Water and Wastewater Engineering
Подождите немного. Документ загружается.


LIME–SODA SOFTENING 7-7
The product of the activity of the ions (approximated by the molar concentration) is a con-
stant for a given compound at a given temperature. In its most general form it is written as
K
AB
A
ab
[][]
[]
ab
B ()s
(7-6)
where [ ] molar concentration, moles/L
( s) solid state, that is the precipitate
Because the precipitate is a solid, [ A
a
B
b
( s)] 1.0 and the equation is conventionally expressed as
KAB
ab
sp
[][]
(7-7)
where K
sp
solubility product constant
This expression forms the fundamental basis for lime-soda softening. K
sp
values are often
reported as pK
sp
where
pK K
sp sp
log
(7-8)
A selected list of K
sp
values is presented in Appendix A.
Le Chatelier’s Principle. The lime-soda reactions are a direct application of Le Chatelier’s prin-
ciple or the law of mass action, which states that a reaction at equilibrium will adjust itself to relieve
any force or stress that disturbs the equilibrium. To soften the water, an ion in common in the solu-
bility
equilibrium is selected to react with calcium or magnesium so the reaction forms more pre-
cipitate. The solubility product equilibrium provides a starting point for selecting the common ion.
From Appendix A, it is apparent that several forms of calcium and magnesium will form a prec
ipi-
tate. The solubility product, as well as public health and economic criteria, are used as the criteria
for selecting the form of precipitate. In the case of calcium, the desired precipitate is CaCO
3
. In the
case of magnesium, the desired precipitate is Mg(OH)
2
. The solubility equilibrium reactions are
Ca
2
3
CO CaCO s
3
2
(
)
(7-9)
Mg OH Mg OH s
2
2
2
()()
(7-10)
The concentration of
CO
3
2
a n d/or OH
i s increased by the addition of chemicals, and the
chemicals drive the reactions given in Equations 7-9 and 7-10 to the right. Insofar as possible, the
naturally occurring bicarbonate alkalinity (
H
CO
3
) is converted to carbonate (
CO
3
2
) b y the addi-
tion of hydroxyl ions (OH
). Hydroxyl ions cause the carbonate buffer system (Equation 6-2) to
shift to the right and, thus, provide the carbonate for the precipitation reaction ( Equation 7-9 ).
The common source of hydroxyl ions is calcium hydroxide [Ca(OH)
2
]. Many water treatment
plants find it more ec onomical to buy quicklime (CaO), commonly called li me, than hydrated
lime [Ca(OH)
2
]. The quicklime is converted to hydrated lime at the water treatment plant by
mixing CaO and water to produce a slurry of Ca(OH)
2
, which is fed to the water for softening.
The conversion process is called slaking:
CaO H O Ca(OH) heat
22
(7-11)

7-8 WATER AND WASTEWATER ENGINEERING
The reaction is exothermic. It yields almost 1 MJ per gram mole of lime. Becau se of this high
heat release, the reaction must be controlled carefully. All safety precautions for handling a
strong base should be observed. Because the chemical is purchased as lime, it is common to
speak of chemical additions as additions of “lime,” when in fact the chemical added is hy-
drated lime (calcium hydrox ide). When carbonate ions must be supplied, the most common
chem ical chosen is sodiu
m carbonate (Na
2
CO
3
). Sodium carbonate is commonly referred to as
soda ash or soda.
Softening Reactions. The softening reactions are regulated by controlling the pH. First, any
free acids are neutralized. Then the pH is raised to precipitate the CaCO
3
; if necessary, the pH is
raised further to remove Mg(OH)
2
. Finally, if necessary,
CO
3
2
i s added to precipitate the non-
carbonate hardness.
Six important softening reactions are discussed below. In each case, the chemical that is
added to the water is printed in bold type. The notation (s) designates the solid form, and indi-
cates that the compound has been removed from the water. The following reaction
s are presented
sequentially, although in reality they occur simultaneously.
1. Neutralization of carbonic acid (H
2
CO
3
).
In order to raise the pH, free acid s must be neutralized first. CO
2
i s the principal acid
present in unpolluted, naturally occurring water.* No hardness is removed in this step.
CO
2
Ca(OH)
2
CaCO (s)HO
3 2
(7-12)
2. Precipitation of carbonate hardness due to calcium.
To prec ipitate calcium carbonate, all of the naturally occurring bicarbonate must be
converted to carbonate. The carbonate then serves as the common ion for the precipita-
tion reaction.
Ca HCO CaCO s HO
3
2
3 2
222
Ca(OH)
2
() (7-13)
3 . Precipitation of carbonate hardness due to magnesium.
To remove carbonate hardness that results from the presence of magnesium, more
lime is added. The reaction may be considered to occur in two stages. The first stage oc-
curs when the bicarbonate in step 2 above is c
onverted to carbonate.
Mg HCO MgCO CaCO s HO
3
2
332
22
Ca(OH)
2
()
(7-14)
The hardness of the water does not change because MgCO
3
i s soluble. With the addition
of more lime, the hardness due to magnesium is removed.
Mg CO Mg(OH)
2
3
2
2
Ca(OH)
2
() ()s CaCO s
3
(7-15)
*CO
2
and H
2
CO
3
in water are essentially the same:
CO H
2
OHCO
2 2
3
+
Thus, the number of reaction units (n) used to calculate the equivalents for CO
2
is two.
LIME–SODA SOFTENING 7-9
4. Removal of noncarbonate hardness due to calcium.
To remove noncarbonate hardness due to calcium, additional carbonate in the form of
soda ash must be added
Ca CaCO (s)2Na
2
3
Na CO
23
(7-16)
5 . Removal of noncarbonate hardness due to magnesium .
To remove noncarbonate hardness due to magnesium, both lime and soda must be
added. The lime provides the hydroxyl ion for precipitation of the magnesium.
Mg Mg OH s Ca
2
2
2
Ca(OH)
2
()()
(7-17)
Although the magnesium is removed, there is no change in the hardness because the
calcium is still in solution. To remove the calcium, soda ash must be added.
Ca
2
Na CO
23
CaCO s Na
3
2() (7-18)
Note that this is the same reaction as the one to remove noncarbonate hardness due to
calcium.
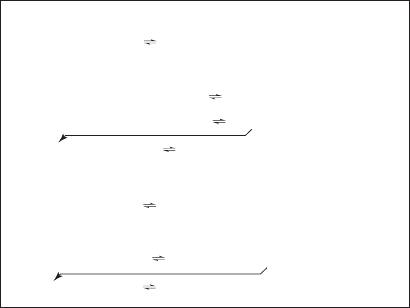
These reactions are summarized in Figure 7-5 . Although the reactions shown above use lime
and soda ash as sourc es of hydroxyl ion and carbonate ion, other sources
may be used. For ex-
ample, sodium hydroxide (NaOH) can be substituted for calcium hydroxide.
pH. Solubility relationships are generally more complex than implied by the discussion to this
point. In addition to the solubility product, other equ
ilibria affect the concentration of the ions
present. Other ions may form salts with less solubility than the ones assumed to result from the
solubility equilibrium. Reactions of the cation or anion with water to form hydroxide complexes or
Mg
2
2HCO
3
–
Ca(OH)
2
MgCO
3
CaCO
3
(s) 2H
2
O
Neutralization of carbonic acid
Precipitation of carbonate hardness
Precipitation of noncarbonate hardness due to calcium
Precipitation of noncarbonate hardness due to magnesium
CO
2
Ca(OH)
2
CaCO
3
(s) H
2
O
Ca
2
2HCO
3
–
Ca(OH)
2
2CaCO
3
(s) 2H
2
O
Ca
2
Na
2
CO
3
CaCO
3
(s) 2Na
Ca
2
Na
2
CO
3
CaCO
3
(s) 2Na
Mg
2
Ca(OH)
2
Mg(OH)
2
(s) Ca
2
MgCO
3
Ca(OH)
2
Mg(OH)
2
(s) CaCO
3
(s)
FIGURE 7-5
Summary of softening reac tions. (Note: The chemical added is
printed in bold type. The precipitate is designated by (s). The
arrow indicates where a compound formed in one reaction is used
in another reaction.) (Source: David and Cornwell, 2008.)

7-10 WATER AND WASTEWATER ENGINEERING
protonated anion species are common. In addition, the cations or anions may form complexes with
other materials in solution, thus, reducing their effective concentration (Sawyer et al., 2003).
Of particular importance is the effect of solution pH on the solubility of cations. For example,
assuming that there are no other com
pounds in solution to react with calcium hydroxide, the solu-
bility product would be
K
sp
22
[Ca ][OH ]
(7-19)
or
log Ca log log OH
sp
[] []
2
2
K
(7-20)
However, log [OH
] is a function of pH:
pHppOHplogOH
KK
ww
[]
(7-21)
or
log OH pH p[]
K
w
(7-22)
S o E q uation 7-20 may be written
log Ca log pH p
sp
[] ( )
2
2
KK
w
(7-23)
The pH not only affects the solubility of metal hydroxides, it also affects other equilibria,
which in turn affects the solubility of the cation. Of particular importance is the relationship of
the carbonate buffer system to pH. As noted in Equations 6-5 and 6-6, carbonate is an anion of the
weak diprotic acid, H
2
CO
3
. The carbonate species both influ ence and are influ enc ed by the pH.
The sum of the carbonate species may be specified as a total concentration of inorganic carbon:
C
T
[][][]H CO HCO CO
33
2
2 3
(7-24)
If the pH is specified, the saturation value for [Ca
2
] can be estimated for a given value of C
T
.
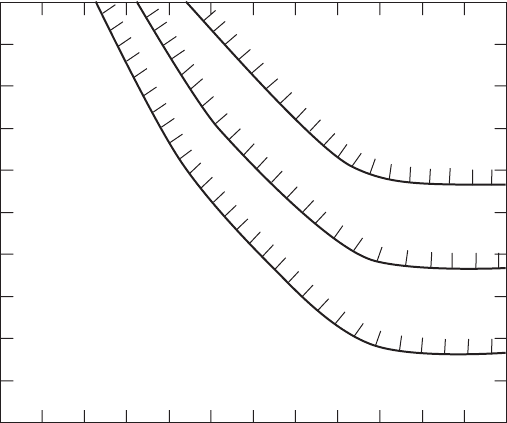
This is illustrated in Figure 7-6 .
In lime-soda softening, the pH is controlled by the addition of lime. To precipitate CaCO
3
,
the pH of the water must be raised to about 10.3. To precipitate magnes ium, the pH must be
raised to a range of about 11 to 11.3 (Horsley et al., 2005).
Process Limitations and Empirical Considerations. Lime-s oda softening cannot produce a
water completely free of hardness because of the solubility of CaCO
3
and Mg(OH)
2
, the physical
limitations of mixing and contact, and the lack of sufficient time for the reactions to go to com-
pletion. Thus, the minimum calcium hardness that can be achieved is about 30 mg/L as CaCO
3
,
and the minimum magnesium hardness is about 10 mg/L as CaCO
3
. Because of the slimy condi-
tion that results when soap is used with a water that is too soft, historically the goal for final total
hardness has been set at between 75 and 120 mg/L as CaCO
3
. In recent years, many utilities have
raised the target hardness to 120 to 150 mg/L as CaCO
3
to reduce chemical costs and residuals *
production (Horsley et al., 2005).
I n o r der to achieve reasonable removal of hardness in a reasonable time period, an extra amount
of Ca(OH)
2
beyond the stoichiometric amount usually is provided. Based on empirical experience, the
minimum extra amount is 20 mg/L of Ca(OH)
2
e xpressed as CaCO
3
(or 0.40 meq).
*Residuals precipitate in the lime-softening process and brine in ion exchange and reverse osmosis softening.
LIME–SODA SOFTENING 7-11
Magnesium in excess of about 40 mg/L as CaCO
3
(0.80 meq) forms scales on heat exchange
elements in hot water heaters. Because of the expense of removing magnesium, normally only
the magnesium that is in excess of 40 mg/L as CaCO
3
i s removed. For magnesium removals less
than 20 mg/L as CaCO
3
, the basic extra amount of lime mentioned above is sufficient to ensure
good results. For magnesium removals between 20 and 40 mg/L as CaCO
3
, an extra amount of
lime equal to the magnesium to be removed is added. For magnesium removals greater than 40
mg/L as CaCO
3
, the extra lime added is 40 mg/L as CaCO
3
. Addition of extra lime in amounts
greater than 40 mg/L as CaCO
3
does not appreciably improve the reaction kinetics.
Because the excess lime adds hardness in the form of Ca
2
, it is removed in a subsequent
process step called recarbonation. Recarbonation is discussed in detail in Section 7.3.
The sequence chemical additions (as CaCO
3
) to soften water are summarized in Table 7-2 :
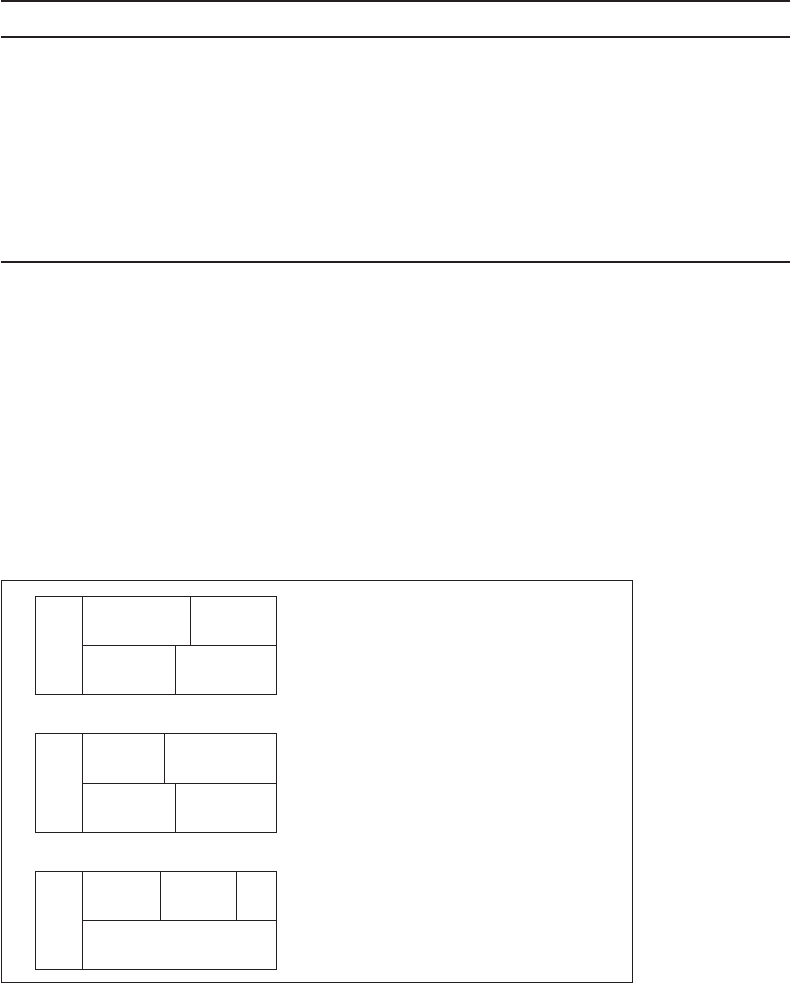
7-3 SOFTENING PROCESSES
The selection of chemicals and their dosage depends on the raw water composition, the desired final
water composition, operational convenience, sludge production, and cost. If a Mg
2
concentration of
40 mg/L as CaCO
3
i s used as a product water criterion, then six cases illustrate the dosage schemes.
Three of the cases occur when the Mg
2
concentration is less than 40 mg/L as CaCO
3
( Figure 7-7a , b,
and c) and three cases occur when Mg
2
is greater than 40 mg/L as CaCO
3
( Figure 7-8a , b, and c).
The process alternatives described below are a selection of the many that may be imple-
mented. The naming convention for the different process alternatives is not standardized and care
should be taken to make sure that the process alternative is well understood by the design team
and the client irrespective of the naming
convention.
log[Ca
2+
]
–10
–8
2144 6 8 10 12
–2
–4
–6
0
pH
CaCO
3
(s)
C
T
=10
–4
C
T
=10
–2
C
T
=10
0
FIGURE 7-6
Logarithmic concentration diagram showing the relationship between pH, C
T
(mol/L of
inorganic carbon), and the equilibrium concentration of Ca
2
with respect to CaCO
3
(s).
(Source: Sawyer et al., 2003.)
7-12 WATER AND WASTEWATER ENGINEERING
Removal of CO
2
Because CO
2
in the raw water behaves as H
2
CO
3
, its removal is the first step in raising the pH
in lime-soda softening. It may be neutralized by the addition of lime, in which case it is not a
“process” in the conventional meaning of the word. When the concentration exceeds 10 mg/L as
CO
2
(22.7 mg/L as CaCO
3
or 0.45 meq/L), the economics of removal by aeration ( stripping ) are
favored over removal by lime neutralization. Air stripping is a separate process. No hardness is
removed in this process.
CO
2
(a)
Ca
2
HCO
3
C1
Mg
2
CO
2
(b)
Ca
2
HCO
3
C1
Mg
2
CO
2
(c)
Ca
2
HCO
3
Mg
2
Na
1. Add lime CO
2
(to raise pH)
2. Add lime HCO
3
(to raise pH)
3. Check is sum of Ca
2
that remains:
NCH Mg
2
120? If yes, remove Ca
2
that is
NCH with soda ash (Ca
2
HCO
3
).
4. Consider excess lime
1. Add lime CO
2
2. Add lime HCO
3
3. Consider excess lime
HCO
3
Ca
2
, therefore, all Ca
2
has been removed.
1. Add lime CO
2
2. Add lime HCO
3
3. Consider excess lime
FIGURE 7-7
Dosage schemes when Mg
2
concentration is less than or equal to 40 mg/L as CaCO
3
and no split
treatment is required. Note that no Mg
2
is removed and that reactions deal with CO
2
and Ca
2
only.
(Source: Davis and Cornwell, 2008.)
TABLE 7-2
Summary of chemical additions to soften water
a
The terms “Lime” and “Soda”refer to mg/L of Ca(OH)
2
and Na
2
CO
3
respectively, as CaCO
3
equal to mg/L of ion (or gas
in the case of CO
2
) as CaCO
3
.
Step
Chemical addition
a
Reason
Carbonate hardness
1. Lime CO
2
Neutralize H
2
CO
3
2. Lime
Raise pH; convert
HCO
3
to
3. Lime Mg
2
to be removed Raise pH; precipitate Mg(OH)
2
4. Lime required excess Drive reaction
Noncarbonate hardness
5. Soda noncarbonate hardness
to be removed
Provide
HCO
3
CO
3
2
CO
3
2
LIME–SODA SOFTENING 7-13
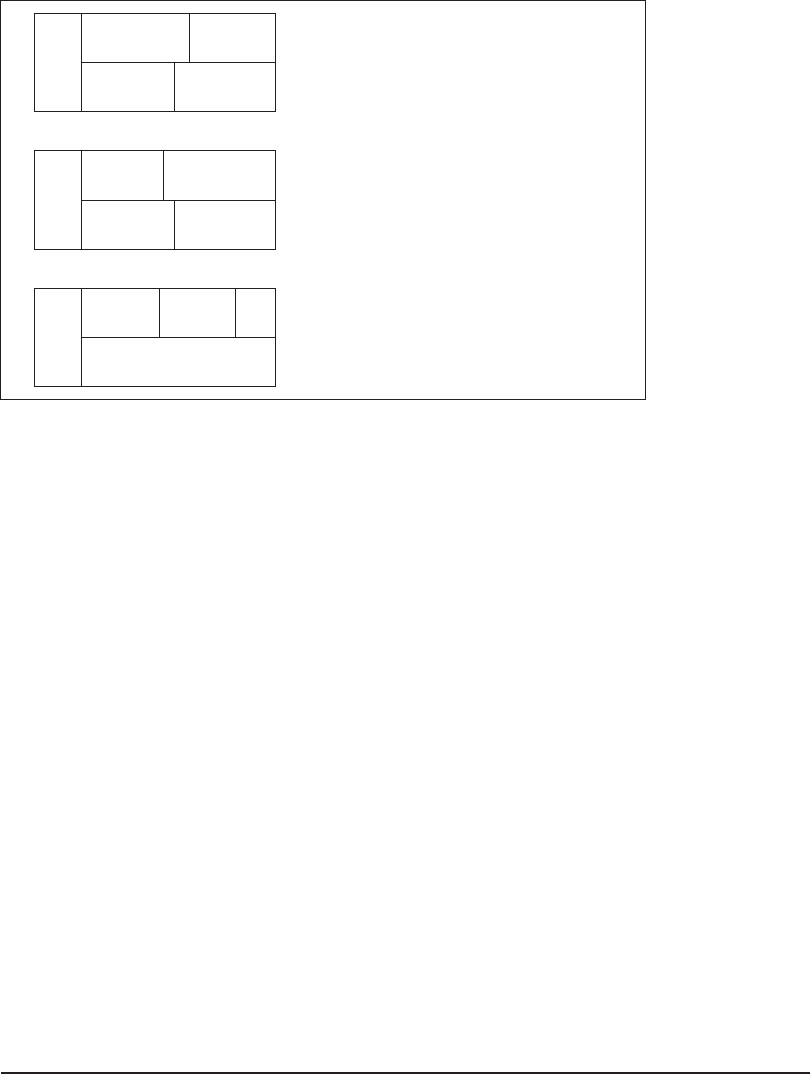
Lime Softening
Also called selective calcium removal, or partial lime softening, this alternative applies to cases
( a ), ( b ), and ( c ) in Figure 7-7 . Only lime is added. The desired c ontrol pH is 10.3. This process
removes only carbonate hardness caused by calcium.
Excess Lime Softening
This alternative applies to cases ( b ) and ( c ) in Figure 7-8 . Only lime is added. The desired control
pH is 11.3. This process removes only the carbonate hardness caused by calcium and magnesium.
Lime-Soda Softening
This alternative applies to cases ( a ), ( b ), and ( c ) in Figure 7-8 . Both lime and soda ash are added.
The desired control pH is 11.3. This process removes both carbonate and noncarbonate hardness
caused by calcium and magnesium.
Softening to Practical Limits
One process to achieve a specified magnesium concentration or to achieve a given hardness
is to treat a portion of the water to the practical limits and then blend the treated water with
the raw water to achieve the desired magnesium concentration or hardness. * Stoichiometric
amounts of lime and soda are added to remove all of the Ca
2
and Mg
2
to the prac tical limits
of softening (that is, 0.60 meq/L or 30 mg/L as CaCO
3
of Ca
2
and 0.20 meq/L or 10 mg/L as
CaCO
3
of Mg
2
).
CO
2
(a)
Ca
2
HCO
3
C1
Mg
2
CO
2
(b)
Ca
2
HCO
3
C1
Mg
2
CO
2
(c)
Ca
2
HCO
3
Mg
2
Na
1. Add lime CO
2
(to raise pH)
2. Add lime HCO
3
(to raise pH)
3. Add lime Mg
2
4. Add soda (Ca
2
Mg
2
) HCO
3
(to remove Ca
2
)
5. Consider excess lime
1. Add lime CO
2
2. Add lime HCO
3
3. Add lime Mg
2
4. Add soda (Ca
2
Mg
2
) HCO
3
5. Consider excess lime
1. Add lime CO
2
2. Add lime HCO
3
(Need all because need to raise pH)
3. Add lime Mg
2
4. No soda ash required
5. Consider excess lime
FIGURE 7-8
Cases when Mg
2
concentration is greater than 40 mg/L as CaCO
3
and split treatment is required.
Note that these cases illustrate softening to the practical limits in the first stage of the split-flow
scheme. (Source: Davis and Cornwell, 2008.)
*Generally, it is not practical to attempt to achieve both a desired magnesium concentration and a specified final hardness with
a single split, and
it is not economical to have multiple splits.
7-14 WATER AND WASTEWATER ENGINEERING
Split Treatment
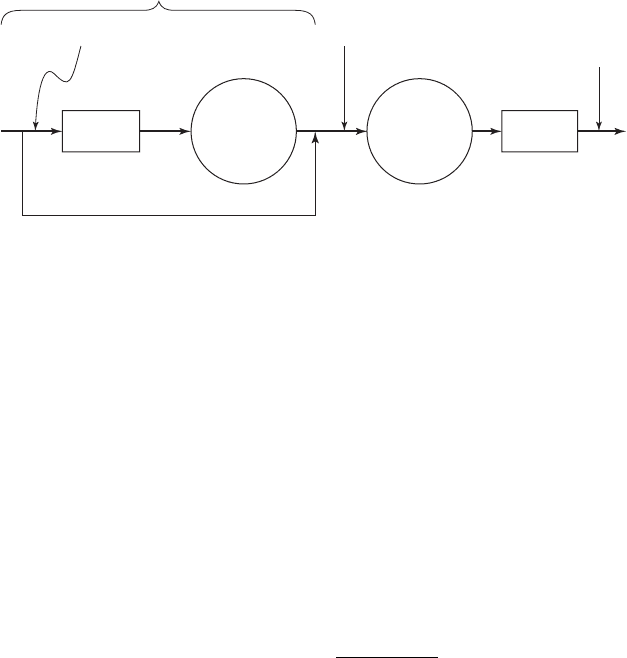
A s shown in Figure 7-9 , in split treatment a portion of the raw water is bypassed around the soft-
ening reaction tank and the settling tank. This serves several functions. First, it allows the water
to be tailored to yield a product water that has 0.80 meq/L or 40 mg/L as CaCO
3
of magnesium
(or any other value above the solubility limit). Second, it allows for a reduction in capital cost of
tankage because the entire flow does not need to be treated. Third, it minimizes operating costs
for chemicals by treating only a fraction of the flow. Fourth, it us es the natural alkalinity of the
water to lower the pH of the product water and ass
ist in stabilization. In many cases a second
sedimentation basin is added after recarbonation and prior to filtration to reduce the solids load-
ing onto the filters.
The fractional amount of the split is calculated as
X
f i
r i
Mg Mg
Mg Mg
(7-25)
where Mg
f
final magnesium concentration, mg/L as CaCO
3
Mg
i
magnesium concentration from first stage ( Figure 7-9 ), mg/L as CaCO
3
Mg
r
raw water magnesium concentration, mg/L as CaCO
3
The first stage is operated to soften the water to the practical limits of softening. Thus, the
value for Mg
i
i s commonly taken to be 10 mg/L as CaCO
3
. Because the desired concentration
of Mg is nom inally set at 40 mg/L as CaCO
3
a s noted previously, Mg
f
i s commonly taken as
40 mg/L as CaCO
3
.
Recarbonation
When the pH of the softened water is greater than the saturation pH, the pH must be reduced to
stop the precipitation reaction that will deposit CaCO
3
in the filters and distribution system pip-
ing because this will c ement them closed. CO
2
(which when dissolved in water forms H
2
CO
3
)
has frequently been found to be the most economical chemical to use in reducing the pH. Alterna-
tively, strong acids such as sulfuric acid may be employed. The stabilization process i s discussed
in detail in Section 7.8.
Raw
water
Softening
Bypass
Sedimentation
“First Stage”
Sedimentation
Filtration
Finished
water
Disinfection
RecarbonationFlow (1X)(Q)
Fraction bypassed (X)(Q)
Q Q
FIGURE 7-9
Split-flow treatment scheme. (Source: Davis and Cornwell, 2008.)

LIME–SODA SOFTENING 7-15
Five reactions that are employed in recarbonation are discussed below. In each case, the
chemical that is added to the water is printed in bold type. The notation (s) designates the solid
form and indicates that the compound has been removed from the water. The following reactions
are presented sequentially
, although in reality they occur simultaneously.
1. Recarbonation after selective calcium removal.
After selective calcium removal, the water will be supersatu rated with c alc ium carbon-
ate and the pH will be between 10.0 and 10.6. The addition of CO
2
lowers the pH to
between 8.5 and 9.0 and converts the carbonate ions to bicarbonate ions.
Ca CO
2
3
2
CO
2
H O Ca 2HCO
32
2
(7-26)
2. Recarbonation after the excess lime process.
After calcium and magnesium removal with excess lime, the pH will be above 11.0. Suf-
ficient CO
2
i s added to convert the excess hydroxyl ions to carbonate ions and then to
convert the carbonate ions to bicarbonate. This will occur in the pH range of 10.0 to 10.5.
Ca OH CaCO s HO
2
3 2
2
CO
2
()
(7-27)
Mg 2OH Mg CO H O
22
2
CO
2
3
2
(7-28)
Additional CO
2
is added to lower the pH to about 8.4 to 8.6.
CaCO (s)HOCa2HCO
3 2
2
3
CO
2
(7-29)
Mg CO H O Mg 2HCO
2
3 2 3
22
CO
2
(7-30)
7-4 CHEMICAL DOSAGES BASED ON STOICHIOMETRY
The estimation of the chemical dosage is used to design the chemical storage silos, chemical feed
systems, and sludge disposal facilities. In the following examples, it is assumed that the reactions
go to completion, that the lime and soda ash are pu
re (100 percent of the chemical), and that the
extra lime added to drive the reaction is removed by recarbonation.
Estimating CO
2
Concentration
CO
2
i s of importance in two instances in softening. In the first instance, it consumes lime that other-
wise could be used to remove Ca
2
and Mg
2
. In the second instance, CO
2
i s used to neutralize the
high pH of the effluent from the softening process. These reactions are an application of the concepts
of the carbonate buffer system discussed in Chapter 6.
The approximate concentration * of CO
2
may be estimated using the equilibrium expressions
for the dissociation of water and carbonic acid with the definition of alkalinity (Equation 6-3).
The pH and alkalinity of the water mus t be determined to make the estimate. The equilibrium
expressions for carbonic acid are
KK
a1
2 3
1
6 35
[][ ]
[]
H
HCO
p
HCO
.
3
a
at C25
(7-31)
*A more accurate estimation technique is described by Benefield and Morgan (1999).

7-16 WATER AND WASTEWATER ENGINEERING
KK
aa22
10 3
[][ ]
[]
HCO
HCO
p
3
2
3
. 33 25at C
(7-32)
where [ ] concentration in moles/L.
For water temperatures other than 25C, the dissociation constants may be estimated as
(Rossum and Merrill, 1983)
K
a1
14 8453 3404 71 0 032786
10
.../T T
(7-33)
K
a2
6 498 2909 39002379
10
.../T T
(7-34)
where T absolute temperature, K.
When the pH is less than 8.3,
HCO
3
is the dominant form of alkalinity, and total alkalinity is
nominally taken to be equal to the concentration of
HCO
3
(Figure 6-8). For most natural waters
this is a reasonable assumption. Thus, we can ignore the dissociation of bicarbonate to form car-
bonate. With this assumption, the procedure to solve the problem is:
a . Calculate the [H
] from the pH.
b. Correct the K
a
value for temperature.
c. Calculate the [
HCO
3
] from the alkalinity.
d. Solve the first equilibrium expression of the carbonic acid dissociation for [H
2
CO
3
].
e. Use the assumption that [CO
2
] [H
2
CO
3
] to estimate the CO
2
concentration.
E xample 7-3 illustrates a simple case where one of the forms of alkalinity predominates.
Example 7-3. What is the estimated CO
2
concentration of a water with a pH of 7.65 and a total
alkalinity of 310 mg/L as CaCO
3
? Assume the water temperature is 25C.
Solution. When the raw water pH is less than 8.3, we can assume that the alkalinity is pre-
dominately
HCO
3
. Thus, we can ignore the dissociation of bicarbonate to form carbonate.
a. The [H
] concentration is
[]H moles/L
10 2 24 10
765 8.
.
b. Because the alkalinity is reported as mg/L as CaCO
3
, it must be converted to mg/L as the
species using Equation 6-7 before the molar concentration may be calculated. The ratio
61/50 is the ratio of the equivalent weight of
HCO
3
to the equivalent weight of CaCO
3
.
The [
HCO
3
] concentration is
[]( )HCO
3
310
61
50
1
mg/L
mg/meq
mg/meq
⎛
⎝
⎜
⎞
⎠
⎟
(()()61 10
620 10
3
3
g/mole mg/g
mole
⎛
⎝
⎜
⎞
⎠
⎟
.
ss/L
