Water and Wastewater Engineering
Подождите немного. Документ загружается.


LIME–SODA SOFTENING 7-17
c. With p K
a 1
6.35, solve Equation 7-31 for [H
2
CO
3
].
[]
[][ ]
HCO
H
2 3
1
1
6 35 7
10 4 47 10
HCO
.
3
.
K
K
a
a
moles/L at C
HCO
moles/L
25
224 10
2 3
8
[]
(.
))( )620 10
447 10
3 11
3
7
.
.
.
moles/L
moles/L
10
4
moles/L
d . Assume that all the CO
2
in water forms carbonic acid. Thus, the estimated CO
2
concen-
tration is
[]CO moles/L
2
4
3 11 10
.
In other units for comparison and calculation:
CO moles/L mg/mole
2
4 3
3 11 10 44 10 13
()()..77
2
mg/L as CO
and
CO mg/L as CO
mg/meq
mg/meq
22
13 7
50
22
()
)
.
⎛
⎝
⎜⎜
⎞
⎠
⎟
3114 311
3
..or mg/L as CaCO
The equivalent weight of CO
2
i s taken as 22 because it effectively behaves as carbonic acid
(H
2
CO
3
) and thus n 2.
Comment. This CO
2
concentration is high enough to warrant consideration of air stripping to
remove it.
Selective Calcium Removal
When the magnesium concentration is less than 40 mg/L as CaCO
3
, lime softening (also
called partial lime softening ) can produce the desired final hardness. The alternative dos-
ing schemes are dependent on the amount of carbonate alkalinity as shown in Figure 7-7 .
In each instance CO
2
removal is shown by lime neutralization. This assumes that this is the
economic alternative. In addition, it shou ld be noted that lime must be added to the stoichio-
metric equivalent of the bicarbonate present regardless of the c oncentration of calcium. If the
bicarbonate is not neutralized, the pH objective of 10.3 required to prec ipitate the c
alcium
will not be achieved.
E xample 7-4 illustrates one case of those shown in Figure 7-7 , using both mg/L as CaCO
3
and milliequivalents/L (meq/L) as units of measure.
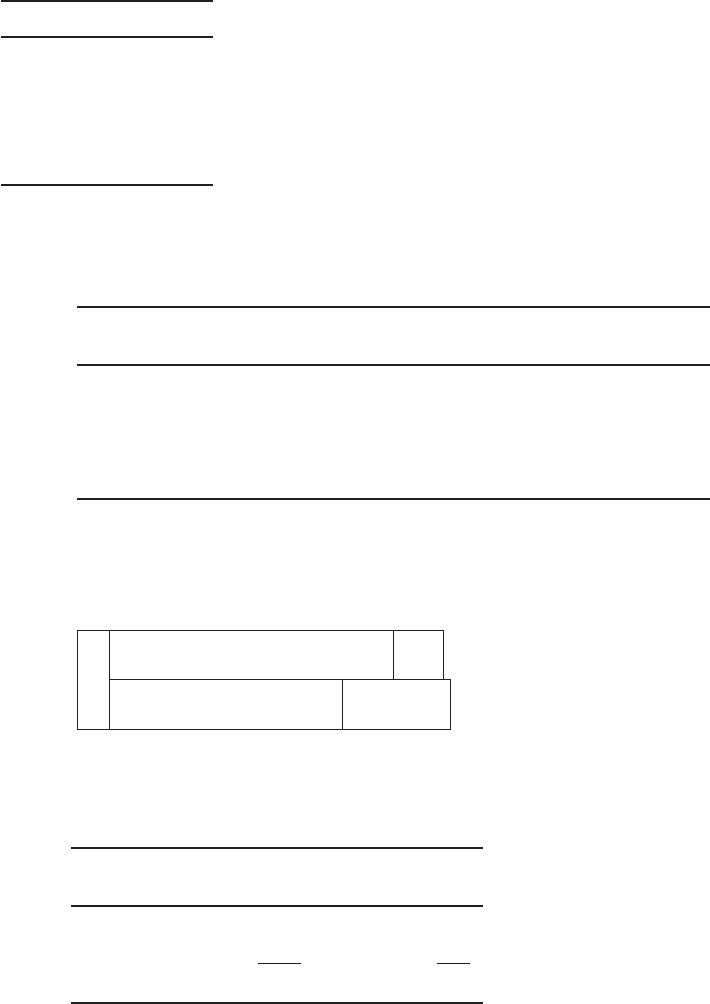
Example 7-4. Prepare a bar chart for Sweetwater’s water analysis given below and determine
the chemical dosage required for selective c alcium removal. Estimate the dosage of quicklime
(CaO) that needs to be added if the purity of lime is 90%.
7-18 WATER AND WASTEWATER ENGINEERING
Sweetwater water analysis
a
Constituent mg/L
CO
2
6.6
Ca
2
80
Mg
2
8.5
HCO
3
200.0
S
O
4
2
73
a
Assume that other ions in the water that are not reported account for the lack of an ion balance.
Solution:
a. Begin by converting all the concentrations to CaCO
3
equivalents and meq.
Constituent, mg/L EW EW CaCO
3
/EW ion
mg/L as
CaCO
3
meq/L
CO
2
6.6 22.0 2.28 15.0 0.30
Ca
2
80 20.0 2.50 200.0 4.00
Mg
2
8.5 12.2 4.12 35.0 0.70
200.0 61.00 0.820 164.0 3.28
73 48.00 1.04 76 1.52
HCO
3
SO
4
2
NOTE: meq/L (mg/L as CaCO
3
)/50 and meq/L (mg/L)/EW.
The bar chart of the raw water in mg/L as CaCO
3
is shown below.
15
164 240
CO
2
Ca
2
Mg
2
HCO
3
SO
4
2
0 200 235
0
b. Bec ause Mg
2
i s less than 40 mg/L as CaCO
3
, removal of magnesium is unnecessary.
The chemical additions are as follows:
Addition
equal to:
Lime,
mg/L as CaCO
3
Lime,
meq/L
CO
2
15.0 0.30
HCO
3
164.0 3.28
179.0 3.58
c. Using the rule of thumb for extra lime, with Mg
2
40 mg/L as CaCO
3
, the extra lime
dosage shou ld be about 20 mg/L as CaCO
3
. The total amount of lime to be added is
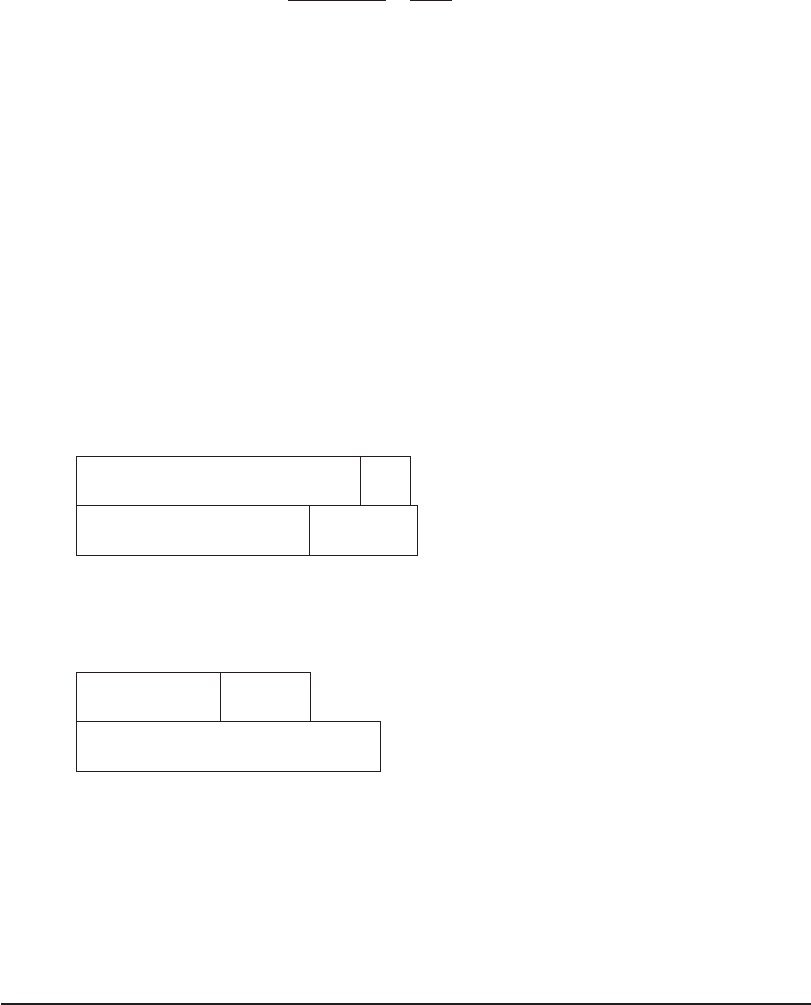
LIME–SODA SOFTENING 7-19
179.0 mg/L as CaCO
3
20 mg/L as CaCO
3
199 mg/L as CaCO
3
. Lime is purchased
and stored as CaO. The amount of 90% pure lime as CaO is
()199
28
50
3
mg/L as CaCO
meq/mg
meq/mg
⎛
⎝
⎜
⎞
⎠
⎟
11
090
123 8 124
.
.
⎛
⎝
⎜
⎞
⎠
⎟
or mg/L as CaO
where the equivalent weight of CaO 28 meq/mg and the equivalent weight of
CaCO
3
50 meq/mg.
d. The total hardness of the finished water (after recarbonation ) is
Ca Initial mg/L as CaCO mg/L as
2
3
200 164
CCaCO removed
with bicarbonate
mg/L as C
3
36 aaCO
Mg mg/L as CaCO
Total hardness
3
2
3
35
36
35 71
3
mg/L as CaCO
e. The changes in the water composition as illustrated by the changes in the bar chart are
shown below.
Bar chart after removal of CO
2
(in mg/L as CaCO
3
).
164 240
Ca
2
Mg
2
HCO
3
SO
4
2
0 200 235
0
Bar chart of the finished water (in mg/L as CaCO
3
).
76
Ca
2
Mg
2
03635
0
SO
4
2
Comments:
1 . Lime neutralization is used because the CO
2
is less than 10 mg/L as CO
2
.
2. The finished water is quite soft and consideration should be given to splitting the flow
to bypass some of the raw water to blend to a higher residual hardness. This would save
capital costs by using smaller tanks and operating costs by reducing chemical usage as
well as
the amount of sludge that has to be disposed.

7-20 WATER AND WASTEWATER ENGINEERING
Softening to Practical Limits
Magnesium is more expensive to remove than calcium, so as much Mg
2
i s left in the water
as possible. It is more expensive to remove noncarbonate hardness than carbonate hardness
because soda ash must be added to provide the
CO
3
2
. Therefore, as much noncarbonate hard-
ness is left in the water as possible. One way to achieve these objectives is to treat a portion
of the water to the practical limits and then blend the treated water with the raw water to
achieve the desired hardness. This form of split treatment does not control the final Mg
2
hardness.
Stoichiometric amounts of lime and soda are added to remove all of the Ca
2
and Mg
2
to
the practical limits of softening, that is 0.60 meq/L or 30 mg/L as CaCO
3
of Ca
2
and 0.20 meq/L
or 10mg/L as CaCO
3
of Mg
2
. Example 7-5 illustrates the technique using both mg/L as CaCO
3
and milliequivalents/L as units of measure.
Example 7-5. Prepare a bar chart for Mineral Wells water analysis given below and determine
the chemical dosages to soften the water to the practical solubility limits. Assume that the lime
and soda are 100% pure.
Mineral Wells water analysis
a
Constituent mg/L
CO
2
9.6
Ca
2
95.2
Mg
2
13.5
Na
25.8
Alkalinity
b
198
Cl
67.8
73
SO
4
2
a
A ssume that other ions in the water account for the lack of an ion balance.
b
mg/L as CaCO
3
Solution:
a. Begin by converting all the concentrations to CaCO
3
equivalents and meq.
Constituent mg/L EW EW CaCO
3
/EW ion mg/L as CaCO3 meq/L
CO
2
9.6 22.0 2.28 21.9 0.44
Ca
2
95.2 20.0 2.50238.0 4.76
Mg
2
13.5 12.2 4.12 55.6 1.11
Na
25.8 23.0 2.18 56.2 1.12
Alkalinity 198 3.96
Cl
67.8 35.5 1.41 95.6 1.91
73 48.0 1.04 76 1.52
SO
4
2
b . Bar chart of raw water in mg/L as CaCO
3
.
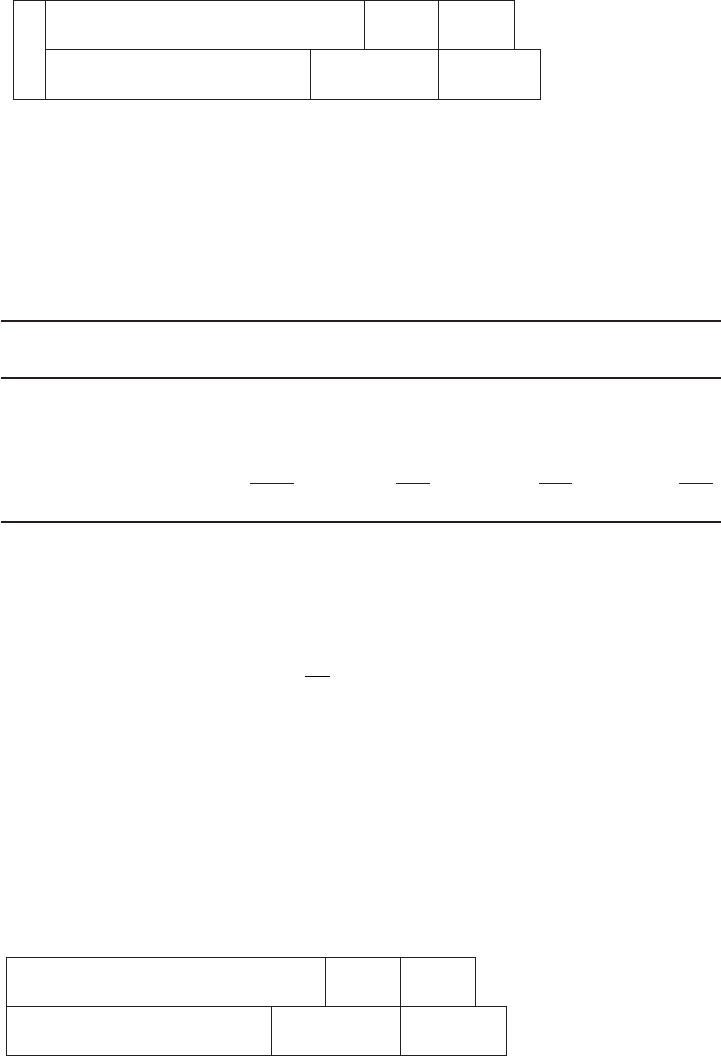
LIME–SODA SOFTENING 7-21
21.9
21.9
198 293.6 369.6
CO
2
Ca
2
Mg
2
Na
Cl
HCO
3
SO
4
2
0 238 293.6 349.8
0
This is similar to the case shown in Figure 7-8a .
Solution:
a. To soften to the practical solubility limits, lime and soda must be added as shown
below.
Addition
equal to:
Lime,
mg/L as CaCO
3
Lime,
meq/L
Soda,
mg/L as CaCO
3
Soda,
meq/L
CO
2
21.9 0.44
198.0 3.96
Ca
2
minus 40 0.80
Mg
2
55.6 1.11 55.6 1.11
Total
275.55.5195.6 1.91
HCO
3
H
CO
3
Because the difference Mg
2
40 15.6 mg/L as CaCO
3
, the minimum excess lime
of 20 mg/L as CaCO
3
i s selected. The total lime addition is 295.5 mg/L as CaCO
3
or
165.5 mg/L as CaO. The soda addition is 95.6 mg/L as CaCO
3
or
95 6
53
50
101 3 100
3
..mg/L as CaCO or m
⎛
⎝
⎜
⎞
⎠
⎟
gg/L as Na CO
2 3
Note that (53/50) is the equivalent weight of Na
2
CO
3
/equivalent weight of CaCO
3
.
b. The total hard ness of the finished water is the sum of the practical solubility limits for
calcium and magnesium, that is 30 mg/L as CaCO
3
10 mg/L as CaCO
3
40 mg/L as
CaCO
3
.
c. The step-wise changes in the bar chart for each of the chemical additions is shown below.
Bar chart after removal of CO
2
(in mg/L as CaCO
3
).
198 293.6 369.9
Ca
2
Mg
2
Na
Cl
HCO
3
SO
4
2
0 238 293.6 349.8
0
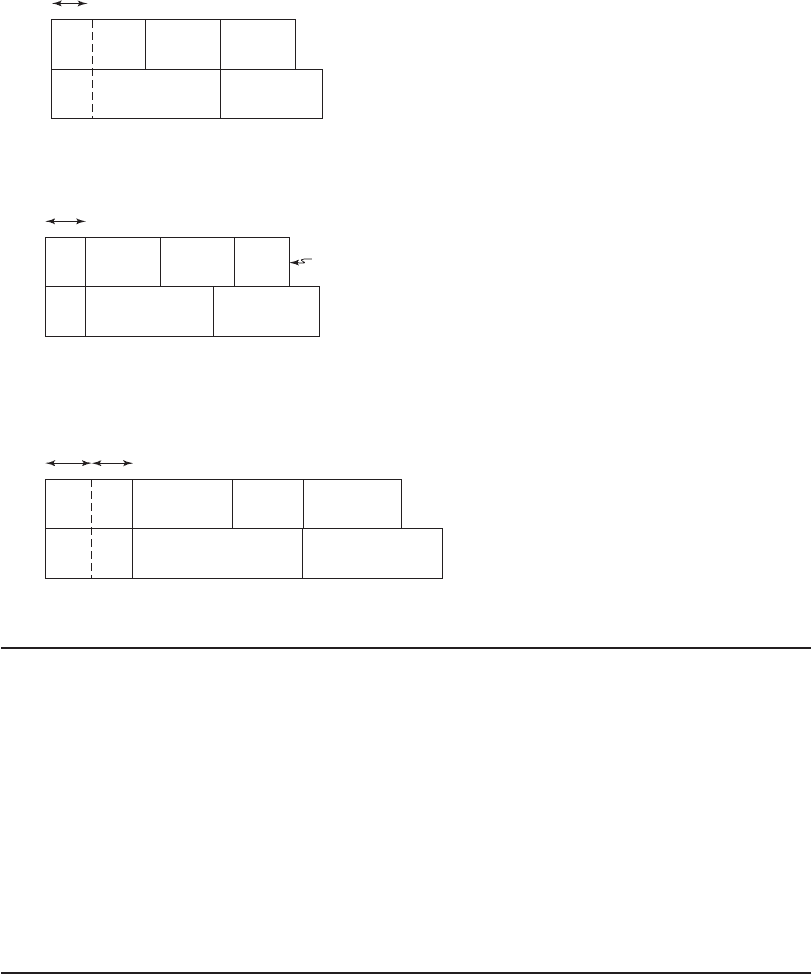
7-22 WATER AND WASTEWATER ENGINEERING
Bar chart after reaction with
HCO
3
(in mg/L as CaCO
3
).
95.6
95.6
171.6
151.8
Ca
2
Ca
2
CO
2
Mg
2
Na
Cl
SO
4
2
3
0
30
40
030
Bar chart after reaction of calcium with soda (in mg/L as CaCO
3
).
95.6
111.8
171.6
151.8
added
Ca
2
Mg
2
40 Na
2
CO
3
Na
Na
Cl
SO
4
2
CO
3
2
30
55.6
Bar chart after reaction of magnesium with lime and soda to yield the finished water (in
mg/L as CaCO
3
).
95.6
56.2
171.6
96.2 151.8
Ca
2
CO
2
Mg
2
Na
Na
Na
Cl
OH
SO
4
2
3
30
0
10
Comment: Lime neutralization of CO
2
is used because the CO
2
is less than 10 mg/L.
Split Treatment
When the magnesium concentration is greater than 40 mg/L as CaCO
3
, the flow is split to achieve
a magnesium hardness of 40 mg/L as CaCO
3
a s noted above. The portion of the flow that is
treated is dosed to achieve the practical solubility limits for calcium and magnesium. The alterna-
tive dosing schemes are dependent on the amount of carbonate alkalinity as shown in Figure 7-8 .
In each instance CO
2
removal is shown by lime neutralization. This assumes that this is the eco-
nomic alternative.
If the total hardness after blending is above the desired final hardness, then further softening in a
second stage is required ( Figure 7-10 ). Because the split is designed to achieve a desired Mg
2
of 40
mg/L as CaCO
3
, no further Mg
2
removal is required. Only treatment of Ca
2
i s required. The dos-
ing scheme for selective calcium removal is employed. Example 7-6 illustrates the dosing scheme.
Example 7-6. Prepare a bar chart for the Hard Times water analysis given below and determine the
chemical dosages to soften the water to meet the following finished water criteria: max
imum magne-
sium hardness of 40 mg/L as CaCO
3
and a total hardness in the range 80 to 120 mg/L as CaCO
3
.
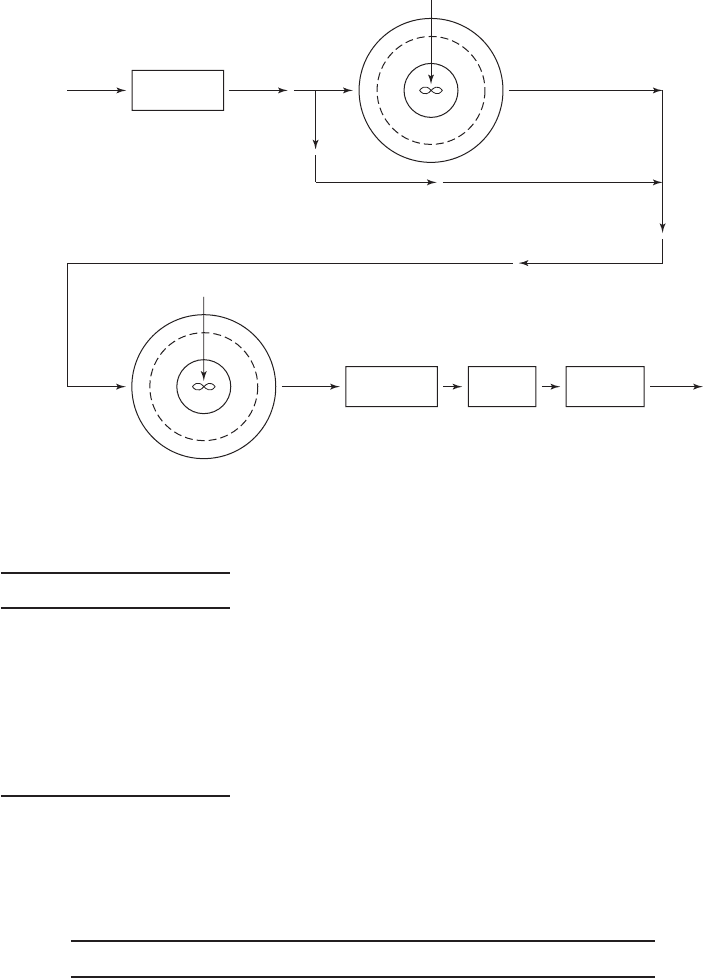
LIME–SODA SOFTENING 7-23
Raw water Aeration
First stage
Second stage
Lime/soda ash
Lime/soda ash
Recarbonation Filtration Disinfection
Flow bypassed XQ
Flow (1X)Q
Mg
r
Mg
i
Mg
f
Q
Q
FIGURE 7-10
Flow diagram for a two stage split-treatment lime–soda ash softening plant.
Solution:
a. Begin by converting all the concentrations to CaCO
3
equivalents and meq.
Constituent mg/L
CO
2
5.5
Ca
2
95.2
Mg
2
22.0
Na
25.8
Alkalinity
b
198
Cl
67.8
SO
4
2
73
Hard Times water analysis
a
a
Assume that other ions in the water account for the lack of an ion balance.
b
mg/L as CaCO
3
.
Constituent mg/L EW EW CaCO
3
/EW ion mg/L as CaCO
3
meq/L
CO
2
5.5 22.0 2.28 12.5 0.25
Ca
2
95.2 20.0 2.50238.0 4.76
Mg
2
22.0 12.2 4.12 90.6 1.80
Na
25.8 23.0 2.18 56.2 1.12
continued
7-24 WATER AND WASTEWATER ENGINEERING
b. Bar chart of raw water in meq/L.
0.25
0.25
3.96 5.87 7.39
CO
2
Ca
2
Mg
2
Na
Cl
HCO
3
SO
4
2
0 4.76 6.56 7.68
0
This is similar to the case shown in Figure 7-8a . Split treatment must be used to achieve
a magnesium concentration goal of 40 mg/L as CaCO
3
.
c. In the first stage the water is softened to the practical s olubility limits; lime and soda
must be added as shown below.
Addition
equal to:
Lime,
mg/L as CaCO
3
Lime,
meq/L
Soda,
mg/L as CaCO
3
Soda,
meq/L
CO
2
12.5 0.25
HCO
3
198.0 3.96
Ca
2
minus
HCO
3
40 0.80
Mg
2
90.6 1.80 90.6 1.80
Total 301.1 6.01 130.6 2.60
d. The split is calculated in terms of mg/L as CaCO
3
:
X
40 10
90 6 10
0 372
.
.
The fraction of water passing through the first stage is then 1 0.372 0.628.
e. The total hardness of the water after passing through the first stage is the sum of the
practical solubility limits, that is, 30 10 40 mg/L as CaCO
3
. Because the total hard-
ness in the raw water is 238 90.6 328.6 mg/L as CaCO
3
, the mixture of the treated
and bypass water has a hardness of:
()( )()(0 372 328 6 0 628 40
3
.. .mg/L as CaCO mg/ LLas CaCO
mg/L as CaCO
3
3
147 4
)
.
This is above the specified finished water criteria range of 80–120 mg/L as CaCO
3
, so
further treatment is required.
f. Because the split is designed to yield 40 mg/L as CaCO
3
of magnesium, no further
magnesium is removed. To achieve the desired total hardness more calcium must be
Constituent mg/L EW EW CaCO
3
/EW ion mg/L as CaCO
3
meq/L
Alkalinity 198.0 3.96
Cl
67.8 35.5 1.41 95.6 1.91
73 48.0 1.04 76 1.52
S
O
4
2
LIME–SODA SOFTENING 7-25
removed. Removal of the calcium equivalent to the bicarbonate will leave 40 mg/L as
CaCO
3
of calcium hardness plus the 40 mg/L as CaCO
3
of magnesium hardness for a
total of 80 mg/L as CaCO
3
. The additions are as follows.
Constituent
Lime
mg/L as CaCO3 Lime meq/L
CO
2
12.5 0.25
HCO
3
198.0 3.96
210.5 4.21
A ddition of lime equal to CO
2
and HCO
3
(even in the second stage) is necessary to
achieve the control pH of 10.3.
g. Excluding the extra lime to drive the reaction, the total chemical additions are in propor-
tion to the flows:
Limeor0 628 313 60372 210 5 2753 275.... .() () mg/L as CaCO
Soda
3
0 628 1306 0372 0 0....() ()82
3
mg/L as CaCO
h. Because the magnesium concentration is greater than 40 mg/L as CaCO
3
, the rule-of-
thumb addition of extra lime to the first stage is 40 mg/L as CaCO
3
.
Comment. In this case the final hardness is at the low end of the acceptable range. Because the
capital cost of installing a second stage is quite high, other alternative process schemes should be
considered. For example, treating more water in the first stage would result in a total hardness in
the acceptable range without the need for a sec
ond stage. The resulting water would have a mag-
nesium concentration lower than the design goal of 40 mg/L as CaCO
3
. An economic analysis
would have to be conducted because the capital cost would be less but the chemical and operating
costs including sludge disposal might be higher.
Other Estimating Methods
The method of estimating dosages used here is a traditional technique that provides a direct
link to the chemical reactions and, with the bar charts , provides a means of illustrating
the chemical processes. An alternative to the stoichiometric approach is the solution of the
simu ltaneous eq
uilibria equations to es timate the dosage. A series of diagrams called the
Caldwell-Lawrence diagrams have been developed to solve these equations graphically.
Examples of their use may be found in AWWA (1978), Merrill (1978), Benefield et al.
(1982), and Benefield and Morgan (1999).
The American Water Works Association has compu ter software for working with the
Caldwell-Lawrenc
e diagrams. It is called The Rothberg, Tamburini, and Winsor Model for
Corrosion Control and Process Chemistry.
Use of Caustic Soda
Caustic soda (NaOH) is an alternative to the use of lime for softening. It has the advantages of
decreased sludge production, reduction in dust generation, and the option of simpler storage and
feed systems because it is purchased as a liquid. There are several disadvantages in using
caustic
7-26 WATER AND WASTEWATER ENGINEERING
soda: the cost is four to six times higher than lime, the potential for hazardous chemical release is
greater because it is a liquid, and freezing problems occur for 50 percent solutions at temperatures
below 13C (Kawamura, 2000). The choice of caustic over lime will fundamentally be driven by
economic evaluation of the cost of caustic, the feed system, and sludge treatment and disposal.
The stoichiometric reactions may be derived by replacing Ca(OH)
2
with NaOH in Equations
7-12 through 7-15 and rebalancing the reactions. Because Ca
2
hardness is not substituted for
Mg
2
, the reactions shown in Equations 7-16 and 7-18 are not required. The sodium carbonate
formed in the reactions of caus tic with carbonate hardness is available to precipitate calcium
noncarbonate hardness.
7 -5 CONCURRENT REMOVAL OF OTHER CONSTITUENTS
Arsenic
Arsenic removal ranging from 60 to 90 percent have been observed in softening plants that use
excess lime for Mg
2
treatment. For single-stage softening plants that remove only Ca
2
, 0 to 40
percent removal has been observed (MWH, 2005). Removal effectiveness is highly dependent on
the oxidation state of the arsenic. Arsenate ( 5) is more readily removed than arsenite ( 3). The
major removal mechanism is by adsorption to the precipitate (MWH, 2005).
Iron and Manganese
The solubility diagrams shown in Figures 7-11 and 7-12 reveal that ferrous hydroxide [Fe(OH)
2
( s)]
and manganese hydroxide [Mn(OH)
2
( s)] precipitate at high pH. Softening processes that achieve a
pH greater than 9.6 remove 100 percent of the iron. Manganese is more difficult to remove. The pH
must be greater than 9.8 to remove 100 percent of the manganese (Kawamura, 2000). Because the
desired control pH for softening processes is 10.3 or greater, iron and manganese are effectively
removed concurrently. The extra mass of li
me added in the softening process is sufficiently great to
provide an excess over the stoichiometric requirements to remove the iron and manganese.
Natural Organic Matter (NOM)
The concurrent removal of NOM in the softening process is of importance in preventing the for-
mation of trihalomethanes (THM) and haloacetic acids (HAA5) when chlorine is used as a dis-
infectant. The effectiveness of lime-soda softening in reducing NOM is different for each water
source. However, some generalizations may be made (Benefield an
d Morgan, 1999):
• Calcium carbonate precipitation generally removes from 10 to 30 percent of the color, total
organic carbon, and disinfection byproduct precursors.
• Magnesium hydroxide precipitation generally removes from 30 to 60 percent of the total
organic carbon and disinfection byproduct precur
sors, and 50 to 80 percent of the color.
• A ddition of iron in the form of ferric sulfate generally removes an additional 5 to 15 percent
of the color, total organic carbon, and disinfection byproduct precursors in either calcium
or magnesium precipitation.
Alum hydroxide is an amphoteric hydrox
ide. That is, it is soluble at both low and high pH. Thus,
at pH values normally encountered in lime-soda softening it is dissolved and is not effective in
enhancing the removal of NOM.
