Wang Zh.M. One-Dimensional Nanostructures
Подождите немного. Документ загружается.

x Contributors
Suklyun Hong
Department of Physics and Institute
of Fundamental Physics,
Sejong University, Seoul 143–747,
Korea
Byoung-Kye Kim
Department of Physics,
Chonbuk National University,
Jeonju, Korea
Hyo-Suk Kim
Department of Physics,
Chonbuk National University,
Jeonju, Korea
Ju-Jin Kim
Department of Physics,
Chonbuk National University,
Jeonju, Korea
Hideo Kohno
Graduate School of Science, Osaka
University 1-1 Machikaneyama,
Toyonaka, Osaka 560-0043, Japan
Thierry Laroche
Institut Charles Delaunay, Universit
´
ede
technologie de Troyes, CNRS FRE
2848 Laboratoire de Nanotechnologie
et d’Instrumentation Optique 12,
rue Marie Curie BP-2060,
10010 Troyes Cedex, France
Jeong-O Lee
Fusion-Biotechnology Research Center,
Advanced Materials Division,
Korea Research Institute of Chemical
Technology, Daejeon 305–600,
Korea
W. Lei
Key Laboratory of Semiconductor
Materials Science, Institute of
Semiconductors, Chinese Academy of
Sciences, PO Box 912, Beijing 100083,
P.R. China
M. Meyyappan
NASA Ames Research Center,
Moffett Field, CA 94035, USA
Guoquan Min
Shanghai Nanotechnology Promotion
Center, Shanghai 200037, China
Otto L. Muskens
FOM Institute for Atomic and
Molecular Physics, AMOLF,
c/o Philips Research Laboratories,
High-Tech Campus 4, 5656 AE
Eindhoven, The Netherlands
Frank Neubrech
Kirchhoff Institute for Physics,
University of Heidelberg,
Im Neuenheimer Feld 227,
69120 Heidelberg, Germany
Garrick Ng
NASA Ames Research Center,
Moffett Field, CA 94035, USA
Xiaoming Niu
Shanghai Nanotechnology Promotion
Center, Shanghai 200037, China
Hyun D. Park
US Naval Research Lab,
4555 Overlook Ave., SW,
Washington, DC 20375, USA
Noejung Park
Department of Applied Physics,
Dankook University,
Yongin 448–701, Korea
S.M. Prokes
US Naval Research Lab,
4555 Overlook Ave., SW, Washington,
DC 20375, USA
Contributors xi
Annemarie Pucci
Kirchhoff Institute for Physics,
University of Heidelberg,
Im Neuenheimer Feld 227,
69120 Heidelberg, Germany
Jaime G
´
omez Rivas
FOM Institute for Atomic
and Molecular Physics, AMOLF,
c/o Philips Research Laboratories,
High-Tech Campus 4, 5656 AE
Eindhoven,
The Netherlands
Hye-Mi So
Fusion-Biotechnology Research
Center, Advanced Materials Division,
Korea Research Institute
of Chemical Technology,
Daejeon 305–600, Korea
Xuhui Sun
NASA Ames Research Center,
Moffett Field, CA 94035, USA
Alexandre Vial
Institut Charles Delaunay,
Universit
´
e de technologie de Troyes,
CNRS FRE 2848 Laboratoire de
Nanotechnologie et d’Instrumentation
Optique 12, rue Marie Curie BP-2060,
10010 Troyes Cedex, France
Z.G. Wang
Key Laboratory of Semiconductor
Materials Science,
Institute of Semiconductors,
Chinese Academy of Sciences,
PO Box 912, Beijing 100083, P.R.
China
Zhiguo Wang
Department of Applied Physics,
University of Eletronic Science
and Technology of China,
Chengdu 610054, P.R. China
William J. Weber
Pacific Northwest National
Laboratory, PO Box 999, Richland,
WA 99352, USA
Hongjun Xiang
Hefei National Laboratory for Physical
Sciences at the Microscale, University
of Science and Technology
of China, Hefei, Anhui 230026, P.R.
China
Jinlong Yang
Hefei National Laboratory for Physical
Sciences at the Microscale,
University of Science and Technology
of China, Hefei, Anhui 230026,
P.R. China
Sung Soo Yi
Philips Lumileds Lighting, 370 West
Trimble Road, San Jose, CA 95131,
USA
Bin Yu
NASA Ames Research Center,
Moffett Field, CA 94035, USA
Yafei Zhang
Research Institute of Micro/Nano
Science and technology,
Shanghai Jiaotong University,
Shanghai 200030, China
Weimin Zhou
Shanghai Nanotechnology Promotion
Center, Shanghai 200037, China
Xiaotao Zu
Department of Applied Physics,
University of Eletronic Science
and Technology of China,
Chengdu 610054,
P.R. China
Chapter 1
Study of Nanowire Growth Mechanisms:
VLS and Si Assisted
Hyun D. Park and S.M. Prokes
Abstract In this chapter, we have examined several of our recent results on InAs
nanowires that have implications to the vapor–liquid–solid (VLS) growth mecha-
nism as well as the newly proposed Si-assisted growth mechanism. In summary,
the study on the effect of oxygen during the nanowire growth showed the inhibiting
effect of oxygen on the VLS growth mechanism. The results on the observation of
size-dependent liquidus depression, more importantly, do not seem applicable on the
results of Ti-catalyzed grown Si nanowires, but bring into question the validity of the
vapor–solid–solid (VSS) growth mechanism in the Au-catalyzed grown GaAs and
InAs nanowires. In the newly proposed nanowire growth mechanism, namely the
Si-assisted mechanism using SiO
x
, a growth model is proposed based on the phase
separation of SiO at higher temperature, which forms a stable SiO
2
and reactive,
nanometer-sized Si clusters. It is suggested that these clusters consequently serve
as the nucleating/catalyst sites for the growth of InAs nanowires with the growth
mechanism different from VLS, VSS, and OA.
1.1 Introduction
Two primary nanowire growth mechanisms have been reported in the literature:
vapor–liquid–solid (VLS) and oxide assisted (OA). On the one hand, the OA growth
mechanism does not use any metal catalyst, but somehow utilizes the reaction of the
metastable oxide. Previously, nanowires such as Ge [1], C [2], GaAs [3], and Si [4]
have been grown using GeO, CO, Ga
2
O
3
, and SiO, respectively. Based on these re-
sults, it has been suggested that the metastable oxides serve as a nucleating center for
subsequent growth of the nanowires, but much work is still needed to fully under-
stand this growth mechanism. On the other hand, the VLS growth mechanism uti-
lizes a metal catalyst (such as Au, Ni, and Fe) to enhance the nanowire growth. The
key feature of this growth mechanism is the liquid state of the metal alloy tip during
the nanowire growth, which occurs by the formation of a eutectic alloy. Once the
1
2 H.D. Park, S.M. Prokes
alloy tip forms a eutectic, any excess material that is adsorbed from the vapor pre-
cipitates out in the form of a nanowire. Over the years, numerous nanowires such as
Si [5,6], Ge [7], and InAs [8–10], to name a few, have been successfully grown using
this growth mechanism. Recently, however, a different growth mechanism, namely
the vapor–solid–solid (VSS) (where the metal alloy tip is supposedly in solid state
during the nanowire growth, unlike the VLS) was proposed to explain the growth of
Au-catalyzed GaAs [11] and InAs [12] nanowires in the CBE and MOVPE growth
systems, respectively. The growth of InAs nanowires in the MOVPE system, in par-
ticular, was shown to occur at temperatures up to 480
◦
C when using an Au catalyst
(this is near the Au–In eutectic temperature of 450
◦
C), but no growth was noted at
higher temperatures. However, when a 13-
˚
ASiO
x
layer was deposited along with
the Au catalyst, the growth of InAs nanowires at temperature of 580
◦
Cwasre-
ported. Based on this preliminary work, it was suggested that the growth at higher
temperature was possible only with the presence of the oxide, where its effect was
to prevent the Au–In tip from melting, thereby extending the VSS growth process.
Our own recent results (where we observed a size-dependent liquidus depression of
Au–In alloy tip) [13], however, reported InAs nanowire growth up to 660
◦
C (with
no SiO
x
), and the Au–In tip retained its liquid feature down to 400
◦
C. Since our
results confirmed that the VLS is valid in the InAs system, there is some question
as to the validity of the VSS growth mechanism in the Au-catalyzed grown GaAs
and InAs nanowires as well as the real role of the oxide employed in the work of
Dick et al.
In addition, in the work of Kolb et al. [14], SiO evaporation and VLS were com-
bined to grow Si nanowires. In this case, the suggested growth model included the
VLS mechanism to grow Si nanowires, and the oxide was thought to deposit only
around the Si core but not on the Au catalyst. In this chapter, we review some of our
recent work, which includes the effect of oxygen in the VLS growth mechanism,
the controversy regarding the VSS–VLS growth mechanisms, and the newly pro-
posed Si-assisted growth mechanism that was used to explain the nanowire growth
using SiO
x
. The outline of the chapter is as follows. In Sect. 1.2, the VLS growth
mechanism is discussed, which includes the basic description (Sect. 1.2.1), the ef-
fect of oxygen on VLS (Sect. 1.2.2), and the controversy regarding VSS and VLS
(Sect. 1.2.3). In Sect. 1.3, the Si-assisted growth mechanism is discussed. Conclud-
ing remarks are given in Sect. 1.4.
1.2 VLS Growth Mechanism
1.2.1 Basic Description
In its simplest term, the VLS growth mechanism is a nanowire growth process,
which utilizes a nanometer-sized metal alloy that is in liquid state during the
nanowire growth. It is perhaps the most well-known nanowire growth mechanism,
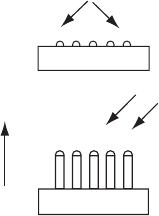
1 Study of Nanowire Growth Mechanisms: VLS and Si Assisted 3
gold nanoparticles
Sivapor
T
Si (111)
Si (111)
Fig. 1.1 VLS growth process of Si nanowires using Au nanoparticles on a Si(111) substrate
first put forward by Wagner and Ellis [15] and subsequently reviewed in detail by
Wagner and Givargizov [16, 17]. The primary feature of this growth mechanism is
the liquid metal alloy droplet which serves as the preferred site for nucleation due to
its large accommodation coefficient compared with the surrounding solid surface.
It should be noted that the dimension of the nanometer-sized droplet also serves to
determine the diameter of the nanowire.
The schematic of the basic steps involved in the VLS growth mechanism is
shown in Fig. 1.1 in the epitaxial growth of Si nanowires using Au nanoparticles on
a Si(111) substrate. The growth proceeds with the initial deposition of Au nanopar-
ticles (Au thin film can also be deposited instead) on an oxide-free Si(111) substrate
using poly-
L-lysine. The Au-deposited Si(111) substrate is then placed inside the
reaction zone and the temperature is raised above the Au–Si eutectic, where the Au
nanoparticles form nanometer-sized Au–Si liquid alloy droplets. At the nanowire
growth temperature, the Si vapor (from such sources as silane, SiCl
4
, or laser ab-
lation of Si target) impinging on these droplets allows supersaturation to occur.
Further incorporation of Si vapor into the droplet then causes the nanowire to grow
by precipitation above the substrate in the 111 growth direction, with the liquid
alloy droplet remaining at the top. When the nanowire growth is finished and cooled
to room temperature, the alloy droplet still remains at the tip of the nanowire, but
in solid form. The remnant of this metallic alloy nanoparticle found at the tip of
the nanowire is typically used as evidence of the VLS growth mechanism. Over the
years, numerous types of nanowire growths have been successfully attributed to this
growth mechanism [5,18–20].
1.2.2 Effect of Oxygen on VLS
Recently, we have examined the effect of oxygen on the VLS growth mechanism
in the growth of InAs nanowires inside a torch-sealed quartz tube [21]. The de-
tails of the experimental procedure for this work can be found elsewhere, but the
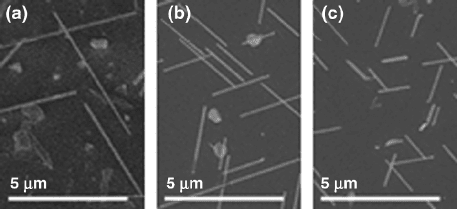
4 H.D. Park, S.M. Prokes
Fig. 1.2 Growth on InAs(111) with 5
◦
miscut as seen from the top with (a) 50 mTorr (no oxygen
backfill). Parts (b)and(c) are with 200 and 800 mTorr of oxygen backfill, respectively (reprinted
with permission from [21], Copyright 2006, American Institute of Physics)
experiment essentially consisted of a quartz tube (initially filled with bare and Au-
deposited InAs substrates) evacuated to 50 mTorr and then backfilled with 200 and
800 mTorr of oxygen and torch sealed. The quartz tube was then annealed inside
the open-end furnace for the nanowire growth time of 30 min. The InAs nanowires
were grown on InAs(111) substrate with 5
◦
miscut with Au catalyst. The results
are shown in the scanning electron microscope (SEM) images in Fig. 1.2a–c, where
there was a corresponding decrease in the nanowire lengths with the increase in the
oxygen level, clearly indicating the deleterious effect of oxygen on the VLS growth
mechanism.
To further ascertain the effect of oxygen on the VLS nanowire growth, nanowires
were first grown for only 5min using an Au catalyst on InAs(111)B substrate. These
nanowires were then oxidized in air at room temperature for 3 days, placed back into
the quartz tube, and then grown for an additional 25 min. The quartz tubes were all
sealed at 50 mTorr. The results are shown in Fig. 1.3a and b for 5 min and addi-
tional 25 min (after room temperature air oxidation), respectively. The nanowires
grown on InAs(111)B substrate under identical, nonoxidized growth condition typ-
ically yielded a length greater than 2µm (not shown), but as can be seen in the
SEM images, we only observed a small change in the length of the nanowires from
5 min (250–300 nm) to 25 additional minutes (300–600 nm). This result indicates a
noticeable decrease in the growth rate after the oxidation step.
From the growth experiments performed at various vacuum pressures, these re-
sults suggest that the presence of oxygen inhibits nanowire growth in the VLS
growth mechanism. Although some nanowires were still seen growing even at
800 mTorr, the inhibiting effect of oxygen was quite evident and it was more pro-
nounced with worsening vacuum conditions. The room temperature oxidation of the
Au–In tips in the air offers additional proof of the deleterious effect of oxygen on
the nanowire growth. For the Au–In alloy, the interdiffusion of Au and In occurs
quickly even at room temperature, and various intermetallic compounds are formed
depending on the wt% of In and Au. After 1 day, intermetallic compounds such
as AuIn
2
, AuIn, and Au
7
In
3
have been identified [22]. As In readily oxidizes, the
In in the Au–In alloy will be subjected to oxidation. Although the literature on the
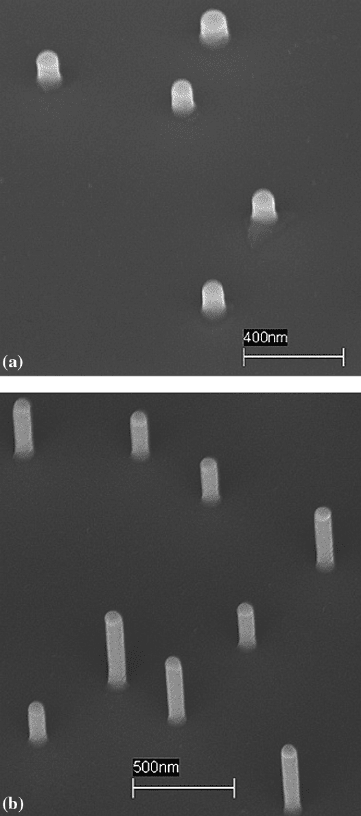
1 Study of Nanowire Growth Mechanisms: VLS and Si Assisted 5
Fig. 1.3 SEM images showing growth at (a) 5 min and (b) 25 additional minutes after 3 days room
temperature oxidation
oxidation of Au–In alloy is scarce, the work of Pasquevich et al. [23] has shown
that In oxidation occurs primarily on the surface, not internally, regardless of the In
content in the Au–In alloy. Consequently, the surface oxide, which forms either at
room or at higher temperature (at 580
◦
C during the growth at poor vacuum pres-
sures), will then serve as a diffusion barrier for impinging In and As atoms, which
are in the vapor state, thus inhibiting the nanowire growth. This is consistent with
our reported growth results.
6 H.D. Park, S.M. Prokes
1.2.3 VLS and VSS
A recent and more debated development regarding the VLS growth mechanism
is the newly proposed VSS growth mechanism that was used to explain the
Au-catalyzed growth of GaAs nanowires in CBE by Persson et al. [11] (and
subsequently by Dick et al. [12] in the Au-catalyzed growth of InAs nanowires
in MOVPE). The basis of the VSS growth mechanism is the solid state of the
metal alloy tip used during the nanowire growth, and not liquid as in the case of
VLS. Generally, the VLS growth mechanism had been quite successful over the
years in explaining the nanowire growth process that used metal catalysts in the
growth. Recently, however, there was one result, namely the growth of Ti-catalyzed
Si nanowires by Kamins et al. [24], that did not seem consistent with the VLS
growth mechanism. In their work, Kamins et al. found the growth of Ti-catalyzed Si
nanowire at approximately 600
◦
C, much below the Si–Ti eutectic temperature (the
lowest published Ti–Si eutectic temperature is about 1,300
◦
C). Because the growth
temperature was considerably lower than that required for the metal alloy tip to
be in liquid state, and with the size-dependent melting point depression interpreta-
tion insufficient, the authors then concluded that the nanowire should have grown
with the metal alloy tip in solid state. Subsequently, Persson et al. investigated the
growth of GaAs nanowires by CBE using an Au catalyst, where Au–Ga alloy is
formed. Then, through in situ transmission electron microscope (TEM) analysis and
X-ray energy dispersive spectroscopy (EDS) of the GaAs nanowires, Persson et al.
observed not only the crystallinity of the Au–Ga seed alloy particle at the growth
temperature, but also a low Ga concentration in the Au–Ga alloy below the level
required for the eutectic melt.
Based on these observations, VSS growth mechanism was then proposed. Soon
thereafter, Dick et al. [12] used this growth mechanism to explain the growth of InAs
nanowires by MOVPE as well (with Au–In alloy particle), suggesting the failure
of the VLS growth mechanism. In their work, the authors also concluded that the
growth of Au-catalyzed InAs nanowires was possible only when the metal alloy tip
of Au–In was in solid state during the nanowire growth, which limited the growth of
InAs nanowires below the eutectic temperature. It should be mentioned that for both
Au-catalyzed GaAs and InAs nanowires, the element As is not present (or present
only in trace amount) in these metal alloy tips and thus only a binary phase diagram
is necessary.
Since then, several contradicting works by Harmand et al. [25] and by us [13,21]
have been published, reexamining the validity of the VSS growth mechanism. In the
work of Harmand et al., the elemental composition of the Au–Ga seed alloy particle
was examined at different growth durations in the case of GaAs nanowires grown in
MBE. From their analysis, the authors have identified three different metallic com-
pounds at room temperature: the hexagonal β
Au
7
Ga
2
structure, the orthorhombic
AuGa structure, and an almost pure Au face-centered cubic structure, and observed
that the final composition of the metallic particle (determined at room temperature)
depended on the growth history of the wire. Thus, it was suggested that the Au–
Ga seed alloy particle was indeed in liquid state during the nanowire growth and
1 Study of Nanowire Growth Mechanisms: VLS and Si Assisted 7
that GaAs nanowires grew via the VLS mechanism in contrast to the VSS growth
mechanism of CBE-grown nanowires.
In our recent work, we studied the size-dependent melting point depression of
a metallic binary system undergoing the VLS growth mechanism. In our work, a
point elemental composition EDS was performed on various Au–In tip sizes (20–
100 nm) in the InAs nanowires that were grown in the temperature range 400–660
◦
C
(not shown). The highest growth temperature reported was approximately 100
◦
C
higher than the temperature (which is above the eutectic temperature) at which InAs
nanowires were grown in the MOVPE system of Dick et al. The results of EDS
analysis are shown in Table 1.1, where tips having diameters approximately 60 nm
and below showed decreased In content compared with diameters larger than 60nm.
The results show a 30% decrease in the In content in all nanowires with diameters
60 nm or less, as compared with the 100-nm nanowires. Interestingly, in the Au–
In phase diagram (not shown), the measured In content for the tip size 100 nm is
within the liquid region above the liquidus line, while the tip sizes 60 nm or less are
in the solid plus liquid region. These observations suggest the occurrence of size-
dependent melting point depression, but to support this claim, the InAs nanowires
were also grown below the Au–In eutectic at 400
◦
C using 20 and 60-nm-sized
Au nanoparticles. The results, as shown in Fig. 1.4, show the liquid-like migration
of Au–In alloy particle (as well as subsequent nanowire growth) despite the tem-
perature being below the level required for the eutectic melt, thereby confirming
the occurrence of size-dependent melting point depression as well as the nanowire
growth via VLS growth mechanism.
One possible origin of the melting temperature depression is the Gibbs–Thomson
effect owing to the finite size of a crystal [26]. To illustrate the order of magnitude
associated with this effect [13], consider a single-component spherical crystal of ra-
dius r. The melting temperature T
r
of the finite size crystal is given by the expression
T
m
−T
r
= 2
γΩ
/r∆S,
Table 1.1 Summary of EDS elemental composition analyses on the gold alloy tip of various
nanowires
Size (nm) per
element
580
◦
C (liq. 26.6%) 610
◦
C (liq. 25.5%) 660
◦
C (liq. 23%)
Au In Au In Au In
25 79.8 19.5
35 80.2 19.5
40 80.4 19.4
60 79.8 19.6 78.1 20.9 80.4 19.3
100 71.3 28.2 71.0 28.3 77.2 22.5
The values are in atm%. The standard deviation was ∼2atm%. Trace amount of As (<0.5atm%)
was also detected in the nanowire tips (reprinted from [13], Copyright 2006, with permission from
Elsevier)
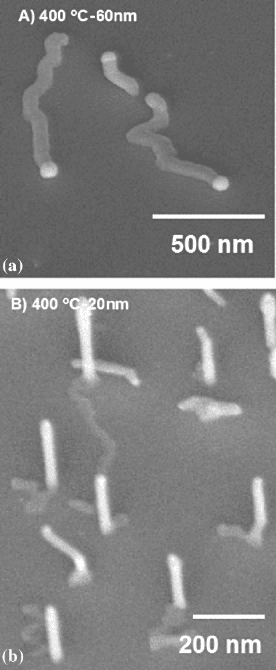
8 H.D. Park, S.M. Prokes
Fig. 1.4 InAs nanowires grown at 400
◦
Cfor(a)60nmand(b) 20 nm sized Au nanoparticles
(reprinted from [21], Copyright 2006, with permission from Elsevier)
where T
m
is the bulk melting temperature,
γ
is the free surface energy,
Ω
is the
molar volume, and ∆S is the molar entropy change for melting. Assuming ∆S to be
approximately independent of temperature, then ∆S = L/T
m
, where L is the molar
latent heat of melting, so that (T
r
−T
m
)/T
m
≈ 2
γΩ
/rL. Using the values for Au
(
γ
= 1.4Jm
−2
,
Ω
= 10.2cm
3
mol
−1
, L = 12.55kJmol
−1
) and a radius of 40 nm
gives a melting temperature reduction of 6%. This would also apply to a single-
component sphere sitting on a “noninteracting” substrate. For the case of Au on the
InAs substrate, however, it is a two-component alloy on an “interacting” substrate.
Nevertheless, since the expressions for Gibbs–Thomson effects in multicomponent
systems have the same dependence on the size of the crystal and similar depen-
dences on the surface thermodynamic parameters [26], it is expected that a finite
size Au–In alloy would display a similar order of magnitude reduction for the liq-
uidus temperature. Also, since the free surface area of the crystal is larger than the
