Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

thereby “focused” into a narrow band about its isoelectric
point that may be as thin as 0.01 pH unit. Hence, this
process is called isoelectric focusing (IEF).
A pH gradient produced by mixing two different buffers
together in continuously varying ratios is unstable in an
electric field because the buffer ions migrate to the elec-
trode of opposite polarity. Rather, the pH gradient in IEF
is formed by a mixture of low molecular mass (600–900 D)
oligomers bearing aliphatic amino and carboxylic acid
groups (Fig. 6-26) that have a range of isoelectric points.
Under the influence of an electric field in solution, these
ampholytes (amphoteric electrolytes) will each migrate to
their isoelectric points. Consequently, the most acidic am-
pholytes gather at the anode and the progressively more
basic ones position themselves ever closer to the cathode.
The pH gradient, which is maintained by the ⬃1000 V elec-
tric field, arises from the buffering action of these am-
pholytes. The convection of the pH gradient is prevented
by carrying out IEF in a lightly cross-linked polyacryl-
amide gel in the form of a rod or slab. IEF gels often con-
tain ⬃6M urea, a powerful protein denaturing agent that,
unlike SDS, is uncharged and hence cannot directly affect
the charge of a protein.
An alternative form of IEF utilizes gels containing im-
mobilized pH gradients. These are made from acrylamide
derivatives that are covalently linked to ampholytes.
Through the use of a gradient maker (e.g., Fig. 6-7), a gel
containing an immobilized pH gradient is polymerized
from a continuously varying mixture of acrylamide deriva-
tives with different pK’s so that the gel’s pH varies
smoothly from one end to the other.
The fact that IEF separates proteins into sharp bands
makes it a useful analytical and preparative tool. In fact,
many protein preparations once thought to be homogen-
eous have been resolved into several components by IEF.
IEF can be combined with electrophoresis in an extremely
powerful two-dimensional separation technique named
Section 6-4. Electrophoresis 151
Molecular mass (kD)
90
0.2
Relative mobility
0.4 0.6 0.8 1.0
80
70
60
50
40
30
20
10
+
–
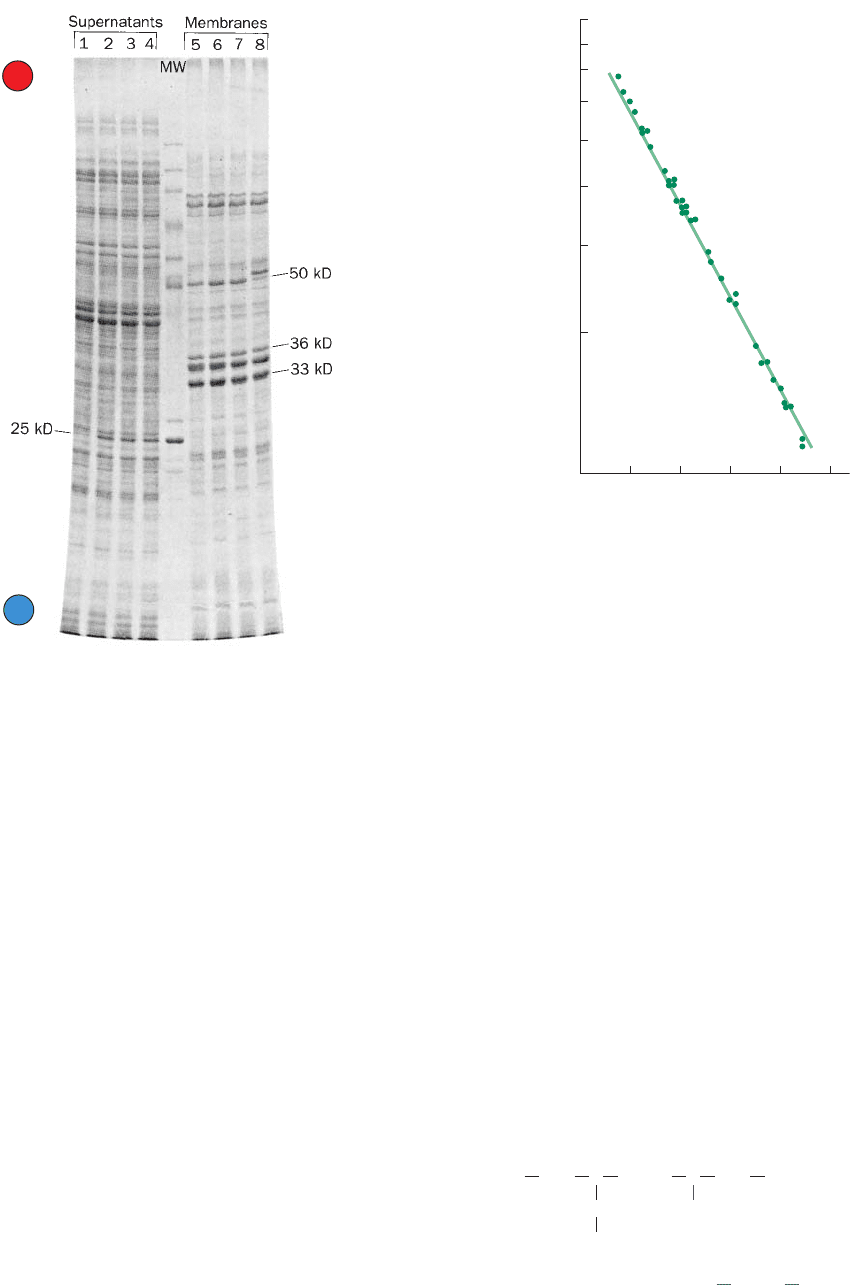
Figure 6-24 SDS–PAGE. The SDS–polyacrylamide disc
electrophoretogram shows separation of proteins from the
supernatant (left) and membrane fractions (right) of various
strains of the bacterium Salmonella typhimurium. Samples of
200 g of protein each were run in parallel lanes on a 35-cm-long,
0.8-mm-thick slab gel containing 10% polyacrylamide. The lane
marked MW contains molecular weight standards. [Courtesy of
Giovanna F. Ames, University of California at Berkeley.]
Figure 6-25 Logarithmic relationship between the molecular
mass of a protein and its relative electrophoretic mobility in
SDS–PAGE. This relationship is plotted for 37 polypeptides
ranging from 11 to 70 kD. [After Weber, K. and Osborn, M., J.
Biol. Chem. 244, 4406 (1969).]
Figure 6-26 General formula of the ampholytes used in
isoelectric focusing.
CH
2
NR
2
(CH
2
)
n
n
(CH
2
)
n
N N
CH
2
(CH
2
)
n
COOH
R
⫽ 2 or 3
R ⫽ H or
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 151

two-dimensional (2D) gel electrophoresis; Fig. 6-27). Up
to 5000 proteins can be observed on a single two-dimen-
sional electrophoretogram. Hence two-dimensional gel
electrophoresis is a valuable tool for proteomics (the
study of the proteome, which, in analogy with the term
“genome,” is defined as the aggregate of all the proteins
expressed by a cell or organism, but with emphasis on
their quantitation, localization, modifications, interactions,
and activities, as well as their identification). Individual
protein bands in a stained gel can be cut out from the gel
(with a scalpel or by a robot guided by a digitized image of
the gel acquired by an optical scanner or a digital camera),
destained, and the protein eluted from the gel fragment
for identification and/or characterization, often by mass
spectrometry (Section 7-1J).Variant proteins can be found
by comparing the positions and intensities of the bands in
2D gels of similar preparations. This can be done with the
aid of a computer after acquiring digitized images of the
stained gels. Numerous reference 2D gels are publicly
available for this purpose in the Web-accessible databases
listed at http://www.expasy.org/swiss-2dpage?de. These
databases contain images of 2D gels of a variety of organ-
isms and tissues and identify many of their component
proteins.
E. Capillary Electrophoresis
Although gel electrophoresis in its various forms is a com-
mon and highly effective method for separating charged
molecules, it typically requires an hour or more for a run
and is difficult to quantitate and automate.These disadvan-
tages are largely overcome through the use of capillary
electrophoresis (CE), a technique in which electrophoresis
is carried out in very thin (10 to 100 m inner diameter)
capillary tubes made of silica, glass, or plastic. Such narrow
capillaries rapidly dissipate heat and hence permit the use
of high electric fields (typically 100 to 300 V ⴢ cm
–1
, about
10 times that of most other electrophoretic techniques),
which reduces separation times to a few minutes. These
rapid separations, in turn, minimize band broadening
caused by diffusion, thereby yielding extremely sharp
separations. Capillaries can be filled with buffer (as in
moving boundary electrophoresis, but here the capillary’s
narrow bore all but eliminates convective mixing),
SDS–polyacrylamide gel (separation according to molecu-
lar mass; Section 6-4C), or ampholytes (isoelectric focus-
ing; Section 6-4D). These CE techniques have extremely
high resolution and can be automated in much the same
way as is HPLC, that is, with automatic sample loading and
on-line sample detection. Since CE can only separate small
amounts of material, it is largely limited to use as an analyt-
ical technique.
5 ULTRACENTRIFUGATION
If a container of sand and water is shaken and then allowed
to stand quietly, the sand will rapidly sediment to the bot-
tom of the container due to the influence of Earth’s gravity
(an acceleration g equal to 9.81 m
.
s
–2
). Yet macromole-
cules in solution, which experience the same gravitational
field, do not exhibit any perceptible sedimentation because
their random thermal (Brownian) motion keeps them uni-
formly distributed throughout the solution. Only when they
are subjected to enormous accelerations will the sedimenta-
tion behavior of macromolecules begin to resemble that of
sand grains.
The ultracentrifuge was developed around 1923 by the
Swedish biochemist The Svedberg. Using this instrument,
Svedberg first demonstrated that proteins are macromole-
cules with homogeneous compositions and that many pro-
teins are composed of subunits.Within a few decades, ultra-
centrifugation became an indispensable tool for the
fractionation of proteins, nucleic acids, and subcellular par-
ticles. Modern ultracentrifuges can attain rotational speeds
as high as 150,000 rpm (revolutions per minute) so as to
generate centrifugal fields in excess of 1 million ⫻ g.In this
section we outline the theory and practice of ultracentrifu-
gation.
A. Sedimentation
The rate at which a particle sediments in the ultracen-
trifuge is related to its mass. The force, F
sedimentation
, acting to
sediment a particle of mass m that is located a distance r
from a point about which it is revolving with angular veloc-
ity (in radians ⴢ s
–1
) is the centrifugal force (m
2
r) on the
152 Chapter 6. Techniques of Protein and Nucleic Acid Purification
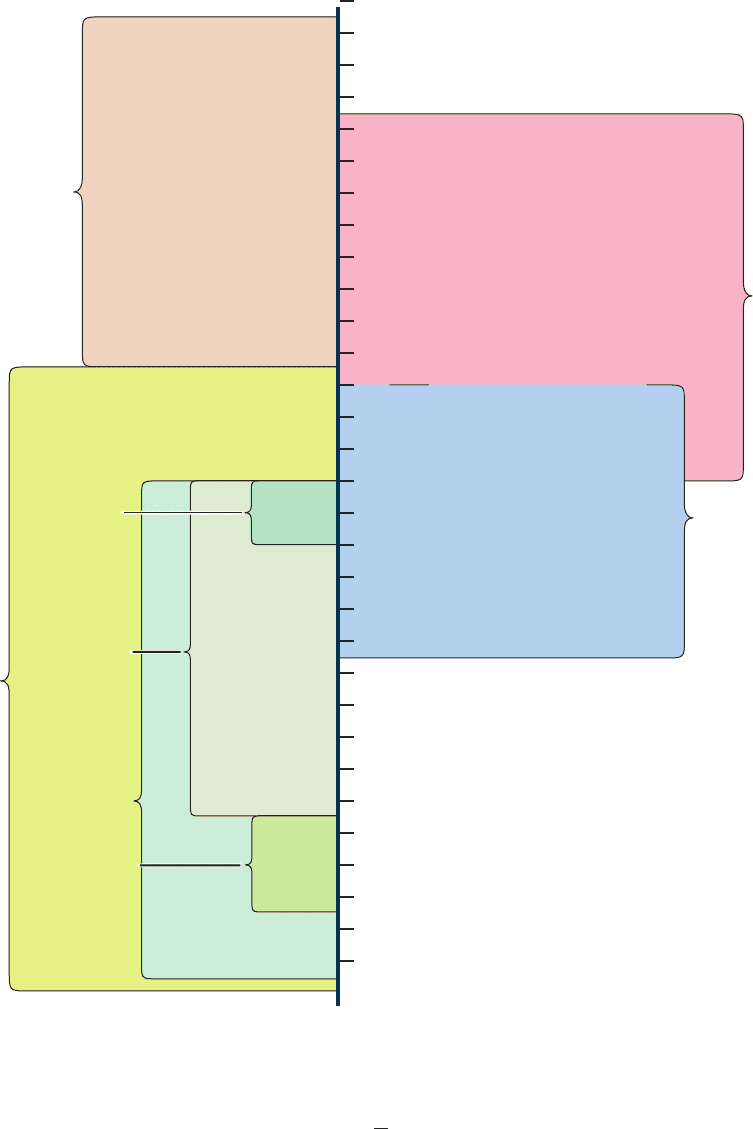
Figure 6-27 Two-dimensional (2D) gel electrophoresis. This
autoradiogram shows the separation of E. coli proteins by 2D gel
electrophoresis (isoelectric focusing horizontally and
SDS–PAGE vertically). A 10-g sample of proteins from E. coli
that had been labeled with
14
C-amino acids was subjected to
isoelectric focusing in a 2.5 ⫻ 130–mm tube of a urea-containing
polyacrylamide gel.The gel was then extruded from its tube,
placed in contact with one edge of an SDS–polyacrylamide slab
gel, and subjected to electrophoresis. Over 1000 spots were
counted on the original autoradiogram, which resulted from an
825-h exposure. [Courtesy of Patrick O’Farrell, University of
California at San Francisco.]
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 152

particle less the buoyant force (V
P
2
r) exerted by the so-
lution:
[6.10]
Here V
P
is the particle volume and is the density of the
solution. However, the motion of the particle through the
solution, as we have seen in our study of electrophoresis, is
opposed by the frictional force:
[6.7]
where v ⫽ dr/dt is the rate of migration of the sedimenting
particle and f is its frictional coefficient. The particle’s fric-
tional coefficient can be determined from measurements
of its rate of diffusion.
Under the influence of gravitational (centrifugal) force,
the particle accelerates until the forces on it exactly bal-
ance:
[6.11]
The mass of 1 mol of particles, M, is
[6.12]
where N is Avogadro’s number (6.022 ⫻ 10
23
). Thus, a par-
ticle’s volume, V
P
, may be expressed in terms of its molar
mass:
[6.13]
where , the particle’s partial specific volume, is the vol-
ume change when 1 g (dry weight) of particles is dissolved
V
V
P
⫽ Vm ⫽
VM
N
M ⫽ mN
m
2
r ⫺ V
P
2
r ⫽ vf
F
friction
⫽ vf
F
sedimentation
⫽ m
2
r ⫺ V
P
2
r
in an infinite volume of the solute. For most proteins dis-
solved in pure water at 20°C, is near 0.73 cm
3
ⴢ g
–1
(Table
6-4). Indeed, for proteins of known amino acid composi-
tion, is closely approximated by the sum of the partial
specific volumes of its component amino acid residues,
thereby indicating that the atoms in proteins are closely
packed (Section 8-3Bc).
a. A Particle May Be Characterized by Its
Sedimentation Rate
Substituting Eqs. [6.12] and [6.13] into Eq. [6.11] yields
[6.14]
Now define the sedimentation coefficient, s, as
[6.15]
The sedimentation coefficient, a quantity that is analo-
gous to the electrophoretic mobility (Eq. [6.9]) in that it
is a velocity per unit force, is usually expressed in units of
10
–13
s, which are known as svedbergs (S). For the sake of
uniformity, the sedimentation coefficient is customarily
corrected to the value that would be obtained at 20°C in
a solvent with the density and viscosity of pure water.
This is symbolized s
20,w
. Table 6-4 and Fig. 6-28 indicate
the values of s
20,w
in svedbergs for a variety of biological
materials.
Equation [6.15] indicates that a particle’s mass, m ⫽
M/N, can be determined from the measurement of its sedi-
mentation coefficient, s, and the solution density, , if its
s ⫽
v
2
r
⫽
1
2
a
d ln r
dt
b⫽
M(1 ⫺ V
)
Nf
vf ⫽
M(1 ⫺ V
)
2
r
N
V
V
Section 6-5. Ultracentrifugation 153
Table 6-4 Physical Constants of Some Proteins
Partial Specific Sedimentation
Molecular
Volume,
Coefficient, Frictional
Protein Mass (kD) (cm
3
ⴢ g
–1
) s
20,w
(S) Ratio, f/f
0
Lipase (milk) 6.7 0.714 1.14 1.190
Ribonuclease A (bovine pancreas) 12.6 0.707 2.00 1.066
Cytochrome c (bovine heart) 13.4 0.728 1.71 1.190
Myoglobin (horse heart) 16.9 0.741 2.04 1.105
␣-Chymotrypsin (bovine pancreas) 21.6 0.736 2.40 1.130
Crotoxin (rattlesnake) 29.9 0.704 3.14 1.221
Concanavalin B (jack bean) 42.5 0.730 3.50 1.247
Diphtheria toxin 70.4 0.736 4.60 1.296
Cytochrome oxidase (P. aeruginosa) 89.8 0.730 5.80 1.240
Lactate dehydrogenase H (chicken) 150 0.740 7.31 1.330
Catalase (horse liver) 222 0.715 11.20 1.246
Fibrinogen (human) 340 0.725 7.63 2.336
Hemocyanin (squid) 612 0.724 19.50 1.358
Glutamate dehydrogenase (bovine liver) 1015 0.750 26.60 1.250
Turnip yellow mosaic virus protein 3013 0.740 48.80 1.470
V
20,w
Source: Smith, M.H., in Sober, H.A. (Ed.), Handbook of Biochemistry and Molecular Biology (2nd ed.), p. C-10, CRC Press (1970).
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 153
frictional coefficient, f, and its partial specific volume, ,
are known. Indeed, before about 1970, most macromolecu-
lar mass determinations were made using the analytical ul-
tracentrifuge, a device in which the sedimentation rates of
molecules under centrifugation can be optically measured
(the masses of macromolecules are too high to be accurately
determined by such classic physical techniques as melting
point depression or osmotic pressure measurements).
V
Although the advent of much simpler molecular mass deter-
mination methods, such as gel filtration chromatography
(Section 6-3Ba) and SDS–PAGE (Section 6-4C), had caused
analytical ultracentrifugation to largely fade from use, re-
cently developed instrumentation has led to a resurgence
in the use of analytical ultracentrifugational measure-
ments. They are particularly useful in characterizing sys-
tems of associating macromolecules.
154 Chapter 6. Techniques of Protein and Nucleic Acid Purification
Figure 6-28 Sedimentation coefficients in svedbergs (S) for some biological materials.
[After a diagram supplied by Beckman Coulter, Inc.]
0
1
2
3
4
5
6
7
8
9
10
20
60
80
100
200
400
600
800
1000
2000
4000
6000
8000
10,000
20,000
40,000
60,000
80,000
100,000
Collagen
Albumin
Luteinizing hormone
Immunoglobulin G
Aldolase
Catalase
-Macroglobulin
Rous sarcoma
Feline leukemia
Bacteriophage T2
Soluble proteins
Yeast tRNA
Nucleic acids
α
E. coli tRNA
Calf liver DNA
40
Vesicular stomatitis virus RNA
Bacteriophage T5 DNA
Bacteriophage T2 & T4 DNAs
Ribosomal subunits
Broad bean mottleRibosomes
Polysomes
Polio
Tobacco mosaic
Equine encephalitis
Viruses
Microsomes
Subcellular
particles
Plasma
membranes
Mitochondria
Svedbergs
2
Cytochrome c
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 154

b. The Frictional Ratio Is Indicative of Molecular
Solvation and Shape
For an unsolvated spherical particle of radius r
p
the fric-
tional coefficient is determined according to the Stokes
equation:
[6.16]
where is the viscosity of the solution. Solvation increases
the frictional coefficient of a particle by increasing its effec-
tive or hydrodynamic volume. Furthermore, f is minimal
when the particle is a sphere.This is because a nonspherical
particle has a larger surface area than a sphere of equal vol-
ume and therefore must, on the average, present a greater
surface area toward the direction of movement than a
sphere.
The frictional coefficient, f, of a particle of known mass
and partial specific volume can be ultracentrifugationally
determined using Eq. [6.15]. The effective or Stokes radius
of a particle in solution can be calculated by solving Eq.
[6.16] for r
p
, given the experimentally determined values of
f and . Conversely, the minimal frictional coefficient of a
particle, f
0
, can be calculated from the mass and the partial
specific volume of the particle by assuming it to be spheri-
cal and unsolvated:
[6.17]
If the frictional ratio, f/f
0
, of a particle is much greater than
unity, it must be concluded that the particle is highly sol-
vated and/or significantly elongated. The frictional ratios
of a selection of proteins are presented in Table 6-4. The
globular proteins, which are known from structural studies
to be relatively compact and spheroidal (Section 8-3B),
have frictional ratios ranging up to ⬃1.5. Fibrous mole-
cules such as DNA and the blood clotting protein fibrino-
gen (Section 35-1Aa) have larger frictional ratios. On de-
naturation, the frictional coefficients of globular proteins
increase by as much as twofold because denatured pro-
teins assume flexible and fluctuating random coil confor-
mations in which all parts of the molecule are in contact
with solvent (Section 8-1D).
B. Preparative Ultracentrifugation
Preparative ultracentrifuges, which as their name implies
are designed for sample preparation, differ from analytical
ultracentrifuges in that they lack sample observation facil-
ities. Preparative rotors contain cylindrical sample tubes
whose axes may be parallel, at an angle,or perpendicular to
the rotor’s axis of rotation, depending on the particular ap-
plication (Fig. 6-29).
In the derivation of Eq. [6.15], it was assumed that sedi-
mentation occurred through a homogeneous medium. Sed-
imentation may be carried out in a solution of an inert sub-
stance, however, such as sucrose or CsCl, in which the
concentration, and therefore the density, of the solution in-
creases from the top to the bottom of the centrifuge tube.
f
0
⫽ 6 a
3MV
4N
b
1>3
(V
P
⫽
4
3
r
3
p
)
f ⫽ 6r
p
The use of such density gradients greatly enhances the re-
solving power of the ultracentrifuge. Two applications of
density gradients are widely employed: (1) zonal ultracen-
trifugation and (2) equilibrium density gradient ultracen-
trifugation.
a. Zonal Ultracentrifugation Separates Particles
According to Their Sedimentation Coefficients
In zonal ultracentrifugation, a macromolecular solu-
tion is carefully layered on top of a density gradient pre-
pared by use of a device resembling that diagrammed in
Fig. 6-7. The purpose of the density gradient is to allow
smooth passage of the various macromolecular zones by
damping out convective mixing of the solution. Sucrose,
which forms a syrupy and biochemically benign solution,
is commonly used to form a density gradient for zonal ul-
tracentrifugation. The density gradient is normally rather
shallow because the maximum density of the solution
must be less than that of the least dense macromolecule
of interest. Nevertheless, consideration of Eq. [6.15] indi-
cates that the sedimentation rate of a macromolecule is a
more sensitive function of molecular mass than density.
Consequently, zonal ultracentrifugation separates simi-
larly shaped macromolecules largely on the basis of their
molecular masses.
During centrifugation, each species of macromolecule
moves through the gradient at a rate largely determined by
its sedimentation coefficient and therefore travels as a
zone that can be separated from other such zones as is dia-
grammed in Fig. 6-30. After centrifugation, fractionation is
commonly effected by puncturing the bottom of the cellu-
loid centrifuge tube with a needle, allowing its contents to
drip out, and collecting the individual zones for subsequent
analysis.
Section 6-5. Ultracentrifugation 155
Figure 6-29 A selection of preparative ultracentrifuge rotors.
The sample tubes of the swinging bucket rotors (rear) are hinged
so that they swing from the vertical to the horizontal position as
the rotor starts spinning, whereas the sample tubes of the other
rotors have a fixed angle relative to the rotation axis. [Courtesy
of Beckman Coulter, Inc.]
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 155
b. Equilibrium Density Gradient Ultracentrifugation
Separates Particles According to Their Densities
In equilibrium density gradient ultracentrifugation [al-
ternatively, isopycnic ultracentrifugation (Greek: isos,
equal ⫹ pyknos, dense)], the sample is dissolved in a rela-
tively concentrated solution of a dense, fast-diffusing (and
therefore low molecular mass) substance, such as CsCl or
Cs
2
SO
4
, and is spun at high speed until the solution
achieves equilibrium. The high centrifugal field causes the
low molecular mass solute to form a steep density gradient
(Fig. 6-31) in which the sample components band at posi-
tions where their densities are equal to that of the solution;
that is, where in Eq. [6.15] is zero (Fig. 6-32).
These bands are collected as separate fractions when the
sample tube is drained as described above.The salt concen-
tration in the fractions and hence the solution density is
easily determined with an Abbé refractometer, an optical
instrument that measures the refractive index of a solution.
The equilibrium density gradient technique is often the
method of choice for separating mixtures whose compo-
nents have a range of densities. These substances include
nucleic acids, viruses, and certain subcellular organelles
such as ribosomes. However, isopycnic ultracentrifugation
is rather ineffective for the fractionation of protein mix-
tures because most proteins have similar densities (high
salt concentrations also salt out or possibly denature pro-
teins). Density gradient ultracentrifugation in an analytical
ultracentrifuge was used to show that DNA is semiconser-
vatively replicated (Section 5-3B).
6 NUCLEIC ACID FRACTIONATION
In the preceding parts of this chapter we considered the
most commonly used procedures for isolating and, to some
extent, characterizing proteins. Most of these methods, of-
ten with some modification, are also regularly used to frac-
tionate nucleic acids according to size, composition, and se-
quence.There are also many techniques that are applicable
only to nucleic acids. In this section we outline some of the
most useful of the procedures employed in the separation
of nucleic acids.
A. Solution Methods
Nucleic acids in cells are invariably associated with pro-
teins. Once cells have been broken open (Section 6-1B),
(1 ⫺ V
)
156 Chapter 6. Techniques of Protein and Nucleic Acid Purification
Stabilizing
sucrose
gradient
Sample
centrifugation
fractionation
Fast-sedimenting
component
Slow-sedimenting
component
1.78
1.74
1.70
1.66
1.62
6.0 6.2 6.4 6.6 6.8 7.0 7.2
Radius (cm)
Density (g
•
mL)
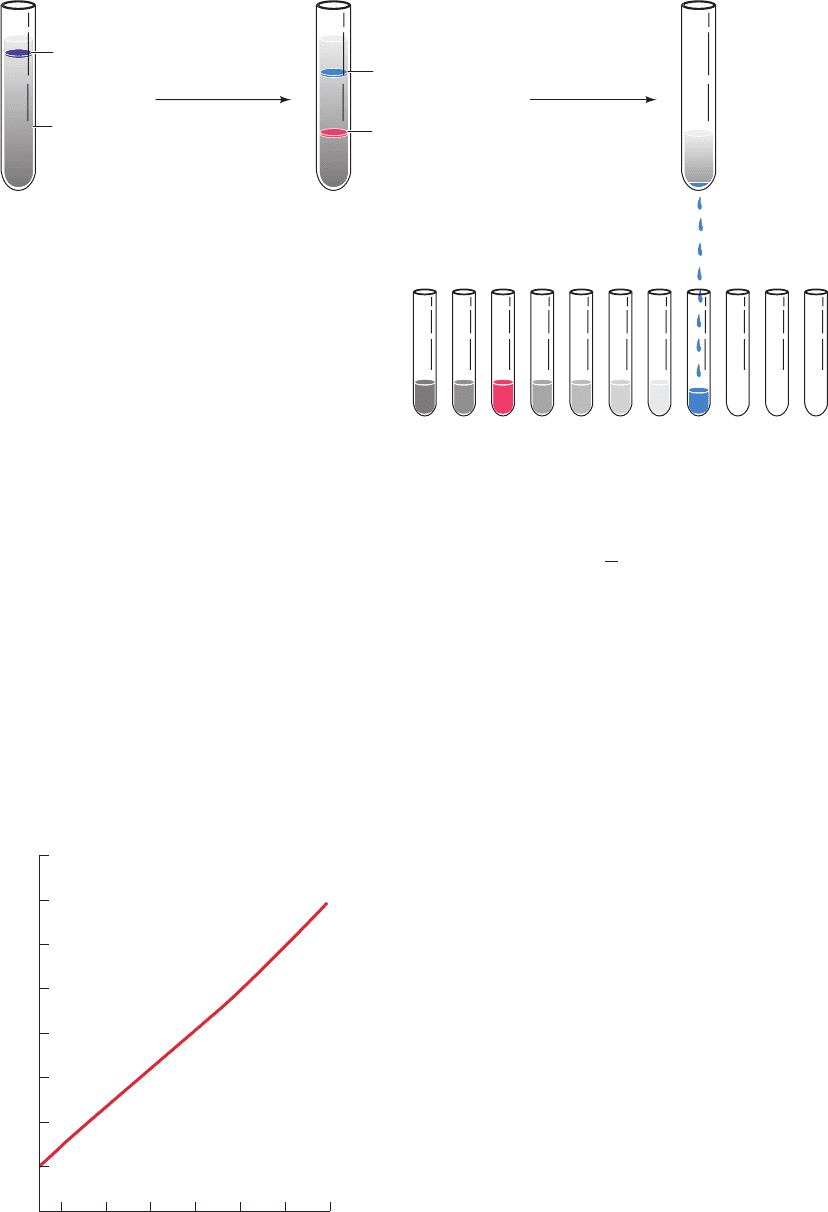
Figure 6-30 Zonal ultracentrifugation. The sample is layered
onto a sucrose gradient (left). During centrifugation (middle),
each particle sediments at a rate that depends largely on its mass.
Figure 6-31 Equilibrium density distribution of a CsCl
solution in an ultracentrifuge spinning at 39,460 rpm. The initial
density of the solution was 1.7 g ⴢ mL
–1
. [After Ifft, J.B.,Voet,
D.H., and Vinograd, J., J. Phys. Chem. 65, 1138 (1961).]
After the end of the run, the centrifugation tube is punctured
and the separated particles (zones) are collected (right).
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 156

their nucleic acids must be deproteinized. This can be ac-
complished by shaking (very gently if high molecular mass
DNA is being isolated; Section 5-3D) the aqueous solution
containing the protein–nucleic acid complex with a 25:24:1
mixture of phenol, chloroform, and isoamyl alcohol. The
protein is thereby denatured and extracted into the water-
immiscible organic phase, which is separated from the nu-
cleic acid–containing aqueous phase by centrifugation
(when a large amount of protein is present, it forms a white
precipitate between the organic and aqueous phases). Al-
ternatively (or in addition), protein can be dissociated from
the nucleic acids by such denaturing agents as detergents,
guanidinium chloride, or high salt concentrations, and/or it
can be enzymatically degraded by proteases. In all cases,
the nucleic acids, a mixture of RNA and DNA, can then be
isolated by precipitation with ethanol.The RNA can be re-
covered from such a precipitate by treating it with pancre-
atic DNase to eliminate the DNA. Conversely, the DNA
can be freed of RNA by treatment with RNase. Alterna-
tively, RNA and DNA may be separated by ultracentrifu-
gation (Section 6-6D).
In all these and subsequent manipulations, the nucleic
acids must be protected from degradation by nucleases
that occur both in the experimental materials and on hu-
man hands. Nucleases may be inhibited by the presence of
chelating agents such as ethylenediaminetetraacetic acid
(EDTA),
CH
2
H
2
C
Ethylenediaminetetraacetic acid (EDTA)
CH
2
COOHCH
2
COOHCH
2
NN
HOOC
H
2
C
HOOC
which sequester the divalent metal ions that nucleases re-
quire for activity. In cases where no nuclease activity can be
tolerated, all glassware must be autoclaved to heat dena-
ture the nucleases and the experimenter should wear plas-
tic gloves. Nevertheless, nucleic acids are generally easier
to handle than proteins because their lack, in most cases, of
a complex tertiary structure makes them relatively tolerant
of extreme conditions.
B. Chromatography
Many of the chromatographic techniques that are used to
separate proteins (Section 6-3) are also applicable to nu-
cleic acids. Oligonucleotides can be readily separated
HPLC, particularly that using reverse-phase chromatogra-
phy. Larger nucleic acids are often separated by procedures
that include ion exchange chromatography and gel filtra-
tion chromatography.
a. Hydroxyapatite Can Be Used to Isolate and
Fractionate DNA
Hydroxyapatite (a form of calcium phosphate; Section
6-3Db) is particularly useful in the chromatographic purifi-
cation and fractionation of DNA. Double-stranded DNA
binds to hydroxyapatite more tightly than do most other
molecules. Consequently, DNA can be rapidly isolated by
passing a cell lysate through a hydroxyapatite column,
washing the column with a phosphate buffer of concentra-
tion low enough to release only the RNA and proteins, and
then eluting the DNA with a more concentrated phosphate
solution. In addition, single-stranded DNA elutes from hy-
droxyapatite at a lower phosphate concentration than does
double-stranded DNA.
Section 6-6. Nucleic Acid Fractionation 157
Centrifugation2.Uniform mixture
of sample and
gradient-forming
substance
1. Gradient is
formed and
samples band at
their isopycnic
positions
3.
Figure 6-32 Isopycnic ultracentrifugation. The centrifugation
starts with a uniform mixture of a macromolecular sample
dissolved in a solution of a dense, fast-diffusing solute such as
CsCl (left).At equilibrium in a centrifugal field, the solute forms
a density gradient in which the macromolecules migrate to their
positions of buoyant density (right).
JWCL281_c06_129-162.qxd 2/22/10 2:25 PM Page 157
b. Messenger RNAs Can Be Isolated
by Affinity Chromatography
Affinity chromatography (Section 6-3C) is useful in iso-
lating specific nucleic acids. For example, most eukaryotic
messenger RNAs (mRNAs) have a poly(A) sequence at
their 3¿ ends (Section 5-4Ac). They can be isolated on an
agarose or cellulose matrix to which poly(U) has been co-
valently attached. The poly(A) sequences specifically bind
to the complementary poly(U) in high salt and at low tem-
peratures and can later be released by altering these condi-
tions. Moreover, if the (partial) sequence of an mRNA is
known (e.g., as inferred from the corresponding protein’s
amino acid sequence), the complementary DNA strand may
be synthesized (via methods discussed in Section 7-6Aa)
and used to isolate that particular mRNA.
C. Electrophoresis
Nucleic acids of a given type may be separated by poly-
acrylamide gel electrophoresis (Sections 6-4B and 6-4C)
because their electrophoretic mobilities in such gels vary
inversely with their molecular masses. However, DNAs of
more than a few thousand base pairs are too large to pene-
trate even a weakly cross-linked polyacrylamide gel. This
difficulty is partially overcome through the use of agarose
gels. By using gels with an appropriately low agarose con-
tent, relatively large DNAs in various size ranges may be
fractionated. In this manner, plasmids, for example, may be
separated from the larger chromosomal DNA of bacteria.
a. Duplex DNA Is Detected by Selectively Staining It
with Intercalation Agents
The various DNA bands in a gel must be detected if
they are to be isolated. Double-stranded DNA is readily
stained by planar aromatic cations such as ethidium ion
and acridine orange.
These dyes bind to duplex DNA by intercalation (slip-
ping in between the stacked base pairs), where they exhibit
a fluorescence under UV light that is far more intense than
that of the free dye. As little as 50 ng of DNA may be de-
tected in a gel by staining it with ethidium bromide (Fig.6-33).
+
H
2
N
NH
2
C
2
H
5
N
+
N
H
N(CH
3
)
2
(CH
3
)
2
N
Ethidium
Acridine orange
Single-stranded DNA and RNA also stimulate the fluores-
cence of ethidium ion but to a lesser extent than does du-
plex DNA. Disadvantages of using these dyes are that they
are mutagens and therefore must be handled and disposed
of with caution and that exposure to UV light damages
DNA (Section 30-5Aa). SYBR Safe, an equally sensitive
nonmutagenic dye that fluoresces when it is bound to dou-
ble-stranded DNA and excited by blue light, avoids these
difficulties.
b. Very Large DNAs Are Separated by Pulsed-Field
Gel Electrophoresis
The sizes of the DNAs that can be separated by conven-
tional gel electrophoresis are limited to ⬃100,000 bp even
when gels containing as little as 0.1% agarose (which
makes an extremely fragile gel) are used. However, the de-
velopment of pulsed-field gel electrophoresis (PFGE) by
Charles Cantor and Cassandra Smith extended this limit to
DNAs with up to 10 million bp (6.6 million kD). The elec-
trophoresis apparatus used in PFGE has two or more pairs
of electrodes arrayed around the periphery of an agarose
slab gel. The different electrode pairs are sequentially
pulsed for times varying from 0.1 to 1000 s depending on
the sizes of the DNAs being separated. Gel electrophoresis
of DNA requires that these elongated molecules worm
their way through the gel’s labyrinthine channels more or
less in the direction from the cathode to the anode. If the
direction of the electric field abruptly changes, these DNAs
must reorient their long axes along the new direction of the
field before they can continue their passage through the
gel. The time required to reorient very long gel-embedded
158 Chapter 6. Techniques of Protein and Nucleic Acid Purification
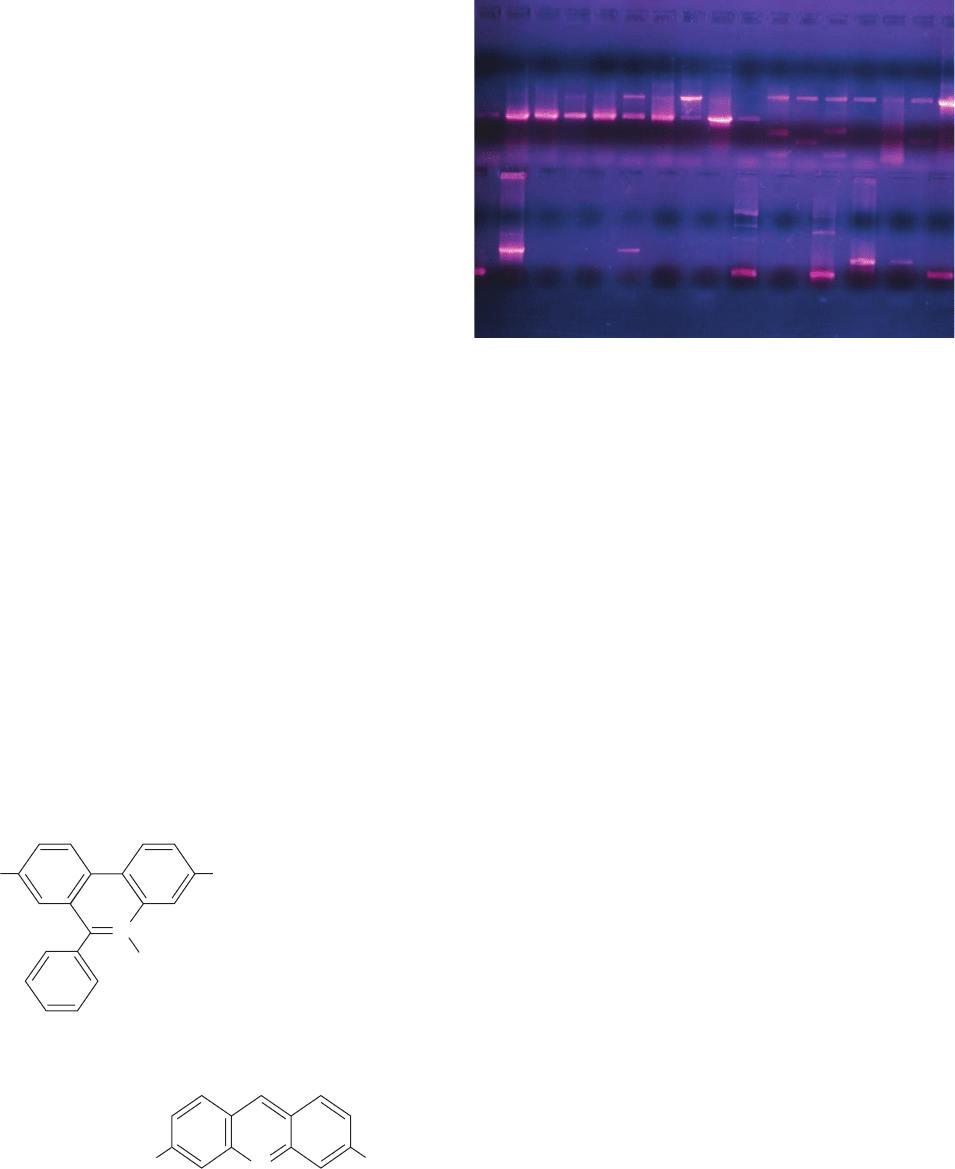
Figure 6-33 Agarose gel electrophoretogram of double helical
DNA. After electrophoresis, the gel was soaked in a solution of
ethidium bromide, washed, and photographed under UV light.
The fluorescence of the ethidium cation is strongly enhanced
by binding to DNA, so each fluorescent band marks a
different sized DNA fragment. [Klaus Guldbrandsen/Photo
Researchers, Inc.]
JWCL281_c06_129-162.qxd 6/3/10 8:24 AM Page 158

DNA molecules evidently increases with their size. Conse-
quently, a judicious choice of electrode distribution and
pulse lengths causes shorter DNAs to migrate through the
gel faster than longer DNAs, thereby effecting their sepa-
ration (Fig. 6-34).
D. Ultracentrifugation
Equilibrium density gradient ultracentrifugation (Section
6-5Bb) in CsCl constitutes one of the most commonly used
DNA separation procedures. The buoyant density, , of
double-stranded Cs
⫹
DNA depends on its base composi-
tion:
[6.18]
where is the mole fraction of G ⫹ C. Hence a CsCl
density gradient fractionates DNA according to its base com-
position. For example,eukaryotic DNAs often contain minor
fractions that band separately from the major species. Some
of these satellite bands consist of mitochondrial and chloro-
plast DNAs. Another important class of satellite DNA is
composed of repetitive sequences that are short segments of
DNA tandemly repeated hundreds, thousands, and in some
cases millions of times in a genome (Section 34-2B). Like-
wise, plasmids may be separated from bacterial chromosomal
DNA by equilibrium density gradient ultracentrifugation.
Single-stranded DNA is ⬃0.015 g ⴢ cm
–3
denser than the
corresponding double-stranded DNA so that the two may
be separated by equilibrium density gradient ultracentrifu-
gation. RNA is too dense to band in CsCl but does so in
Cs
2
SO
4
solutions. RNA–DNA hybrids will band in CsCl
but at a higher density than the corresponding duplex
DNA.
RNA may be fractionated by zonal ultracentrifugation
through a sucrose gradient (Section 6-5Ba). RNAs are sep-
arated by this technique largely on the basis of their size. In
fact, ribosomal RNA, which constitutes the major portion
X
G⫹C
⫽1.660 ⫹ 0.098X
G⫹C
of cellular RNA, is classified according to its sedimentation
rate; for example, the RNA of the E. coli small ribosomal
subunit is known as 16S RNA (Section 32-3A).
Chapter Summary 159
Figure 6-34 Pulsed-field gel electrophoresis (PFGE) of a set of
yeast chromosomes. The yeast chromosomes, which were run as
identical samples in the 13 inner lanes, have sizes 260, 290, 370,
460, 580/600, 700, 780, 820, and 850 kb. The two outer lanes, which
provide molecular mass standards, contained twenty successively
larger multimers of a ⬃43.5-kb bacteriophage DNA (top to
bottom) to an observed limit of ⬃850 kb. [Electrophoretogram by
Margit Burmeister, University of Michigan, in Wilson, K. and
Walker, J., Principles and Techniques of Biochemistry and Molecular
Biology (6th ed.), p. 477, Cambridge University Press (2005).]
1 Protein Isolation Macromolecules in cells are solubil-
ized by disrupting the cells by various chemical or mechanical
means such as detergents or blenders. Partial purification by
differential centrifugation is used after cell lysis to remove cell
debris or to isolate a desired subcellular component.When out
of the protective environment of the cell, proteins and other
macromolecules must be treated so as to prevent their de-
struction by such influences as extremes of pH and tempera-
ture, enzymatic and chemical degradation, and rough mechan-
ical handling.The state of purity of a substance being isolated
must be monitored throughout the purification procedure by a
specific assay.
2 Solubilities of Proteins Proteins are conveniently pu-
rified on a large scale by a fractional precipitation process
called salting out, in which protein solubilities are varied by
changing the salt concentration or pH.
3 Chromatographic Separations Ion exchange chro-
matography employs support materials such as cellulose or
cross-linked dextran gels. Separations are based on differential
electrostatic interactions between charged groups on the ion
exchange materials and those on the substances being sepa-
rated. Molecules may be located through their UV absorbance,
fluorescence, radioactivity, or enzymatic activity. In gel filtra-
tion chromatography, molecules are separated according to
their size and shape through the use of cross-linked dextran,
polyacrylamide, or agarose beads that have pores of molecular
dimensions. A calibrated gel filtration column can be used to
estimate the molecular masses of macromolecules. Affinity
chromatography separates biomolecules according to their
unique biochemical abilities to bind other molecules specific-
ally. High-performance liquid chromatography (HPLC) uti-
lizes any of the foregoing separation techniques but uses high-
resolution chromatographic materials, high solvent pressures,
and automatic solvent mixing and monitoring systems so as to
obtain much greater degrees of separation than are achieved
with the more conventional chromatographic procedures.
CHAPTER SUMMARY
JWCL281_c06_129-162.qxd 2/22/10 2:26 PM Page 159

General
Ahmed, H., Principles and Reactions of Protein Extraction, Purifi-
cation, and Characterization, CRC Press (2005).
Bonner, P.L.R., Protein Purification, Taylor & Francis (2007).
Boyer, R.F., Biochemistry Laboratory: Modern Theory and Tech-
niques, Benjamin-Cummings (2006).
Burgess, R.R. and Deutscher, M.P. (Eds.), Guide to Protein Purifi-
cation (2nd ed.), Methods Enzymol. 463, (2009).
Harding, S.E. and Chowdhry, B.Z. (Eds.)., Protein Ligand Interac-
tions: Structure and Spectroscopy. A Practical Approach,
Oxford University Press (2001). [Contains descriptions of a
variety of physical techniques for studying proteins and their
interactions with other molecules.]
Meyers, R.A., Proteins. From Analytics to Structural Genomics,
Vol. 2, Chapters 20–24, Wiley–VCH (2007).
Ninfa,A.J., Ballou, D.P., and Benore, M., Fundamental Laboratory
Approaches for Biochemistry and Biotechnology (2nd ed.),
Wiley (2010).
Pingoud,A., Urbanke, C., Hoggett, J., and Jeltsch,A., Biochemical
Methods. A Concise Guide for Students and Researchers,
Wiley–VCH (2002).
Roe, S. (Ed.), Protein Purification Techniques. A Practical Ap-
proach (2nd ed.); and Protein Purification Applications. A
Practical Approach (2nd ed.), Oxford University Press (2001).
Tinoco, I., Sauer, K., Wang, J.C., and Puglisi, J.C., Physical Chem-
istry. Principles and Applications in Biological Sciences (4th ed.),
Chapter 6, Prentice-Hall (2002).
Simpson, R.J.,Adams, P.D., and Golemis, E.A. (Eds.), Basic Meth-
ods in Protein Purification and Analysis. A Laboratory Man-
ual, Cold Spring Harbor Laboratory Press (2009).
Structural Genomics Consortium, et al., Protein production and
purification, Nature Methods 5, 135–146 (2008). [Discusses
methods for most efficiently producing and purifying recombi-
nant proteins.]
Walker, J.M. (Ed.), The Protein Protocols Handbook (2nd ed.),
Humana Press (2002).
Wilson, K. and Walker, J.M. (Eds.), Principles and Techniques of
Biochemistry and Molecular Biology (6th ed.), Chapters 10
and 11, Cambridge University Press (2005).
Solubility and Crystallization
Arakawa, T. and Timasheff, S.N., Theory of protein solubility,
Methods Enzymol. 114, 49–77 (1985).
Ducruix, A. and Giegé, R. (Eds.), Crystallization of Nucleic Acids
and Proteins. A Practical Approach, (2nd ed.), Oxford Univer-
sity Press (1999).
McPherson, A., Crystallization of Biological Macromolecules,
Cold Spring Harbor Laboratory Press (1999).
Chromatography
Dean, P.D.G., Johnson, W.S., and Middle, F.A. (Eds.), Affinity
Chromatography. A Practical Approach, IRL Press (1985).
Fischer, L., Gel filtration chromatography (2nd ed.), in Work, T.S.
and Burdon, R.H. (Eds.), Laboratory Techniques in Biochem-
istry and Molecular Biology, Vol. 1, Part II, North-Holland
Biomedical Press (1980).
Meyer,V.R., Practical High-Performance Liquid Chromatography
(2nd ed.),Wiley (1994).
Oliver, R.W.A. (Ed.), HPLC of Macromolecules. A Practical
Approach (2nd ed.), IRL Press (1998).
Rossomando, E.F., HPLC in Enzymatic Analysis (2nd ed.), Wiley
(1998).
Weston, A. and Brown, P.R., HPLC and CE. Principles and Prac-
tice,Academic Press (1997).
Electrophoresis
Altria, K.D., Capillary Electrophoresis Guidebook, Humana Press
(1996).
Baker, D.R., Capillary Electrophoresis, Wiley (1995).
Burmeister, M. and Ulanovsky, L., Pulsed-Field Gel Electrophore-
sis, Humana Press (1992).
Gersten, D.M., Gel Electrophoresis: Proteins, Wiley (1996).
Griffin, T.J. and Aebersold, R., Advances in proteome analysis
by mass spectrometry, J. Biol. Chem. 276, 45497–45500
(2001).
Hames, B.D. (Ed.), Gel Electrophoresis of Proteins. A Practical
Approach (3rd ed.), IRL Press (1998).
Jones, P., Gel Electrophoresis: Essential Techniques,Wiley (1999).
REFERENCES
Adsorption chromatography, thin layer chromatography (TLC),
reverse-phase chromatography (RPC), hydrophobic interac-
tion chromatography (HIC), and metal chelate affinity chrom-
atography also have valuable biochemical applications.
4 Electrophoresis In electrophoresis, charged molecules
are separated according to their rates of migration in an electric
field on a solid support such as paper, cellulose acetate, cross-
linked polyacrylamide, or agarose. Gel electrophoresis employs
a cross-linked polyacrylamide or agarose gel support, so that
molecules are separated according to size by gel filtration as
well as according to charge.The separated molecules may be vis-
ualized by means of stains, autoradiography, or immunoblot-
ting.The anionic detergent sodium dodecyl sulfate (SDS) dena-
tures proteins and uniformly coats them so as to give most
proteins a similar charge density and shape. SDS–PAGE may be
used to estimate macromolecular masses. In isoelectric focusing
(IEF), macromolecules are immersed in a stable pH gradient
and subjected to an electric field that causes them to migrate to
their isoelectric positions. In capillary electrophoresis, the use of
thin capillary tubes and high electric fields permits rapid and
highly resolved separations of small amounts of material.
5 Ultracentrifugation In ultracentrifugation, molecules
are separated by subjecting them to gravitational fields large
enough to counteract diffusional forces. Molecules may be
separated and their molecular masses estimated from their
rates of sedimentation through a solvent or a preformed gra-
dient of an inert low molecular mass material such as sucrose.
Alternately, molecules may be separated according to their
buoyant densities in a solution with a density gradient of a
dense, fast-diffusing substance such as CsCl. The deviation of
a molecule’s frictional ratio from unity is indicative of its de-
grees of solvation and elongation.
6 Nucleic Acid Fractionation Nucleic acids can be frac-
tionated by many of the techniques that are used to separate
proteins. Hydroxyapatite chromatography separates single-
stranded DNA from double-stranded DNA. Polyacrylamide or
agarose gel electrophoresis separates DNA largely on the basis
of size. Very large DNAs can be separated by pulsed-field gel
electrophoresis (PFGE) on agarose gels. DNAs may be fraction-
ated according to their base composition by CsCl density gradi-
ent ultracentrifugation. Different species of RNA can be sepa-
rated by zonal ultracentrifugation through a sucrose gradient.
160 Chapter 6. Techniques of Protein and Nucleic Acid Purification
JWCL281_c06_129-162.qxd 7/20/10 7:44 PM Page 160
