sludge is 1.0.
15-5. What mass of water must be removed from the sludge in Problem 15-4 to achieve 25%
solids?
15-6. Dr. Cornwell derived Equation 15-16 based on the stoichiometric chemis
try of iron.
Using Equation 6-10, show how Dr. Cornwell arrived at the coefficient of 2.9 for the
iron dose in Equation 15-16.
15-7. An alternate form of Equation 15-14 is one based on the stoichiometric chemistry of
ferric chloride assuming that the ferric chloride is purchased in solution and that there
are no waters of hydration. Use Equ
ation 6-10 to derive the coefficient for FeCl
3
as-
suming the ferric chloride dose in Equation 15-16 is mg/L as FeCl
3
instead of Fe;
that is, find “x” in the following equation:
M
s
86 4.Q FerricchlorideSSM(( ) )x
15-8. Compare the approximate form for estimating sludge volume reduction and the rigor-
ous method by computing the percent error for the following conditions:
S p e cific gravity of solids 1.2
Solids fraction of wet sludge 1%
Solids fraction of dewatered sludge 25%
Volume of wet sludge 66.0 m
3
/ d
15-9. Compare the approximate form for estimating sludge volume reduction and the rigor-
ous method by computing the percent error for the following conditions:
S p e cific gravity of solids 2.4
Solids fraction of wet sludge 10%
Solids fraction of dewatered sludge 50%
Volume of wet
sludge 66.0 m
3
/ d
15-10. Using a spreadsheet you have written, plot a graph of percent error versus the wet
sludge solids concentrations of 1, 2, 3, and 4% for the data in Problem 15-8.
15-11. Using a spreadsheet you have written, plot a graph of percent error versus the wet
sludge solids concentrations of 2, 5, 10, and 15% for the data in Problem 15-9.
15-12. The city of Pherri
c’s coagulation treatment plant sedimentation tank is being designed
to treat a flow of 20,000 m
3
/ d. Based on jar tests, the design dose of ferric chloride is
estimated to be 18.0 mg/L and the polymer dose is estimated to be 0.1 mg/L. The design
raw water suspended solids concentration is 19.4 mg/L. The design effluent suspended
solids conc
entration from the settling tank is 3.0 mg/L. The following sludge charac-
teristics have been selected for the design: sludge solids content 0.010%, specific
gravity of the sludge solids 1.2. What volume of sludge from the settling tank must
be disposed of each day? Ass
ume that the ferric chloride is fed as FeCl
3
.
15-13. The city of Calcareous’ upflow solids contact tank design flow is 30,000 m
3
/ d. The
water source is the Raccoon River. The plant will remove 114 mg/L of calcium
carbonate hardness as CaCO
3
b y the addition of lime. No other hardness removal is

15-54 WATER AND WASTEWATER ENGINEERING
required. The maximum turbidity of the Raccoon River at flood stage is 71 mg/L. Ferric
chloride is to be added at a dose of 25 mg/L as FeCl
3
in the coagulation step following
softening. Based on experience with similar plants, an effluent suspended solids con-
centration of 2.0 mg/L is being used for the design. The design sludge solids content is
10% and the design specific gravity of the sludge solids is 1.9. What volume of s
ludge
from the upflow solids contact unit must be disposed of each day during flood stage?
15-14. A MF membrane filter has been selected for providing 136,000 m
3
/ d of treated water
for the city of Tumanipharmz. The design recovery rate is 90%. The suspended solids
concentration in the raw water is 29.1 mg/L. Estimate the feed flow rate to produce
136,000 m
3
/ d of treated water, the reject flow rate, the suspended solids concentration of
the concentrate, the sludge production (kg/d), and the percent solids concentration.
15-15. A small RO plant will be used to soften water for Saline. The design flow rate is
3,800 m
3
/ d. The design recovery rate is 75%. The TDS of the feed water is 2,800 mg/L.
Estimate the feed flow rate required to produce 3,800 m
3
/ d, the reject flow rate, and
TDS concentration of the concentrate.
15-16. The treated water (3,800 m
3
/ d) from Saline’s RO plant ( Problem 15-15 ) is blended
with raw water for delivery to the customers so that the water has a TDS of 250 mg/L.
Determine the flow rate (m
3
/ d) of raw water to blend with the RO water to achieve a
TDS of 250 mg/L.
15-17. Because the settled water from Pherric ( Problem 15-12 ) has a very low percent sol-
ids, a thickener is being considered for treatment of the sludge before dewatering.
Using the following batch settling curve data, design three alternative sludge
thickeners with the following underflow solids con
centrations: 0.30%, 0.40%, and
0.50%. An overflow rate of 2.5 m/h has been selected for the design.
A completed design will include the tank diameter, tank depth, and the running
torque for the rake. Assume two day’s storage at an average P
s
of 0.004.
Batch settling data for Pherric’s coagulation sludge
v
s
, m/d SS, kg/m
3
0.5 4.6
1.7 3.9
2.6 2.7
5.5 2.0
7.9 1.75
14 1.4
24 1.0
38 0.75
500.5
60 0.3
Note: you will need the solution from Problem 15-12 to solve his problem.

WATER PLANT RESIDUALS MANAGEMENT 15-55
15-18. The reject from the MF membrane filter at Tumanipharmz ( Problem 15-14 ) is to be
treated with polymer and thickened. Because the potential for Cryptosporidium con-
centration in the backwash water, an overflow rate equal to or less than 0.2 m/h is
required (Cornwell, 1999). Design three alternative sludge thickeners with the follow-
ing underflow solids concentrations: 3%, 4%, and 5%. The following batch
settling
curve data are assumed to be representative.
A completed design will include the tank diameter, tank depth, and the running
torque for the rake. Assume two day’s storage at an average P
s
of 0.04.
Batch settling data for Tumanipharmz’s backwash water solids
v
s
, m/d SS, kg/m
3
0.538
1.7 26
2.6 23
5.5 19
7.9 16
14 13
24 10.2
388
504
60 1
Note: you will need the solution from Problem 15-14 to solve his problem.
15-19. Rework Example 15-5 using Equation 15-27 and compare the result. Assume
the specific gravity of the sludge is 1.3 and a sludge loading rate of 80 kg dry
solids/m
2
.
15-20. Using the GLUMRB guidance, estimate the surface area of a lagoon to store the
sludge from Calcareous ( Problem 15-13 ).
15-21. Compare the area calculated in Problem 15-20 with the area estimated using Equations
15-26 and 15-27. Use the following assumptions: a 1.5 m depth, one use per year,
spec
ific gravity of sludge 2.0, 10% solids, a dry climate.
15-22. In the c ontinuing management of Pherric’s coagulant treatment sludge ( Problems
15-12 and 15-17 ), your firm has been asked to perform a preliminary design of a
sand drying bed. The sludge from a thickener at 2%
solids is to be applied to the bed
at a loading rate of 10 kg/m
2
. A drained solids concentration of 6.3%, an initial re-
siduals depth of 0.50 m, and a final solids concentration of 20% have been selected
for the design. The following precipitation and pan evaporation data were obtained
in the general geographic region of Pherric. For this preliminary design, estimate the
area required for the sand drying beds. Assume the slud
ge will be stored on the beds
even when there is no evaporation.

15-56 WATER AND WASTEWATER ENGINEERING
Hydrologic and sludge production data for Pherric
Month Precipitation, cm Pan evaporation, cm Sludge production, kg/d
JAN 1.3 1.1 194
FEB 1.0 1.0 150
MAR 1.6 1.8 240
APR 2.3 10.8 345
MAY 4.5 14.4 690
JUN 4.6 16.8 690
JUL 3.4 20.8 510
AUG 3.2 19.6 480
SEP 2.7 12.4 405
OCT 1.6 10.8 240
NOV 1.2 10.2 180
DEC 1.2 9.6 180
15-23. In the continuing management of Tumanipharmz’s backwash solids ( Problems 15-14
and 15-18 ), your firm has been asked to perform a preliminary design of a sand
drying bed. The sludge from a thickener at 4% solids is to be applied to the bed
at a loading rate of 15 kg/m
2
. A drained solids concentration of 18%, an initial resid-
uals depth of 2.20 m, and a final solids concentration of 30% have been selected for
the design. The following precipitation and pan evaporation data were obtained in the
general geographic region of Pherric. For this preliminary design, estimate the area
required for the sand drying beds. Assume the sludge will be
stored on the beds even
when there is no evaporation.
Hydrologic and sludge production data for Tumanipharmz
Month Precipitation, cm Pan evaporation, cm Sludge production, kg/d
JAN 8.0 0.0 3,320
FEB 5.9 0.0 2,450
MAR 8.0 0.0 3,320
APR 5.9 25.3 2,450
MAY 10.2 29.9 4,230
JUN 10.6 31.9 4,400
JUL 10.0 32.4 4,150
AUG 7.3 29.0 3,030
SEP 6.7 25.1 2,780
OCT 5.4 24.2 2,240
NOV 6.3 14.4 2,610
DEC 5.9 5.4 2,450
15-24. In the continuing management of Pherric’s coagulant treatment sludge, your firm
has been asked to perform a preliminary selection of a centrifuge. The sludge from a
thickener at 2% solids is to be pumped to the centrifuge. Using Table 15-8 , select an
