Wallace J.M., Hobbs P.V. Atmospheric Science. An Introductory Survey
Подождите немного. Документ загружается.


44 The Earth System
that settle to the ocean floor are converted into lime-
stone (CaCO
3
) rocks. This inorganic carbon reservoir
of the Earth’s crust is the largest of the carbon reser-
voirs in the Earth system.
2.3.3 Carbon in the Oceans
The carbon in the oceanic reservoir exists in three
forms: (1) dissolved CO
2
or H
2
CO
3
, also known as
carbonic acid, (2) carbonate ions paired
with Ca
2
and Mg
2
and other metallic cations,
and (3) bicarbonate ions. The third
form is by far the largest of the oceanic carbon
reservoirs (Table 2.3). The dissolved carbon dioxide
concentration equilibrates with atmospheric con-
centrations through the reaction
(2.7)
An increase in the atmospheric concentration of CO
2
thus tends to raise the equilibrium concentration of
dissolved CO
2
. Carbonic acid, in turn, dissociates to
form bicarbonate ions and hydrogen ions
(2.8)
thereby causing the water to become more acidic.
The increasing concentration of H
ions shifts the
equilibrium between carbonate and bicarbonate ions
(2.9)
toward the left. The net effect, obtained by adding
(2.7), (2.8), and the reverse of (2.9) is
(2.10)
which incorporates the added carbon into the bicar-
bonate reservoir without any net increase in the
acidity of the ocean. The ability of the ocean to take
up and buffer CO
2
in this manner is limited by the
availability of ions in the carbonate reservoir.
Marine organisms incorporate bicarbonate ions
into their shells and skeletons through the reaction
(2.11)
A fraction of the calcium carbonate created in
Eq. (2.11) settles on the sea floor and forms lime-
stone deposits, while the remainder dissolves through
the reverse reaction.
Ca
2
2HCO
3
: CaCO
3
H
2
CO
3
CO
2
CO
2
3
H
2
O M 2HCO
3
HCO
3
M
H
CO
2
3
H
2
CO
3
M H
HCO
3
CO
2
H
2
O M H
2
CO
3
(HCO
3
)
(CO
2
3
)
(2.12)
Limestone deposits tend to be concentrated in con-
tinental shelves beneath shallow tropical seas that
provide an environment hospitable to coral. At these
levels in the ocean, the acidity of the water is low
enough that shells and skeletons deposited on the
ocean floor do not dissolve.
The Ca
2
ions that marine organisms incorporate
into their shells enter the ocean by way of the
weathering of rocks in rain water that is carried to
the oceans in rivers. Some of these ions are derived
from the weathering of calcium-silicate rocks in the
reaction
(2.13)
The net effect of Eqs. (2.11) and (2.13), in combina-
tion with Eq. (2.7), is
(2.14)
which has the effect of taking up CO
2
from the
atmospheric and oceanic reservoirs and incorporating
it into the much larger reservoir of inorganic carbon
sedimentary rocks in the Earth’s crust.
From a climate perspective, the chemical reactions
(2.7)–(2.14) are virtually instantaneous. In contrast,
the timescale over which the oceanic reservoirs
adjust to changes in atmospheric CO
2
is governed by
the ventilation time for the deeper layers of the
ocean, which is on the order of centuries. Calcium
carbonate formation is limited by the availability of
calcium ions, which is determined by the rate of
weathering of calcium silicate rocks, as described in
the following subsection.
2.3.4 Carbon in the Earth’s Crust
The organic and inorganic carbon reservoirs in the
Earth’s crust are both very large, the exchange rates
in and out of them (apart from the burning of fossil
fuels) are very slow, and residence times are on
the order of many millions of years. Carbon enters
both of these reservoirs by way of the biosphere,
as described earlier. Most deposits of the organic
carbon in natural gas, oil, coal, and shales and
other sedimentary rocks were formed in anoxic
ocean basins. The even larger inorganic carbon
reservoir, consisting mostly of calcium carbonate
CaSiO
3
CO
2
: CaCO
3
SiO
2
SiO
2
H
2
O
CaSiO
3
H
2
CO
3
: Ca
2
2HCO
3
CaCO
3
H
2
CO
3
: Ca
2
2HCO
3
P732951-Ch02.qxd 9/12/05 7:40 PM Page 44

2.4 Oxygen in the Earth System 45
(CaCO
3
), is almost exclusively a product of the
marine biosphere.
Weathering exposes organic carbon in sedimentary
rock to the atmosphere, allowing it to be oxidized,
thereby completing the loop in what is sometimes
referred to as the long term inorganic carbon cycle.
Currently the burning of fossil fuels is returning as
much carbon to the atmosphere in a single year as
weathering would return in hundreds of thousands
of years! The mass of carbon that exists in a form
concentrated enough to be classified as “fossil fuels”
represents only a small fraction of the organic carbon
stored in the Earth’s crust, but it is nearly an order of
magnitude larger than the mass of carbon currently
residing in the atmosphere.
On timescales of tens to hundreds of millions of
years, plate tectonics and volcanism play an essential
role in renewing atmospheric CO
2
. This “inorganic
carbon cycle,” summarized in Fig. 2.25, involves sub-
duction, metamorphism, and weathering. Limestone
sediments on the sea floor are subducted into the
Earth’s mantle along plate boundaries where conti-
nental plates are overriding denser oceanic plates. At
the high temperatures within the mantle, limestone is
transformed into metamorphic rocks by the reaction
(2.15)
The CO
2
released in this reaction eventually returns
to the atmosphere by way of volcanic eruptions. The
metamorphic rocks containing calcium in chemical
combination with silicate are recycled in the form of
newly formed crust that emerges in the mid-ocean
ridges. The metamorphism reaction (2.15), in combi-
nation with weathering, and the carbonate formation
reaction (2.14) form a closed loop in which carbon
atoms cycle back and forth between the atmospheric
CaCO
3
SiO
2
: CaSiO
3
CO
2
CO
2
reservoir and the inorganic carbon reservoir in
the Earth’s crust on a timescale of tens to hundreds
of millions of years.
At times when the rate at which CO
2
is injected
into the atmosphere by volcanic eruptions exceeds
the rate at which calcium ions are made available by
weathering, atmospheric CO
2
concentrations increase
and vice versa. The injection rate is determined by
rate of metamorphism of carbonate rocks, which, in
turn, depends on the rate of plate movement along
convergent boundaries where subduction is occur-
ring. The rate of weathering, however, is proportional
to the rate of cycling of water in the atmospheric
branch of the hydrologic cycle, which increases with
increasing temperature. The fact that weathering
involves the chemical reaction (2.13) makes the
temperature dependence even stronger. Hence, high
ambient temperatures and slow plate movements
are conducive to a draw-down of atmospheric CO
2
and vice versa. The changes in atmospheric CO
2
in
response to imbalances between (2.14) and (2.15) on
timescales of tens of millions of years are believed to
have been quite substantial.
2.4 Oxygen in the Earth System
Earth is unique among the planets of the solar sys-
tem with respect to the abundance of atmospheric
oxygen O
2
and the presence of an ozone (O
3
) layer.
Atmospheric oxygen accounts for only a very small
fraction of the “free” oxygen (i.e., oxygen not bound
to hydrogen atoms in water molecules) in the Earth
system. Much larger quantities of free oxygen are
present in the form of oxidized minerals in sediments
and in the crust and upper mantle. The current level
of oxidation of the Earth system as a whole is much
higher than it was at the time when the planets first
formed.
The formation of the Earth’s molten metallic iron
had the effect of enriching oxygen concentrations in
the mantle, the source of volcanic emissions. Yet geo-
logical evidence suggests that oxygen was only a trace
atmospheric constituent early in the Earth’s history.
Iron in sedimentary rock formations that date back
more than 2.2 billion years is almost exclusively in the
partially oxidized ferrous (FeO) form. Had substantial
amounts of oxygen been present in the atmosphere
and oceans at the time when these sediments formed,
the iron in them would have been fully oxidized to
ferric oxide (Fe
2
O
3
). To account for the large concen-
trations of Fe
2
O
3
that currently reside in the Earth’s
Carbon Calcium Silicon
metamorphic
rocks
W
W
limestone
limestone
ions
quartz
oceans
atmosphere
M
M
M
S
S
W
Fig. 2.25 Schematic of the long-term inorganic carbon
cycle, also referred to as the carbonate–silicate cycle. The
symbol S denotes sedimentation, M denotes metamorphosis,
and W denotes weathering.
P732951-Ch02.qxd 9/12/05 7:40 PM Page 45
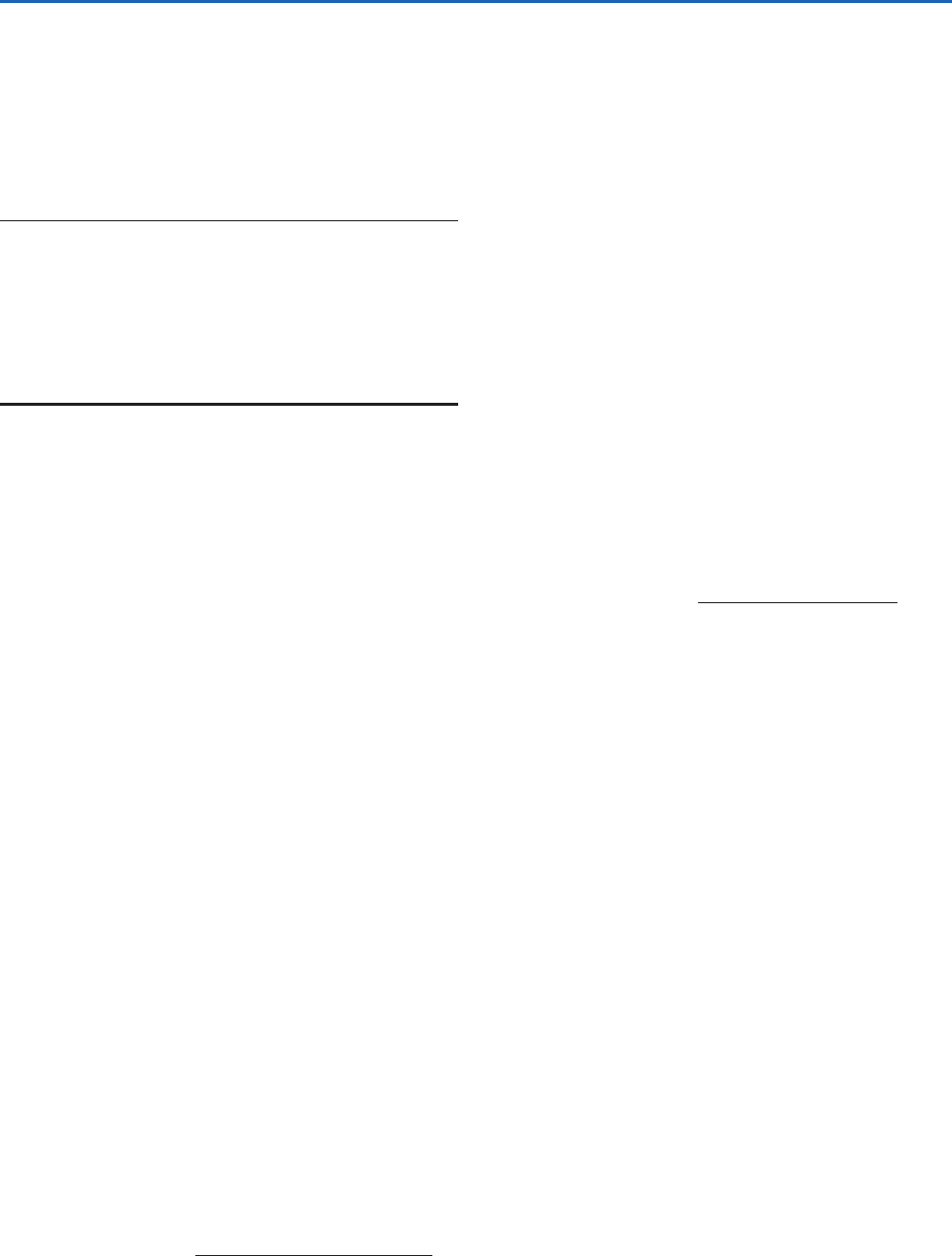
46 The Earth System
crust and upper mantle, it is necessary to invoke the
existence of a source of free oxygen that came into
play later in the history of the Earth system. Free
oxygen is also required to account for the formation of
carbonates in the Earth’s crust by marine organisms,
as illustrated in the following exercise.
Exercise 2.5 Estimate the mass of oxygen required
to form the carbonate deposits in the Earth’s crust.
Solution: From Table 2.3, the mass of carbon
in the carbonate reservoir in the Earth’s crust is
80,000 kg m
2
. Combining (2.7), (2.8), (2.9), and
(2.11), we obtain
The two hydrogen ions generated as a by-product of
this reaction combine with a single oxygen ion to
form water. Hence, the final result is
(2.16)
from which it is clear that one “free” oxygen atom is
paired with each carbon atom that resides in the
Earth’s crust in the form of carbonates. Hence, the mass
of free oxygen required to account for the carbonates is
or 100,000 kg m
2
, which is the basis for the estimate
in Table 2.4. ■
80,000 kg m
2
(16
12)
80,000 kg m
2
molecular weight of oxygen
molecular weight of carbon
CO
2
Ca
2
O
2
: CaCO
3
H
2
O CO
2
Ca
2
: CaCO
3
2H
2.4.1 Sources of Free Oxygen
The photosynthesis reaction (2.5) does not affect the
overall state of oxidation of the Earth system because
for every liberated O
2
molecule, an atom of organic
carbon is stored in sedimentary rocks in the Earth’s
crust. That carbon storage can be used to estimate
the mass of carbon liberated by photosynthesis, as
illustrated in the following exercise.
Exercise 2.6 On the basis of data in Table 2.3, esti-
mate the oxygen liberated by photosynthesis over
the lifetime of the Earth.
Solution: From Table 2.3, the mass of organic
carbon in sedimentary rocks in the Earth’s crust is
20,000 kg m
2
. From (2.5) it is evident that the burial
of each carbon atom in the Earth’s crust has liber-
ated one O
2
molecule. Hence, the mass of oxygen
liberated by photosynthesis is
or 50,000 kg m
2
. ■
Until quite recently, photosynthesis was believed to
have been the major source of free oxygen on Earth.
However, it is evident from Exercise 2.6 that a much
larger source is needed to account for the current
degree of oxidation of the Earth system.The only other
possible candidate is redox reactions that ultimately
lead to the escape of the hydrogen atoms to space, rais-
ing the state of oxidation of the Earth system. The rate
of escape of hydrogen atoms is proportional to their
number concentration in the upper atmosphere. The
principal source of free hydrogen is a four-step process.
1. Subduction or deep burial of rocks containing
hydrated minerals (i.e., minerals with chemically
bound H
2
O molecules or interstitial H
2
O
molecules in their lattices).
2. Breakdown of these hydrated minerals at
the high temperatures in the Earth’s mantle,
releasing the water in the form of steam.
3. The reaction
(2.17)
in which the steam oxidizes ferrous oxide in the
crust or mantle to ferric oxide.
4. The ejection of the hydrogen in volcanism or
metamorphism.
2FeO H
2
O : Fe
2
O
3
H
2
20,000 kg m
2
molecular weight of O
2
molecular weight of C
Table 2.4 Mass of oxygen (in units of 10
3
kg m
2
) averaged
over the surface of the Earth required to raise the level of
oxidation of the various reservoirs in the Earth system to
their present state, starting from their state at the time of
formation of the Earth
a
Reservoir Mass
Atmospheric O
2
2.353
Oceans and sediments 31
Crust Fe
3
100
Crust CO
3
100
Crust (other) 100
Mantle Fe
3
100
a
The estimates for the crust and mantle represent conservative lower limits.
[Based on data adapted with permission from Catling, D. C., K. J. Zahnle and
C. P. McKay, “Biogenic Methane, Hydrogen Escape, and the Irreversible
Oxidation of Early Earth,” Science, 293, p. 841. Copyright 2001 AAAS.]
P732951-Ch02.qxd 9/12/05 7:40 PM Page 46

2.4 Oxygen in the Earth System 47
The escape of hydrogen from the Earth’s atmos-
phere is detectable (Fig. 2.26), but the rate at which it
is occurring is far too small to account for the degree
to which the minerals in the Earth’s crust and mantle
have become oxidized over the lifetime of the Earth.
The rate of escape is slow because the two gases that
supply hydrogen atoms to the upper atmosphere
(i.e., CH
4
and H
2
O) are present only in concen-
trations of a few parts per million by volume. Air
entering the upper atmosphere from below loses
most of its water vapor as it passes through the cold
equatorial tropopause (see Sections 1.3.3 and 3.5).
There are indications that atmospheric methane con-
centrations may have been much higher in the past,
as discussed in the next section.
Photosynthesis and the escape of hydrogen oper-
ate independently to liberate oxygen. The crust of a
lifeless planet could become highly oxidized due
solely to the action of plate tectonics. Conversely,
photosynthesis could occur on a planet on which the
minerals in the crust and the gases in the atmosphere
were in a highly reduced state. However, of the two
mechanisms, only photosynthesis is capable of pro-
ducing atmospheric oxygen, and only the escape of
hydrogen from the Earth system is capable of liber-
ating oxygen in the quantities required to account for
the present degree of oxidation of the minerals in the
crust and mantle.
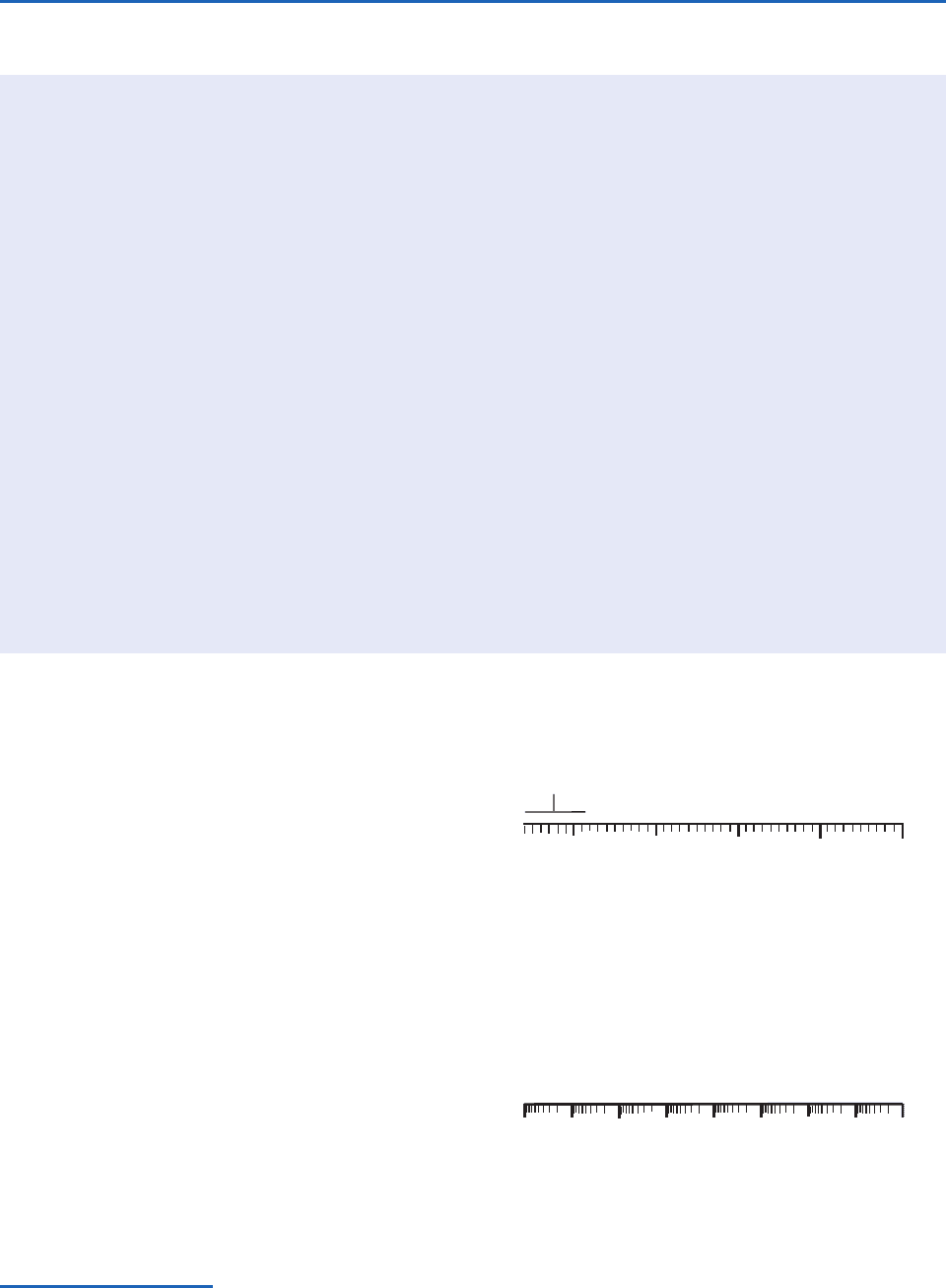
Fig. 2.26 The corona around the Earth in this image is due to
the scattering of solar radiation by hydrogen atoms that are
escaping from the atmosphere. [Image from NASADynamics
Explorer/Spin-Scan Auroral Imaging. Courtesy of David Catling.]
Isotopes of a given element are atoms with differ-
ent numbers of neutrons in their nuclei. Unstable
isotopes, such as
14
C, which spontaneously change
form by radioactive decay with a known “half-
life,” are used for dating ice, tree and sediment
cores, fossils, and rock samples. By comparing the
abundance of the isotope in the sample with the
current atmospheric abundance, it is possible to
infer how long the sample has been out of contact
with the atmosphere (provided, of course, that the
atmospheric abundance has not changed since the
sample was deposited). Relative abundances of
stable isotopes, such as
13
C, vary in accordance
with local (and in some cases regional or even
global) environmental conditions that prevailed at
the time when the sample was deposited. Some of
the more widely used isotopes are the following.
• The relative abundance of deuterium (
2
H,
or D)
15
in the snow samples recovered from
ice cores depends on (and hence can be used
as a proxy for) the temperature of the surface
from which the water vapor that condensed to
form the snow was evaporated.The greater
the relative abundance of HDO in the core
sample, the warmer that evaporating surface
2.1 Isotope Abundances: Proxies for Climate Data
15
The relative abundance of D is given by
where R is a reference value. Positive (negative) values of
D are indicative of an enrichment (depletion) of D relative to the reference
value, expressed in parts per thousand (ooo).The same formalism applies to the isotopes in subsequent bullets.
D(o/oo)
D
H R
R
Continued on next page
P732951-Ch02.qxd 9/12/05 7:40 PM Page 47
2.5 A Brief History of Climate
and the Earth System
This section describes evolution of the Earth system on
a logarithmically telescoping time line, as depicted in
the bottom panel of Fig. 2.27, with subsections focusing
on (1) the lifetime of the Earth, (2) the past 100 million
years, (3) the past million years, and (4) the past 20,000
years. If the life (to date) of a 20-year-old student were
viewed on an analogous and proportional time line, the
respective subsections would focus on hisher entire
20-year life span, the past 6 months, the past 2 days, and
the past hour.
2.5.1 Formation and Evolution
of the Earth System
The sun and the planets are believed to have formed
4.5 billion years ago from the gravitational collapse of
a cold cloud of interstellar gas and dust.
16
The absence
of the noble gases neon, xenon, and krypton
17
in the
48 The Earth System
must have been at the time the sample was
deposited.
• Oxygen-18 (
18
O) abundance in marine sediment
cores containing carbonates reflects the temper-
ature of the water in the euphotic zone where
the carbonate formation took place.The lower
the temperature of the water, the greater the
relative abundance of
18
C that was incorporated
into the shells and skeletons of the marine
organisms from which the carbonates formed.
• Worldwide
18
O abundances also depend on
the volume of the continental ice sheets.
16
O
evaporates more readily than
18
O, so a
disproportionately large fraction of
16
O tends
to be incorporated into the snow that falls on,
and becomes incorporated into, the ice sheets.
When the ice sheets grow, ocean waters
throughout the world are enriched in
18
O.
Hence
18
O abundance in marine sediment
cores and ice cores can be used as a proxy for
ice volume.
• Carbon-13 (
13
C) abundances in deposits
of organic carbon reflect the ambient
CO
2
concentrations at the time that
photosynthesis occurred. Plants prefer the
lighter isotope
12
C, and the higher the
ambient CO
2
level, the more strongly they
exert this preference. Hence, low relative
abundances of
13
C in organic carbon
deposits are indicative of high ambient
CO
2
concentrations, and vice versa.
• Carbon-13 is also an indicator of the sources
and sinks of atmospheric CO
2
. Emissions
from the decay of plants, forest fires and
agricultural burning, and the consumption of
fossil fuels tend to be low in
13
C, whereas CO
2
outgassed from the oceans has the same
13
C
abundance as atmospheric CO
2
. In a similar
manner, the presence of a biospheric CO
2
sink should tend to raise atmospheric
13
C
levels, whereas the presence of an oceanic
sink should not.
2.1 Continued
0
1
2
3
4
Years before present × 10
9
lifetime of Earth
Hadean Epoch
origin of life
Years before present
10
8
10
7
10
6
10
5
10
4
10
3
10
2
10
9
lifetime of Earth
rise of oxygen
Pangaea
K-T boundary
plates collide, Himalayas rise
Panama closes gap
begin Quaternary Epoch
previous interglacial
last glacial maximum
Younger Dryas
“climatic optimum”
Medieval Warm Period
Little Ice Age
rise of oxygen
Pangaea
K-T boundary
Quaternary Epoch
Fig. 2.27 Time line for the history of the Earth system on a
linear scale (top) and on a logarithmic scale (bottom).
16
The age of the of the Earth is inferred from a comparison of the ratios of radiogenic (formed by decay of uranium) and nonradiogenic
isotopes of lead in meteorites and rocks of various ages.
17
Argon in the Earth’s atmosphere is a product of the radioactive decay of the radioactive isotope
40
K in the crust.
P732951-Ch02.qxd 9/12/05 7:40 PM Page 48

2.5 A Brief History of Climate and the Earth System 49
atmospheres of the Earth and the other planets, rela-
tive to their cosmic abundance, is evidence that the
planets formed from the coalescence of the dust into
chunks of solid materials called planetesimals that
were drawn together by gravitation. Present within the
condensing cloud were volatile compounds (i.e., water,
methane, ammonia, and other substances with low
boiling points), mainly in the form of ices. When the
sun formed, the inner part of the cloud should have
warmed, driving out most of the volatiles: hence the
relatively low concentrations of these substances in
the atmospheres of the inner planets.
During the first 700–800 million years of its history,
referred to by geologists as the Hadean Epoch, Earth
was still under continual bombardment by smaller
planetesimals. The heating and degassing resulting
from impacts of these collisions should have liberated
water vapor and other volatile substances, forming a
primordial atmosphere and the oceans. The energy
released by the larger objects may well have been suf-
ficient to entirely vaporize the oceans from time to
time. The formation of the moon has been attributed
to one of these impacts. The bombardment gradually
subsided and, by 3.8 billion years ago, conditions on
Earth had become stable enough to allow early micro-
bial life forms to develop in the oceans. Cataclysmic
collisions still occur occasionally, as evidenced by the
K–T meteorite impact
18
that took place only 65 million
years ago. Earth is still being bombarded by vast num-
bers of much smaller objects, as illustrated in Fig. 2.28,
but their impact on the Earth system is minimal.
The emission of radiation from stars increases
gradually over their lifetime due to the increasing
rate of fusion within their cores, which contract and
heat up as progressively more of the hydrogen is
fused into helium. The luminosity of the sun is
believed to have increased by 30% over the lifetime
of the solar system. Geological evidence indicates
that, with the exception of a few relatively brief
intervals, the oceans have been largely free of ice
throughout the Earth’s history. That the Earth’s
surface was not perpetually frozen during its early
history, when the sun was relatively faint, suggests
that its early atmosphere must have contained sub-
stantially higher concentrations of greenhouse gases
at that time than it does now.
Over the lifetime of the Earth, its atmosphere has
continuously been recycled and renewed by volcanism
and plate tectonics.The makeup of present-day volcanic
emissions is 80–90% steam, 6–12% CO
2
,1–2% SO
2
,
and traces of H
2
,CO,H
2
S, CH
4
, and N
2
. The relative
concentrations of the reduced gases H
2
, CO, and CH
4
could have been much higher earlier in the Earth’s his-
tory when the mantle was less oxidized than it is today.
An important milestone in the evolution of the
Earth system was the rise of atmospheric oxygen.
Cyano-(blue-green) bacteria capable of liberating
oxygen are believed to have been present in the oceans
for at least 3.0 and perhaps as long as 3.8 billion years,
yet geological evidence indicates that oxygen did not
begin to accumulate in the atmosphere until about
2.4–2.2 billion years ago. Oxygen liberated by photo-
synthesis early in the Earth’s history would have been
consumed quickly by H
2
and other reduced gases
emanating from the Earth’s crust, formed in reactions
such as (2.17) or by the oxidation of minerals exposed
to the atmosphere by weathering. Only after the
18
The distinctive marker of this event is the distinctive iridium-enriched layer produced by the explosion that attended the impact. This
layer, which is evident in sediments worldwide, occurs at the boundary between Cretaceous and Tertiary sediments in the Earth’s crust (hence
the name K–T). It has been hypothesized that this event was responsible for the extinction of many species of life forms, including dinosaurs.
10
–12
10
–10
10
–8
10
–6
10
–6
10
8
10
8
10
10
10
6
10
6
10
4
(cm) (m) (km)
10
3
10
4
10
2
10
2
10
–4
10
–4
10
–2
10
–2
1
1
Years between impacts
Impactors on the space shuttle surface
(30 µ s)
(
µ m)
(30 s)
(1 year)
(10,000 years)
Shooting stars
Meteorites
Arizona crater
Sudbury, Ontario,
impact feature
Diameter of striking object (m)
Fig. 2.28 Frequency of occurrence of collisions of objects
with the Earth as a function of the size of the objects.
[Reprinted with permission from L. W. Alvarez, “Mass extinc-
tions caused by large bolide impacts,” Physics Today, 40, p. 27.
Copyright 1987, American Institute of Physics.]
P732951-Ch02.qxd 9/12/05 7:40 PM Page 49

minerals in the crust became more highly oxidized
due to the gradual escape of hydrogen from the Earth
system and reactions such as (2.17) slowed down,
would it have it possible for oxygen liberated by
photosynthesis to begin to accumulate in the atmos-
phere. In this sense, atmospheric O
2
can be viewed as
“surplus” oxygen in the Earth system.
An array of geological evidence indicates that the
rise of atmospheric oxygen, once it began, was quite
rapid, with concentrations rising from less than 0.01%
of the present concentration 2.4 billion years ago to at
least 1–3% of the present concentration 1.9 billion
years ago. Concurrent with the rise of oxygen came
the formation of ozone layer. Photochemical models
based on the equations in Section 5.7.1 indicate that
atmospheric oxygen concentrations of even a few per-
cent of today’s values should have been capable of
supporting an ozone layer thick enough to protect life
on Earth from the harmful effects of solar ultraviolet
radiation.
The conditions that existed on Earth prior to the
rise of oxygen have been the subject of considerable
speculation. Given today’s rates of production of
methane, it is estimated that, in the absence of atmos-
pheric oxygen, methane concentrations could have
been two or three orders of magnitude greater than
their present concentration of 1.7 ppmv, in which
case, methane might well have been the dominant
greenhouse gas. With higher methane concentrations,
the number densities of hydrogen atoms in the upper
50 The Earth System
Geological evidence suggests that major
glaciations, extending all the way into the trop-
ics, occurred three times in Earth’s history:
the first around 2.2–2.4 billion years ago, con-
current with the rise of oxygen,
19
the second
between 600 and 750 million years ago, and the
latest so-called Permian Glaciation, 280 million
years ago. Until quite recently, most climate
researchers discounted this evidence on the
grounds that had the oceans ever been in a
completely ice-covered state, they would have
remained so. These skeptics argued that the
albedo of an ice-covered planet would be so low
that very little of the incident solar radiation
would be absorbed. Hence, the surface of the
Earth would have been so cold that the ice could
not have melted. Recently, this argument has
been called into question by proponents of the
following “freeze-fry scenario.”
i. During an unusually cold period, a
sufficiently large fraction of the Earth’s
surface becomes ice covered so that the
ice-albedo feedback mechanism described
in Section 10.3 renders the climate unstable:
the expanding ice cover cools the Earth’s
surface, the cooling causes the ice to expand
2.2 “Snowball Earth”
Continued on next page
19
It has been hypothesized that the decline in atmospheric methane concentrations brought about by the rise of oxygen might have
precipitated a sudden global cooling at this time.
Fig. 2.29 Artist’s conception of the onset of a world-
wide glaciation. [Courtesy of Richard Peltier.]
still farther, and the process continues until
the entire Earth becomes ice covered, as
depicted schematically in Fig. 2.29.
ii. During the ensuing snowball Earth phase,
the carbonate formation reaction (2.11)
cannot occur because the oceans are frozen.
P732951-Ch02.qxd 9/12/05 7:40 PM Page 50
2.5 A Brief History of Climate and the Earth System 51
atmosphere could have been orders of magnitude
higher than their current values. Under those condi-
tions, large numbers of hydrogen atoms would have
escaped to space, gradually raising the level of oxida-
tion of the Earth system. The abrupt transition of the
atmosphere from an anoxic state, with relatively high
concentrations of CH
4
,CO,H
2
S, and other reduced
gases, to a more oxidized state was marked by the
extinction of anaerobic life forms that are intolerant
of O
2
.The rise of O
2
set the stage for the evolution of
more complex life forms.
2.5.2 The Past 100 Million Years
During the Cretaceous epoch, which ended 65 mil-
lion years ago, surface air temperatures were sub-
stantially higher than they are today, especially at the
higher latitudes. This view is supported by the discov-
ery of remains of dinosaurs and lush tropical plants
dating back to that time in Siberia, Canada, and
other subarctic sites. Geological evidence indicates
that atmospheric concentrations of CO
2
were about
an order of magnitude higher at that time than they
are today. The Cretacous epoch was followed by an
extended interval of cooling and declining CO
2
con-
centrations, culminating in Pleistocene glaciation,
20
which began around 2.5 million years ago.
The cooling that set the stage for the Pleistocene
glaciation is widely attributed to the role of plate
tectonics in regulating the reactions (2.14) and (2.15)
in the carbonate–silicate cycle. Geological evidence
indicates that the rate of movement of the plates has
slowed down over the past 100 million years. A
reduced rate of ingestion of limestone sediments into
the mantle implies reduced metamorphism, which
would favor reduced volcanic emissions of CO
2
.
Meanwhile, the rise of the Himalayas following the
collision of the Indian and Asian plates (Fig. 2.30) is
believed to have increased the rate of weathering of
CaSiO
3
rocks, making more Ca
2
ions available for
However, continental drift continues and,
with it, metamorphism and volcanic
emissions of CO
2
into the atmosphere. In
the absence of a carbon sink, atmospheric
CO
2
concentrations increase.
iii. Eventually, the combination of the increasing
greenhouse effect and the blackening of
parts of the ice sheets by windblown dust
raise the surface temperature up to the
threshold value at which ice in the tropical
oceans begins to melt. Once this process
begins, the ice-albedo feedback exerts a
powerful warming influence, which abruptly
flips the Earth system into an ice-free state.
iv. With the thawing of the oceans, carbonate
production resumes, but, compared to the
reaction time of the cryosphere in (iii), this
is a slow process, limited by the rate at which
weathering supplies Ca
2
ions. Hence, for
several million years, the Earth system
resides in the hothouse phase, with global-
mean temperatures initially as high as 50 °C.
The occurrence of an extended hothouse phase is
supported by the existence of rock formations
(i.e., banded iron formations and cap carbonates)
suggestive of a period of very high temperatures
around the time of the Permian glaciation.
2.2 Continued
20
Pleistocene (from the Greek: pleistos: most, and ceno: new) in reference to geological sediments. The Pleistocene epoch and the
subsequent Holocene (all new) epoch comprise the Quaternary period in the Earth’s history.
Fig. 2.30 Continental configuration 65 million years ago, at
the end of the Cretaceous epoch. Note the separation
between the Indian and Eurasian plates. [Courtesy of the U.S.
Geological Survey.]
P732951-Ch02.qxd 9/12/05 7:40 PM Page 51
limestone formation in the oceans, thereby accelerat-
ing the removal of CO
2
from the atmosphere and
oceans. A decreasing source and increasing sink of
atmospheric CO
2
would account for the apparent
decline in atmospheric CO
2
levels, and the conse-
quent weakening of the greenhouse effect is consis-
tent with the observed cooling.
Another factor that contributed to the cooling was
glaciation of the Antarctic continent 15–30 million
years ago as it drifted into higher latitudes, which
would have increased the fraction of the incident
solar radiation reflected back to space. Other impor-
tant milestones in the drift of the continents toward
their present configuration were the opening of the
Drake passage in the southern hemisphere 15–30
million years ago, which gave rise to the formation of
the Antarctic circumpolar current, and the joining
of the North and South American continents at the
isthmus of Panama 3 million years ago. It has been
suggested that these events could have caused major
reorganizations of the oceanic thermohaline circula-
tion, resulting in a reduction of the poleward heat
flux in the North Atlantic, thereby accelerating the
cooling of the Arctic.
2.5.3 The Past Million Years
The past 2.5 million years have been marked by
climatic swings back and forth between extended
glacial epochs in which thick ice sheets covered
large areas of North America, northern Europe, and
Siberia and shorter interglacial epochs such as the
present one, in which only Antarctica (and some-
times Greenland) remain ice covered. Carbon and
oxygen isotopes (see Box 2.1) and the remains of
living organisms buried in marine sediments in
anoxic ocean basins around the world reveal a great
deal about the history of the pronounced climatic
swings during this so-called Quaternary period of
Earth’s history.
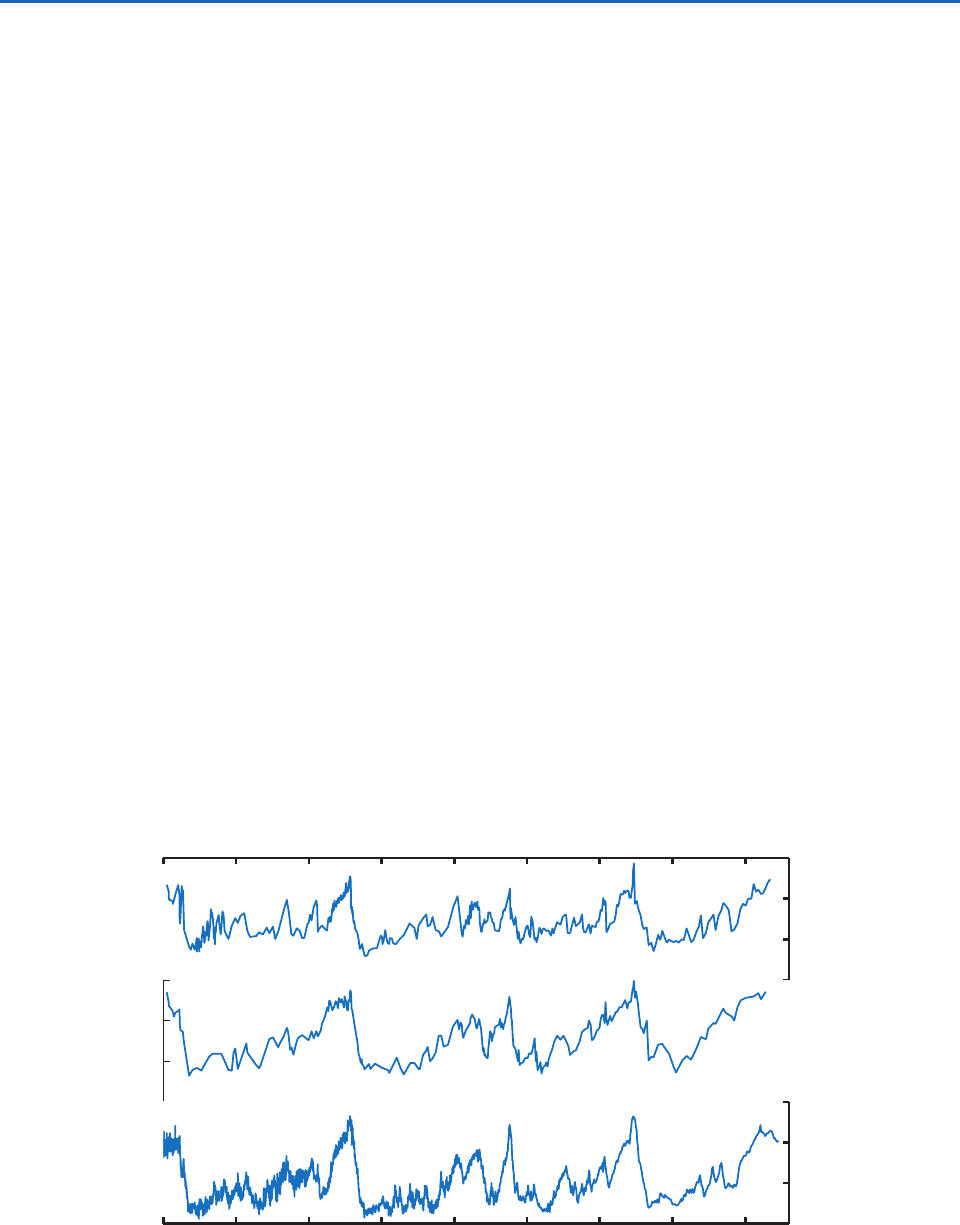
A more accurate and detailed history of the
climate swings of the past few hundred thousand
years, as revealed by an ice core extracted from
the dome of the Antarctic ice sheet, is shown in
Fig. 2.31. The temperature variations shown in
Fig. 2.31 are inferred from changes in concen-
trations of deuterium in the ice, as explained in
Box 2.1. Atmospheric concentrations of CO
2
and
CH
4
are inferred from microscopic bubbles of air
that became trapped in the ice as the snow from
which it was formed was compressed and consoli-
dated. Because these gases tend to be well mixed,
their concentrations in the cores are indicative of
global conditions. The chemical signature of the
dust in the cores provides a basis for identifying it
with one or more specific source regions such as
the Gobi Desert, where fine particles of soil are
exposed directly to strong winds.
52 The Earth System
200
400
600
800
CH
4
(pptv)
150
200
250
300
CO
2
(ppmv)
0 50 100 150 200 250 300 350 400
−10
−5
0
5
Age (thousands of years)
Temperature change (°C)
Fig. 2.31 Comparison of methane, carbon dioxide, and estimated temperature (from oxygen and deuterium isotope ratios) from
the Vostok ice core, Antarctica, over the last 440 thousand years. The location of Vostok is indicated by the red dot in Fig. 2.13.
Note that the time axis runs from right to left. [Adapted from J. R. Petit et al., “Climate and atmospheric history of the past
420,000 years from the Vostok ice core, Antarctica.” Nature, 399, p. 431, 1999. Courtesy of Eric Steig.]
P732951-Ch02.qxd 9/12/05 7:40 PM Page 52
2.5 A Brief History of Climate and the Earth System 53
Based on the analysis of ice core records like
the ones shown in Fig. 2.31 and comparisons with
numerous (but less highly resolved and accurately
dated) marine sediment cores, it is clear that on
timescales of tens of thousands of years or longer,
temperature and a number of other climate-
related parameters vary coherently with one
another and that these variations are global in
extent. Global temperatures cooled at an irregular
rate during the extended glacial epochs and rose
much more rapidly at the beginning of the inter-
glacials, which have been recurring at intervals of
roughly 100,000 years. The last glacial maximum
occurred around 20,000 years ago. Atmospheric
CO
2
and CH
4
concentrations have risen and fallen
synchronously with temperature, and the colder
epochs have been dustier, perhaps because of
higher wind speeds or drier conditions over the
source regions.
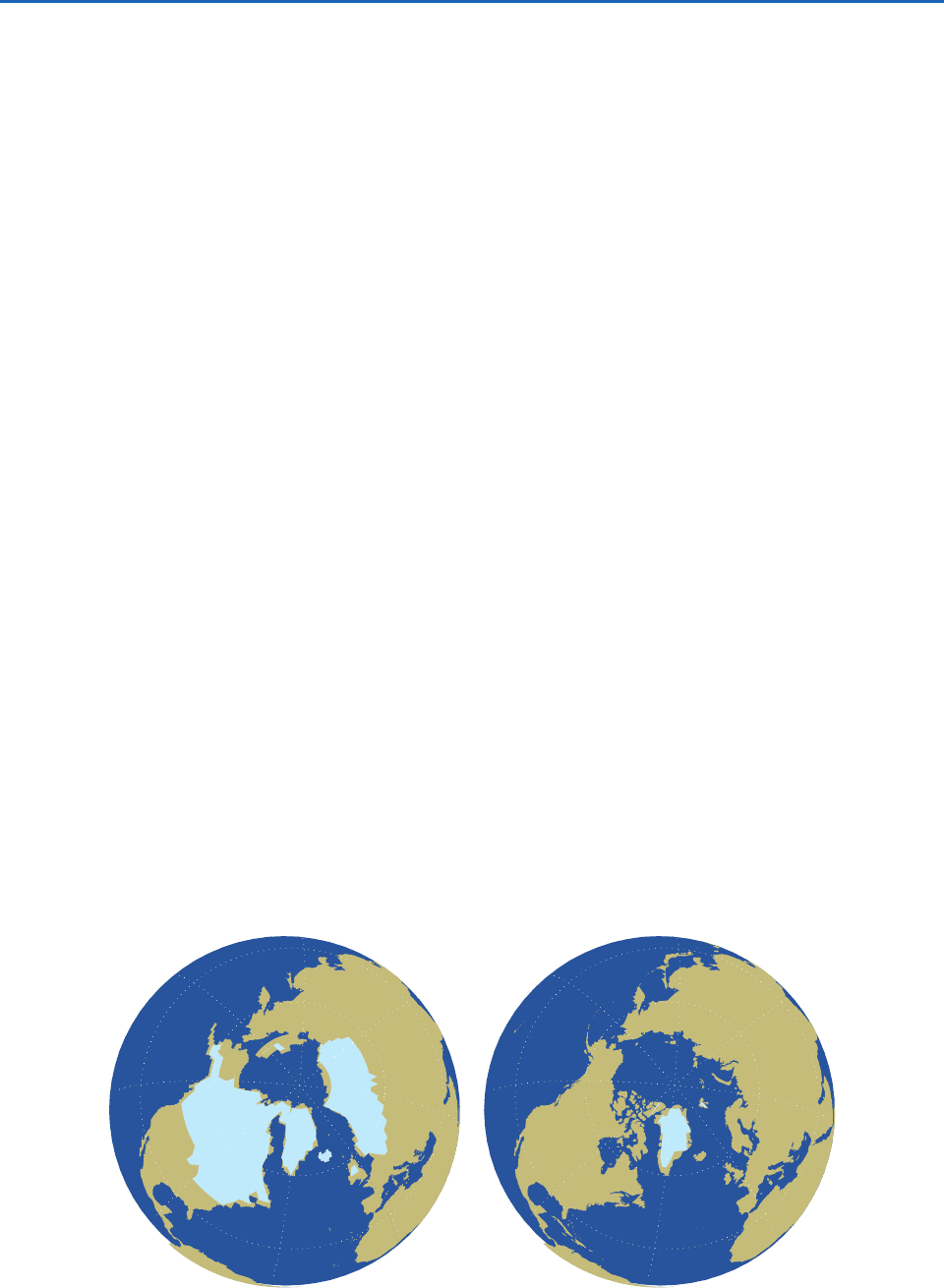
Figure 2.32 contrasts the extent of the northern
hemisphere continental ice sheets at the time of the
last glacial maximum with their current extent.
Parts of Canada were covered by ice as thick as
3 km. As a consequence of the large amount of
water sequestered in the ice sheets, the global sea
level was 125 m lower than it is today. The con-
centration of atmospheric CO
2
was 180 ppm, as
compared with a mean value of 260 ppm during
the current interglacial prior to the industrial
revolution. Hence, the Earth’s albedo must have
been higher at the time of the last glacial maximum
than it is today and the greenhouse effect must
have been weaker, both of which would have
favored lower surface temperatures. Temperatures
in Greenland were 10 °C lower than they are
today and tropical temperatures are estimated to
have been 4 °C lower.
There is evidence of a small time lag between
fluctuations in atmospheric CO
2
concentrations
and fluctuations in the volume of ice stored in the
continental ice sheets, with ice volume leading
CO
2
. Hence, it would appear that the cause of these
fluctuations is intimately related to the growth and
shrinkage of the continental ice sheets. The CO
2
fluctuations represent a positive feedback that
amplifies the temperature contrasts between glacial
and interglacial epochs, as discussed further in
Section 10.3.2.
The pronounced climatic swings during the
Quaternary period are believed to be driven by sub-
tle variations in the Earth’s orbit that affect the sum-
mer insolation (i.e., the average intensity of incident
solar radiation) at high latitudes of the northern
hemisphere. During intervals when the summer inso-
lation is relatively weak, snow deposited during win-
ter does not completely melt, leaving a residual,
which, over a time span of thousands of years, accu-
mulates to form thick ice sheets. The high reflectivity
of the growing ice sheets exacerbates the coolness of
the summers, amplifying the orbital forcing. Based on
the same reasoning, it is believed that the continental
ice sheets are most prone to melting during periods
Fig. 2.32 The extent of the northern hemisphere continental ice sheets at the time of the last glacial maximum 20,000 years
ago (left) as compared with their current extent (right). [Courtesy of Camille Li.]
P732951-Ch02.qxd 9/12/05 7:40 PM Page 53
