Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.


Problems 161
Karger,B.L., Chu,Y.-H., and Foret, F., Capillary electrophoresis of
proteins and nucleic acids, Annu. Rev. Biophys. Biomol. Struct.
24, 579–610 (1995).
Monaco, A.P. (Ed.), Pulsed Field Gel Electrophoresis. A Practical
Approach, IRL Press (1995).
Righetti, P.G., Immobilized pH gradients: Theory and methodol-
ogy, in Burdon, R.H. and van Knippenberg, P.H. (Eds.), Labo-
ratory Techniques in Biochemistry and Molecular Biology,Vol.
20, Elsevier (1990). [Discusses isoelectric focusing.]
Wehr, T., Rodríeguez-Diaz, R., and Zhu, M., Capillary Elec-
trophoresis of Proteins, Marcel Dekker (1999).
Ultracentrifugation
Graham, J., Biological Centrifugation, Bios Scientific Publishers
(2001).
Harding, S.E., Rowe, A.J., and Horton, J.C. (Eds.), Analytical Ul-
tracentrifugation in Biochemistry and Polymer Science, Royal
Society of Chemistry (1992).
Hesley, P., Defining the structure and stability of macromolecular
assemblages in solution: The re-emergence of analytical ultra-
centrifugation as a practical tool, Structure 4, 367–373 (1996).
Laue, T., Biophysical studies by ultracentrifugation, Curr. Opin.
Struct. Biol. 11, 579–583 (2001); and Laue, T.M. and Stafford,
W.F., III, Modern applications of analytical ultracentrifuga-
tion, Annu. Rev. Biophys. Biomol. Struct. 28, 75–100 (1999).
Mächtle, W. and Börger, L., Analytical Ultracentrifugation,
Springer-Verlag (2006).
Schachman, H.K., Ultracentrifugation in Biochemistry, Academic
Press (1959). [A classic treatise on ultracentrifugation.]
Schuster,T.M. and Toedt, J.M., New revolutions in the evolution of
analytical ultracentrifugation, Curr. Opin. Struct. Biol. 6,
650–658 (1996).
Stafford, W.F., III, Sedimentation velocity spins a new weave for
an old fabric, Curr. Opin. Biotech. 8, 14–24 (1997).
1. What are the ionic strengths of 1.0M solutions of NaCl,
(NH
4
)
2
SO
4
, and K
3
PO
4
? In which of these solutions would a pro-
tein be expected to be most soluble; least soluble?
2. An isotonic saline solution (one that has the same salt con-
centration as blood) is 0.9% NaCl.What is its ionic strength?
3. In what order will the following amino acids be eluted from
a column of P-cellulose ion exchange resin by a buffer at pH 6:
arginine, aspartic acid, histidine, and leucine?
4. In what order will the following proteins be eluted from a
CM-cellulose ion exchange column by an increasing salt gradient
at pH 7: fibrinogen, hemoglobin, lysozyme, pepsin, and ribonucle-
ase A (see Table 6-1)?
5. What is the order of elution of the following proteins from
a Sephadex G-50 column: catalase, ␣-chymotrypsin, concanavalin
B, lipase, and myoglobin (see Table 6-4)?
6. Estimate the molecular mass of an unknown protein that
elutes from a Sephadex G-50 column between cytochrome c and
ribonuclease A (see Table 6-4).
7. A gel chromatography column of Bio-Gel P-30 with a bed
volume of 100 mL is poured. The elution volume of the protein
hexokinase (96 kD) on this column is 34 mL.That of an unknown
protein is 50 mL.What are the void volume of the column,the vol-
ume occupied by the gel, and the relative elution volume of the
unknown protein?
8. What chromatographic method would be suitable for
separating the following pairs of substances? (a) Ala-Phe-Lys,
Ala-Ala-Lys; (b) lysozyme, ribonuclease A (see Table 6-1); and
(c) hemoglobin, myoglobin (see Table 6-1).
9. What is the order of the R
f
values of the following amino
acids in their paper chromatography with a water/butanol/acetic
acid solvent system in which the pH of the aqueous phase is 4.5:
alanine, aspartic acid, lysine, glutamic acid, phenylalanine, and
valine?
10. What fractionation procedure could be used to purify Pro-
tein 1 from a mixture of three proteins whose amino acid compo-
sitions are as follows?
1. 25% Ala, 20% Gly, 20% Ser, 10% Ile, 10% Val, 5% Asn,
5% Gln, 5% Pro
2. 30% Gln, 25% Glu, 20% Lys, 15% Ser, 10% Cys
3. 25% Asn, 20% Gly, 20% Asp, 20% Ser, 10% Lys, 5% Tyr
All three proteins are similar in size and pI, and there is no anti-
body available for Protein 1.
*11. Purification tables are often used to keep track of the
yield and purification of a protein.The specific activity is the ratio
of the amount of the protein of interest, here myoglobin (Mb), ob-
tained at given step (in mol or enzyme units) divided by the
amount (mg) of total protein. The yield is the ratio of the amount
of the protein of interest obtained at a given purification step (in
mol or enzyme units) divided by the original amount present in
the crude extract, often converted to percent yield by multiplying
by 100. The fold purification is the ratio of the specific activity of
the purified protein to that of the crude extract.
(a) For the purification table below, calculate the specific ac-
tivity, % yield, and fold purification for the empty cells.
(b) Which step, DEAE or affinity chromatography, causes the
greatest loss of Mb?
(c) Which step causes the greater purification of Mb?
(d) If you could use only one purification step, which tech-
nique would be best?
PROBLEMS
mg Total mol Specific Activity Fold
Purification Step Protein Mb (mol protein/mg total protein) % Yield Purification
1. Crude extract 1550 0.75 100 1
2. DEAE-cellulose chromatography 550 0.35
3.Affinity chromatography 5.0 0.28
Purification Table (for Problem 11)
JWCL281_c06_129-162.qxd 5/31/10 2:10 PM Page 161
162 Chapter 6. Techniques of Protein and Nucleic Acid Purification
12. The neurotransmitter ␥-aminobutyric acid is thought to
bind to specific receptor proteins in nerve tissue. Design a proce-
dure for the partial purification of such a receptor protein.
13. A mixture of amino acids consisting of arginine, cysteine,
glutamic acid, histidine, leucine, and serine is applied to a strip of
paper and subjected to electrophoresis using a buffer at pH 7.5.
What are the directions of migration of these amino acids and
what are their relative mobilities?
*14. Sketch the appearance of a fingerprint of the following
tripeptides: Asn-Arg-Lys, Asn-Leu-Phe, Asn-His-Phe, Asp-Leu-
Phe, and Val-Leu-Phe.Assume the paper chromatographic step is
carried out using a water/butanol/acetic acid solvent system (pH 4.5)
and the electrophoretic step takes place in a buffer at pH 6.5.
15. What is the molecular mass of a protein that has a relative
electrophoretic mobility of 0.5 in an SDS–polyacrylamide gel such
as that of Fig. 6-25?
16. Explain why the molecular mass of fibrinogen is signifi-
cantly overestimated when measured using a calibrated gel filtra-
tion column (Fig. 6-10) but can be determined with reasonable
accuracy from its electrophoretic mobility on an SDS–polyacryl-
amide gel (see Table 6-4).
17. (a) What would be the relative arrangement of the follow-
ing proteins after they had been subjected to isoelectric focusing:
insulin, cytochrome c, histone, myoglobin, and ribonuclease A?
(b) Sketch the appearance of a two-dimensional gel electrophore-
togram of cytochrome c, myoglobin, and ribonuclease A (see
Tables 6-1 and 6-4).
18. Calculate the centrifugal acceleration, in gravities (g’s), on
a particle located 6.5 cm from the axis of rotation of an ultracen-
trifuge rotating at 60,000 rpm (1 g ⫽ 9.81 m ⴢ s
–2
).
19. In a dilute buffer solution at 20°C, rabbit muscle aldolase
has a frictional coefficient of 8.74 ⫻ 10
–8
g ⴢ s
–1
, a sedimentation co-
efficient of 7.35 S, and a partial specific volume of 0.742 cm
3
ⴢ g
–1
.
Calculate the molecular mass of aldolase assuming the density of
the solution to be 0.998 g ⴢ cm
–3
.
*20. The sedimentation coefficient of a protein was measured
by observing its sedimentation at 20°C in an ultracentrifuge spin-
ning at 35,000 rpm.
Time, t Distance of Boundary from
(min) Center of Rotation, r (cm)
4 5.944
6 5.966
8 5.987
10 6.009
12 6.032
The density of the solution is 1.030 g ⴢ cm
–3
, the partial specific vol-
ume of the protein is 0.725 cm
3
ⴢ g
–1
, and its frictional coefficient is
3.72 ⫻ 10
–8
g ⴢ s
–1
. Calculate the protein’s sedimentation coeffi-
cient, in svedbergs, and its molecular mass.
JWCL281_c06_129-162.qxd 2/22/10 2:26 PM Page 162
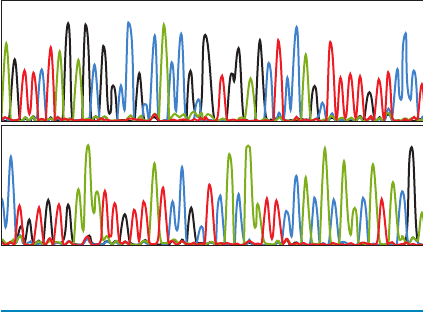
GG G G GGG
200 210 220 230 240
AAA A AA AAAAAA AACCCCCCCCCCCCCCCTTT TTTTT T TT T
GGGGGGG N
120 130 140 1501100
GG GG G G GAAA A AA ACCCCCCCCC CC CCCCCTT T T T TTTT TT TTT
163
CHAPTER 7
Covalent Structures
of Proteins and
Nucleic Acids
1 Primary Structure Determination of Proteins
A. End Group Analysis: How Many Different Types of Subunits?
B. Cleavage of the Disulfide Bonds
C. Separation, Purification, and Characterization of the
Polypeptide Chains
D. Specific Peptide Cleavage Reactions
E. Separation and Purification of the Peptide Fragments
F. Sequence Determination
G. Ordering the Peptide Fragments
H. Assignment of Disulfide Bond Positions
I. Peptide Characterization and Sequencing by Mass
Spectrometry
J. Peptide Mapping
2 Nucleic Acid Sequencing
A. The Sanger Method
B. Genome Sequencing
C. Next Generation DNA Sequencing Technologies
D. Nucleic Acid Sequencing versus Amino Acid Sequencing
3 Chemical Evolution
A. Sickle-Cell Anemia: The Influence of Natural Selection
B. Species Variations in Homologous Proteins: The Effects of
Neutral Drift
C. Evolution through Gene Duplication
4 Bioinformatics: An Introduction
A. Sequence Databases
B. Sequence Alignment
C. Construction of Phylogenetic Trees
5 Chemical Synthesis of Polypeptides
A. Synthetic Procedures
B. Problems and Prospects
6 Chemical Synthesis of Oligonucleotides
A. Synthetic Procedures
B. DNA Microarrays
C. SELEX
many other molecules. In the form of muscle fibers and
other contractile assemblies, proteins generate the coor-
dinated mechanical motion of numerous biological
processes, such as the separation of chromosomes during
cell division and the movement of your eyes as you read
this page. Proteins, such as rhodopsin in the retina of your
eye, acquire sensory information that is processed
through the actions of nerve cell proteins. The proteins of
the immune system, such as the immunoglobulins, form
an essential biological defense system in higher animals.
Proteins are major active elements in, as well as products
of, the expression of genetic information. However, pro-
teins also have important passive roles, such as that of col-
lagen, which provides bones, tendons, and ligaments with
their characteristic tensile strength. Clearly, there is con-
siderable validity to the old cliché that proteins are the
“building blocks” of life.
The function of DNA as the genetic archive and the as-
sociation of RNA with protein synthesis have been known
since the mid-twentieth century. However, it was not until
the 1970s that it became clear that RNA can form struc-
tures whose complexities rival those of proteins, and it was
not until the mid-1980s that it was shown that RNA has
biologically important catalytic functions.
Protein and nucleic acid function can best be under-
stood in terms of their structures, that is, the three-dimen-
sional relationships between their component atoms. The
structural descriptions of proteins and nucleic acids, as
well as those of other polymeric materials, have been tra-
ditionally described in terms of four levels of organization
(Fig. 7-1):
1. Primary structure (1° structure), which for a protein
is the amino acid sequence of its polypeptide chain(s) and
for a nucleic acid is its base sequence.
2. Secondary (2°) structure, which is the local spatial
arrangement of a polypeptide’s or a nucleic acid’s back-
bone atoms without regard to the conformations of their
side chains or bases.
3. Tertiary (3°) structure, which refers to the three-
dimensional structure of an entire polypeptide or polynu-
cleotide chain. The distinction between secondary and ter-
tiary structures is, of necessity, somewhat vague; in practice,
the term “secondary structure” alludes to easily character-
ized structural entities such as helices.
Proteins are at the center of the action in biological
processes. They function as enzymes, which catalyze the
complex set of chemical reactions that are collectively re-
ferred to as life. Proteins serve as regulators of these reac-
tions, both directly as components of enzymes and indi-
rectly in the form of chemical messengers, known as
hormones, as well as the receptors for those hormones.
They act to transport and store biologically important
substances such as metal ions, O
2
, glucose, lipids, and
JWCL281_c07_163-220.qxd 6/1/10 7:03 AM Page 163
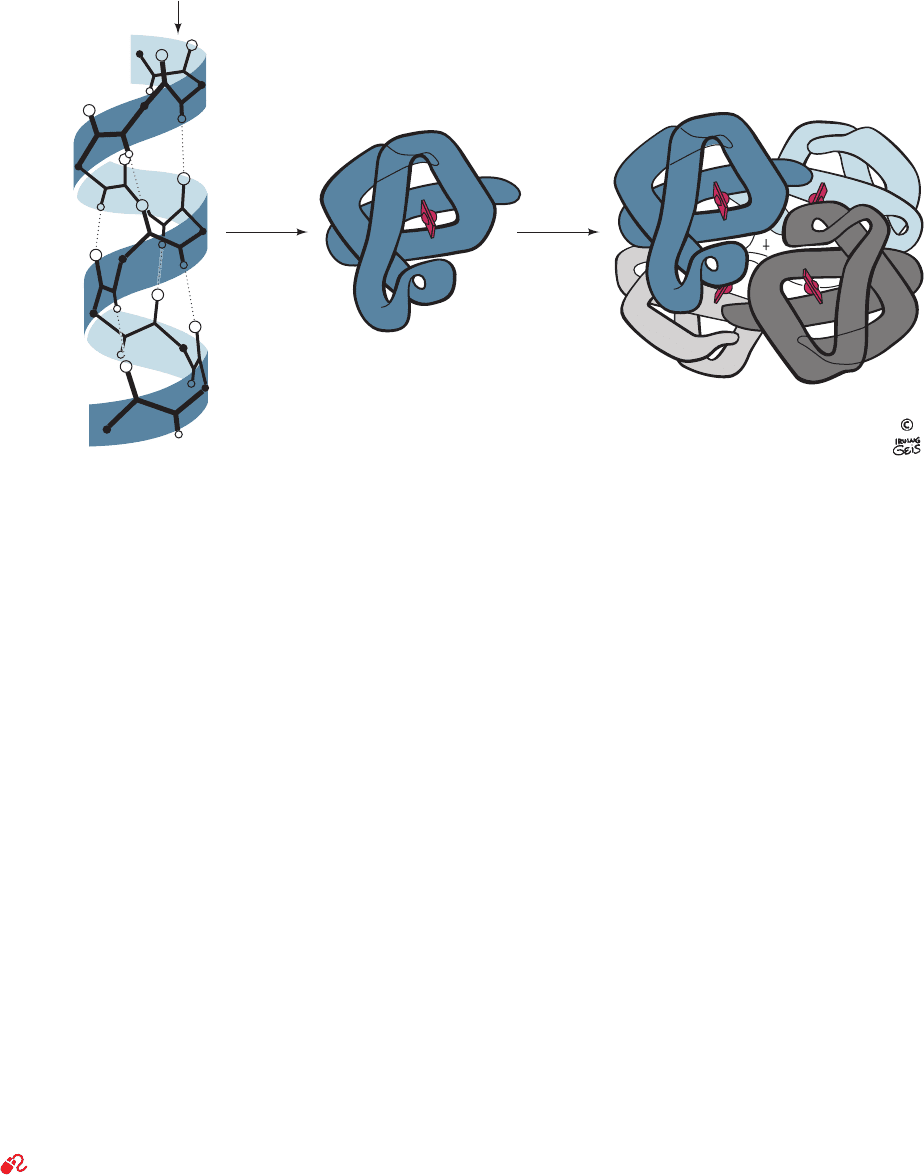
4. Most proteins are composed of two or more polypep-
tide chains, loosely referred to as subunits, which associate
through noncovalent interactions and, in some cases, disul-
fide bonds. A protein’s quaternary (4°) structure refers to
the spatial arrangement of its subunits. A nucleic acid’s
quaternary structure is similarly defined.
In this chapter, we discuss the 1° structures of proteins
and nucleic acids: how they are elucidated and their bio-
logical and evolutionary significance. We also survey the
field of bioinformatics as well as methods of chemically
synthesizing polypeptide and oligonucleotide chains. The
2°, 3°, and 4° structures of proteins and nucleic acids, as we
shall see, are a consequence of their 1° structures. For pro-
teins, these topics are treated in Chapters 8 and 9, whereas
for nucleic acids, they are discussed mainly in Chapters 29,
31, and 32.
1 PRIMARY STRUCTURE
DETERMINATION OF PROTEINS
See Guided Exploration 4: Protein sequence determination The
first determination of the complete amino acid sequence of
a protein, that of the bovine polypeptide hormone insulin
by Frederick Sanger in 1953, was of enormous biochemical
significance in that it definitively established that proteins
have unique covalent structures. Since that time, the amino
acid sequences of tens of thousands of proteins have been
elucidated. This extensive information has been of central
importance in the formulation of modern concepts of bio-
chemistry for several reasons:
1. The knowledge of a protein’s amino acid sequence is
essential for an understanding of its molecular mechanism
of action as well as being prerequisite for the elucidation
of its three-dimensional structure by both X-ray crystal-
lography and nuclear magnetic resonance (NMR) spec-
troscopy (Section 8-3A).
2. Sequence comparisons among analogous proteins
from the same individual, from members of the same
species, and from members of related species have yielded
important insights into how proteins function and have indi-
cated the evolutionary relationships among the proteins and
the organisms that produce them.These analyses, as we shall
see in Section 7-3, complement and extend corresponding
taxonometric studies based on anatomical comparisons.
3. Amino acid sequence analyses have important clini-
cal applications because many inherited diseases are
caused by mutations leading to an amino acid change in a
protein. Recognition of this fact has led to the develop-
ment of valuable diagnostic tests for many such diseases
and, in many cases, to symptom-relieving therapy.
The elucidation of the 51-residue primary structure of
insulin (Fig. 7-2) was the labor of many scientists over a
164 Chapter 7. Covalent Structures of Proteins and Nucleic Acids
Secondary
structure
(helix)
Primary structure (amino acid sequence in a polypeptide chain)
Quaternary structure:
the four separate chains
of hemoglobin assembled
into an oligomeric protein
Tertiary structure:
one complete protein chain
( chain of hemoglobin)
β
2
21
1
(b)
(a)
(c) (d)
– Lys – Ala – His – Gly – Lys – Lys – Val – Leu – Gly - Ala –
β
β
α
β
α
Figure 7-1 The structural hierarchy in proteins. (a) Primary structure, (b) secondary structure,
(c) tertiary structure, and (d) quaternary structure. [Illustration, Irving Geis. Image from the
Irving Geis Collection, Howard Hughes Medical Institute. Reprinted with permission.]
JWCL281_c07_163-220.qxd 8/10/10 12:36 AM Page 164

period of a decade that altogether utilized ⬃100 g of pro-
tein. Procedures for primary structure determination have
since been so refined and automated that proteins of simi-
lar size can be sequenced by an experienced technician in a
few days using only a few micrograms of protein. The se-
quencing of the 1021-residue enzyme -galactosidase in
1978 signaled that the sequence analysis of almost any pro-
tein could be reasonably attempted. Despite these techni-
cal advances, the basic procedure for primary structure de-
termination using the techniques of protein chemistry is
that developed by Sanger.The procedure consists of three
conceptual parts, each of which requires several laboratory
steps:
1. Prepare the protein for sequencing:
a. Determine the number of chemically different
polypeptide chains (subunits) in the protein.
b. Cleave the protein’s disulfide bonds.
c. Separate and purify the unique subunits.
2. Sequence the polypeptide chains:
a. Fragment the individual subunits at specific points
to yield peptides small enough to be sequenced di-
rectly.
b. Separate and purify the fragments.
c. Determine the amino acid sequence of each pep-
tide fragment.
d. Repeat Step 2a with a fragmentation process of
different specificity so that the subunit is cleaved
at peptide bonds different from before. Separate
these peptide fragments as in Step 2b and deter-
mine their amino acid sequences as in Step 2c.
3. Organize the completed structure:
a. Span the cleavage points between one set of pep-
tide fragments by the other. By comparison, the
sequences of these sets of polypeptides can be
arranged in the order that they occur in the sub-
unit, thereby establishing its amino acid sequence.
b. Elucidate the positions of the disulfide bonds, if
any, between and within the subunits.
We discuss these various steps in the following sections.
A. End Group Analysis: How Many Different Types
of Subunits?
Each polypeptide chain (if it is not chemically blocked or
circular) has an N-terminal residue and a C-terminal
residue. By identifying these end groups, we can establish
the number of chemically distinct polypeptides in a protein.
For example, insulin has equal amounts of the N-terminal
residues Phe and Gly, which indicates that it has equal
numbers of two nonidentical polypeptide chains.
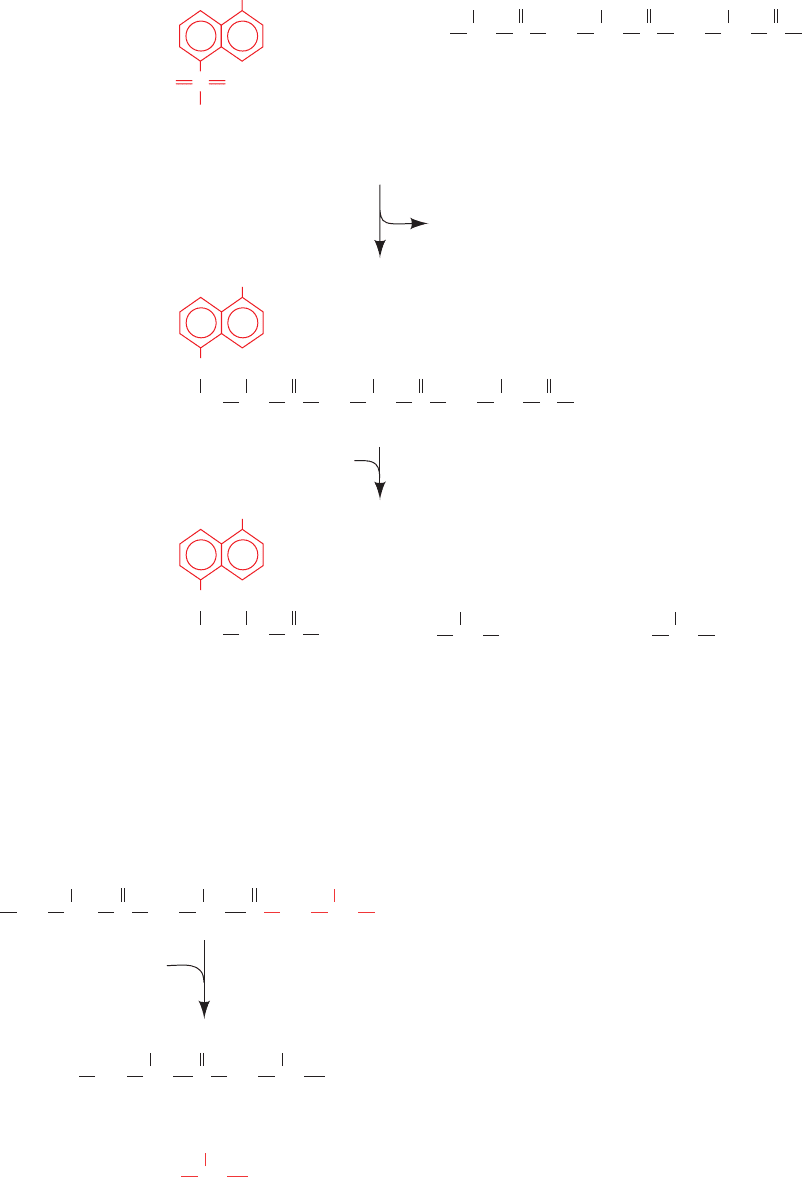
a. N-Terminus Identification
There are several effective methods by which a polypep-
tide’s N-terminal residue may be identified. 1-Dimethyl-
aminonaphthalene-5-sulfonyl chloride (dansyl chloride)
reacts with primary amines (including the ε-amino group of
Lys) to yield dansylated polypeptides (Fig. 7-3). Acid hy-
drolysis liberates the N-terminal residue as a dansylamino
acid, which exhibits such intense yellow fluorescence that it
can be chromatographically identified from as little as 100
picomoles of material [1 picomole (pmol) 10
12
mol].
In the most useful method of N-terminal residue identi-
fication, the Edman degradation (named after its inventor,
Pehr Edman), phenylisothiocyanate (PITC, Edman’s
reagent) reacts with the N-terminal amino groups of pro-
teins under mildly alkaline conditions to form their
phenylthiocarbamyl (PTC) adduct (Fig. 7-4). This product
is treated with an anhydrous strong acid such as trifluo-
roacetic acid, which cleaves the N-terminal residue as its
thiazolinone derivative but does not hydrolyze other pep-
tide bonds. The Edman degradation therefore releases the
N-terminal amino acid residue but leaves intact the rest of
the polypeptide chain.The thiazolinone-amino acid is selec-
tively extracted into an organic solvent and is converted to
the more stable phenylthiohydantoin (PTH) derivative by
treatment with aqueous acid.This PTH-amino acid is most
commonly identified by comparing its retention time on
HPLC with those of known PTH-amino acids.
The most important difference between the Edman
degradation and other methods of N-terminal residue
identification is that we can determine the amino acid se-
quence of a polypeptide chain from the N-terminus inward
by subjecting the polypeptide to repeated cycles of the Ed-
man degradation and, after every cycle,identifying the newly
liberated PTH-amino acid. This technique has been auto-
mated, resulting in great savings of time and materials
(Section 7-1F).
b. C-Terminus Identification
There is no reliable chemical procedure comparable to
the Edman degradation for the sequential end group analy-
sis from the C-terminus of a polypeptide. This can be done
enzymatically, however, using exopeptidases (enzymes that
Section 7-1. Primary Structure Determination of Proteins 165
Figure 7-2 Primary structure of bovine insulin. Note the intrachain and interchain disulfide
bond linkages.
Ala Tyr
Asn
Gly
Asn
GlnGlu Leu
Ile
Val Cys CysSer Val Cys Ser Glu
2110
Tyr Gln Leu
15
5
Cys
CysLeu
His
GlnAsnValPhe
S S
Gly
HisSer Leu Val AlaGlu
Leu
Tyr
Leu Val Cys
S
S
S
S
20
1510
Gly Glu
Arg
Gly
Phe Phe
25
Tyr
Thr
Pro Lys
Ala
30
5
B chain
A
chain
JWCL281_c07_163-220.qxd 2/22/10 9:10 PM Page 165
cleave a terminal residue from a polypeptide). One class of
exopeptidases, the carboxypeptidases, catalyzes the hy-
drolysis of the C-terminal residues of polypeptides:
Carboxypeptidases, like all enzymes, are highly specific (se-
lective) for the chemical identities of the substances whose
reactions they catalyze (Section 13-2). The side chain spe-
cificities of the various carboxypeptidases in common use
are listed in Table 7-1. The second type of exopeptidase
NH C
...
CH
OR
n–2
NH CCH
OR
n–1
NH C
...
CH
OR
n–2
COO
–
NH CH
R
n–1
NH CH
R
n
H
3
N
COO
–
CH
R
n
+
+
carboxypeptidase
COO
–
H
2
O
listed in Table 7-1, the aminopeptidases, sequentially
cleave amino acids from the N-terminus of a polypeptide
and have been similarly used to determine N-terminal se-
quences.
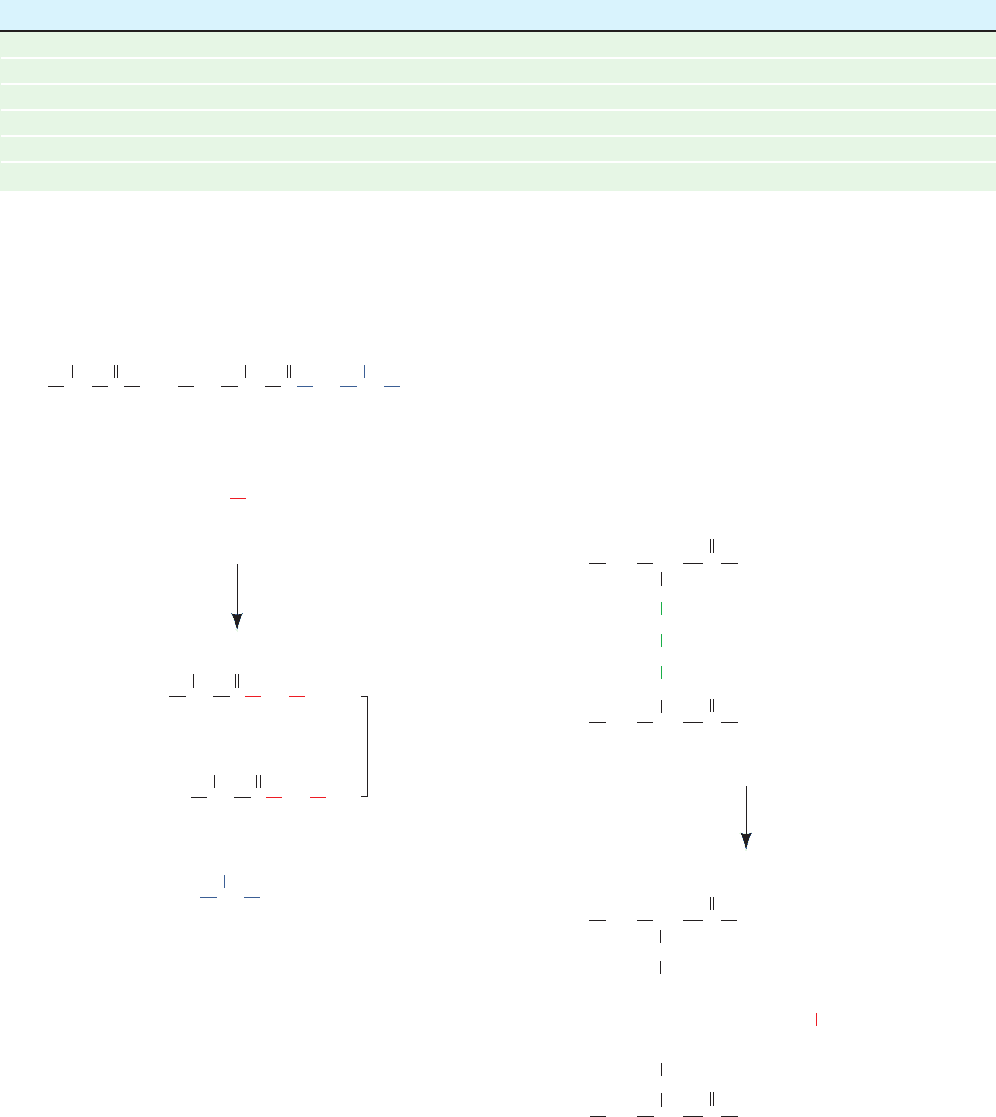
Why can’t carboxypeptidases be used to determine
amino acid sequences? If a carboxypeptidase cleaved all
C-terminal residues at the same rate, irrespective of their
identities, then by following the course of appearance of the
various free amino acids in the reaction mixture (Fig. 7-5a),
the sequence of several amino acids at the C-terminus
could be determined. If, however, the second amino acid
residue, for example, were cleaved at a much faster rate
than the first, both amino acids would appear to be re-
leased simultaneously (Fig. 7-5b). Carboxypeptidases, in
fact, exhibit selectivity toward side chains, so their use, ei-
ther singly or in mixtures, rarely reveals the order of more
than the first few C-terminal residues of a polypeptide.
C-Terminal residues with a preceding Pro residue are
not subject to cleavage by carboxypeptidases A and B
(Table 7-1). Chemical methods are therefore usually em-
ployed to identify their C-terminal residue. In the most re-
liable such chemical method, hydrazinolysis, a polypeptide
is treated with anhydrous hydrazine at 90°C for 20 to 100 h
166 Chapter 7. Covalent Structures of Proteins and Nucleic Acids
Figure 7-3 The reaction of dansyl chloride in end group analysis.
NH CCH
OR
2
H
2
N
CH
R
1
C
O
COOHCH
R
2
+
NH CCH
OR
3
Polypeptide1-Dimethylaminonaphthalene-
5-sulfonyl chloride (dansyl chloride)
OH
–
H
2
O
HCl
NH CCH
OR
2
CH
R
1
C
O
NH
CCH
OR
3
Dansyl polypeptide
NH
...
H
+
CH
R
1
C
O
Dansylamino acid
(fluorescent)
NH
...
OH +
H
3
N
+
H
3
N
+
+
CH
R
3
+
Free amino acids
...
N(CH
3
)
2
N(CH
3
)
2
N(CH
3
)
2
OSO
Cl
SO
2
SO
2
COOH
JWCL281_c07_163-220.qxd 2/22/10 9:11 PM Page 166
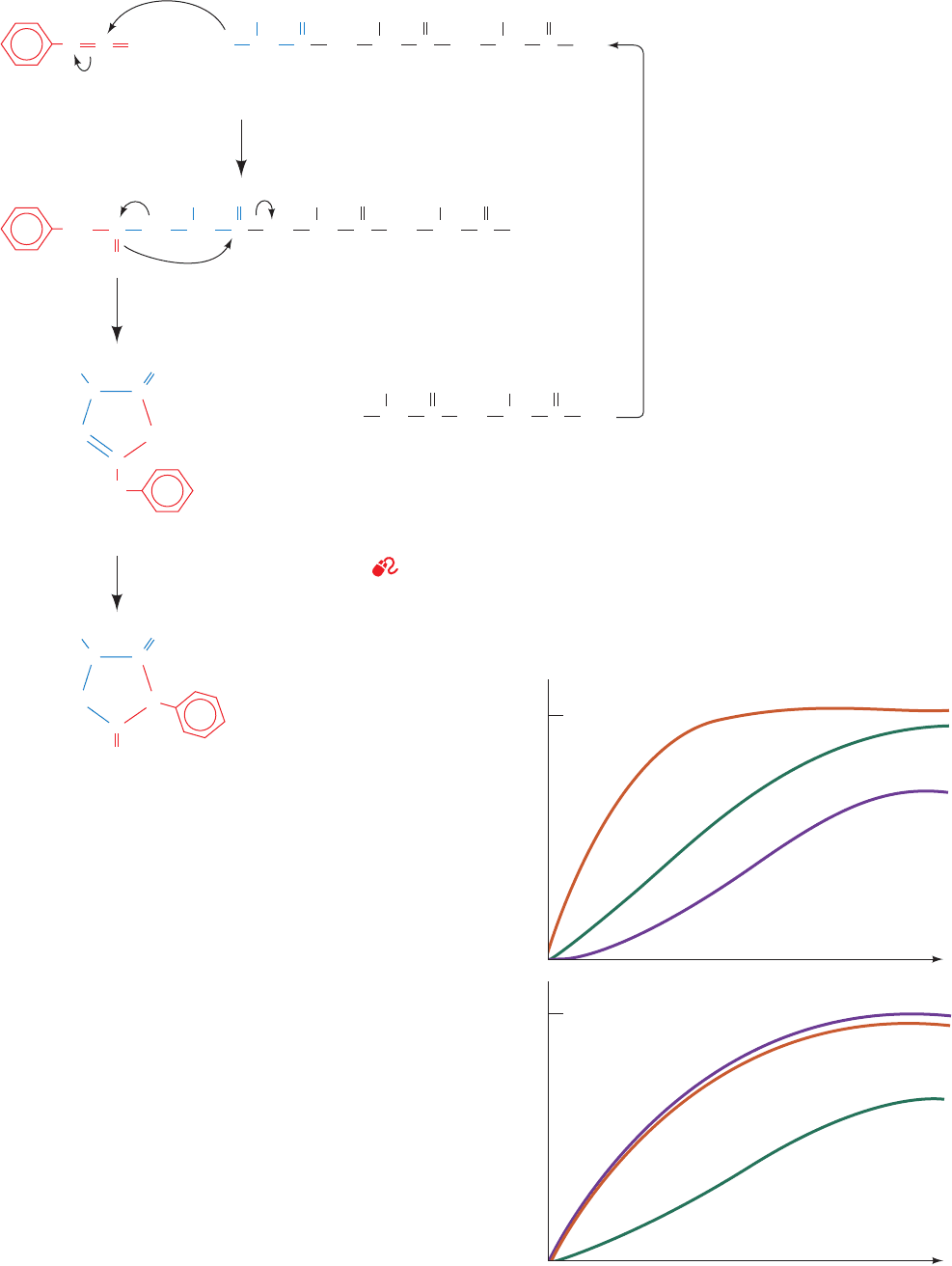
Section 7-1. Primary Structure Determination of Proteins 167
Figure 7-4 The Edman degradation. Note that the reaction occurs in three separate
stages that each require quite different conditions.Amino acid residues can therefore be
sequentially removed from the N-terminus of a polypeptide in a controlled stepwise
fashion.
See the Animated Figures
Figure 7-5 Hypothetical rate of the carboxypeptidase-catalyzed
release of amino acids from peptides having the indicated
C-terminal sequences. (a) All bonds cleaved at the same rate.
(b) Ser removed slowly, Tyr cleaved rapidly, and Leu cleaved at
an intermediate rate.
NH CCH
OR
2
H
2
N
CH
R
1
C
O
+
..
NH CCH
OR
3
PolypeptidePhenylisothiocyanate
(PITC)
anhydrous
F
3
CCOOH
NH
CCH
OR
2
CH
R
1
C
O
..
NH CCH
OR
3
PTC polypeptide
NH
...
HC C
N
N
H
OH
–
R
1
+
CCH
OR
2
NH CCH
OR
3
H
3
N
+
Original polypeptide less
its N-terminal residue
Thiazolinone derivative
H
+
HC C
HN
NCS
C
S
NH
C
S
C
N
S
R
1
O
PTH-amino acid
...
...
O
1.0
0
0
1.0
Time
Ser
Tyr
Leu
Ser
Tyr
Leu
(a) Starting peptide: –Ser–Leu–Tyr
(b) Starting peptide: –Leu–Tyr–Ser
Molar equivalents of amino acid released
JWCL281_c07_163-220.qxd 2/22/10 9:11 PM Page 167
in the presence of a mildly acidic ion exchange resin (which
acts as a catalyst):
All the peptide bonds are thereby cleaved, yielding the
aminoacyl hydrazides of all the amino acid residues except
that of the C-terminal residue, which is released as the free
amino acid and therefore can be identified chromato-
graphically. Unfortunately, hydrazinolysis is subject to a
great many side reactions that have largely limited its ap-
plication to carboxypeptidase-resistant polypeptides.
B. Cleavage of the Disulfide Bonds
The next step in the sequence analysis is to cleave the disul-
fide bonds between Cys residues.This is done for two reasons:
1. To permit the separation of polypeptide chains (if
they are disulfide linked).
CCH
O O
...
Polypeptide
Free amino acid
Hydrazine
acidic ion exchange
resin catalyst
Aminoacyl
hydrazides
R
1
H
3
N
R
n1
NH CCH
NH
2
NH
2
R
n
NH COO
CH
CCH
O
R
1
H
3
N
COO
CH
R
n
H
3
N
NH
NH
2
NH NH
2
...
H
3
N
R
n1
CCH
O
2. To prevent the native protein conformation, which is
stabilized by disulfide bonds, from obstructing the action of
the proteolytic (protein-cleaving) agents used in primary
structure determinations (Section 7-1D).
Disulfide bond locations are established in the final step of
the sequence analysis (Section 7-1H).
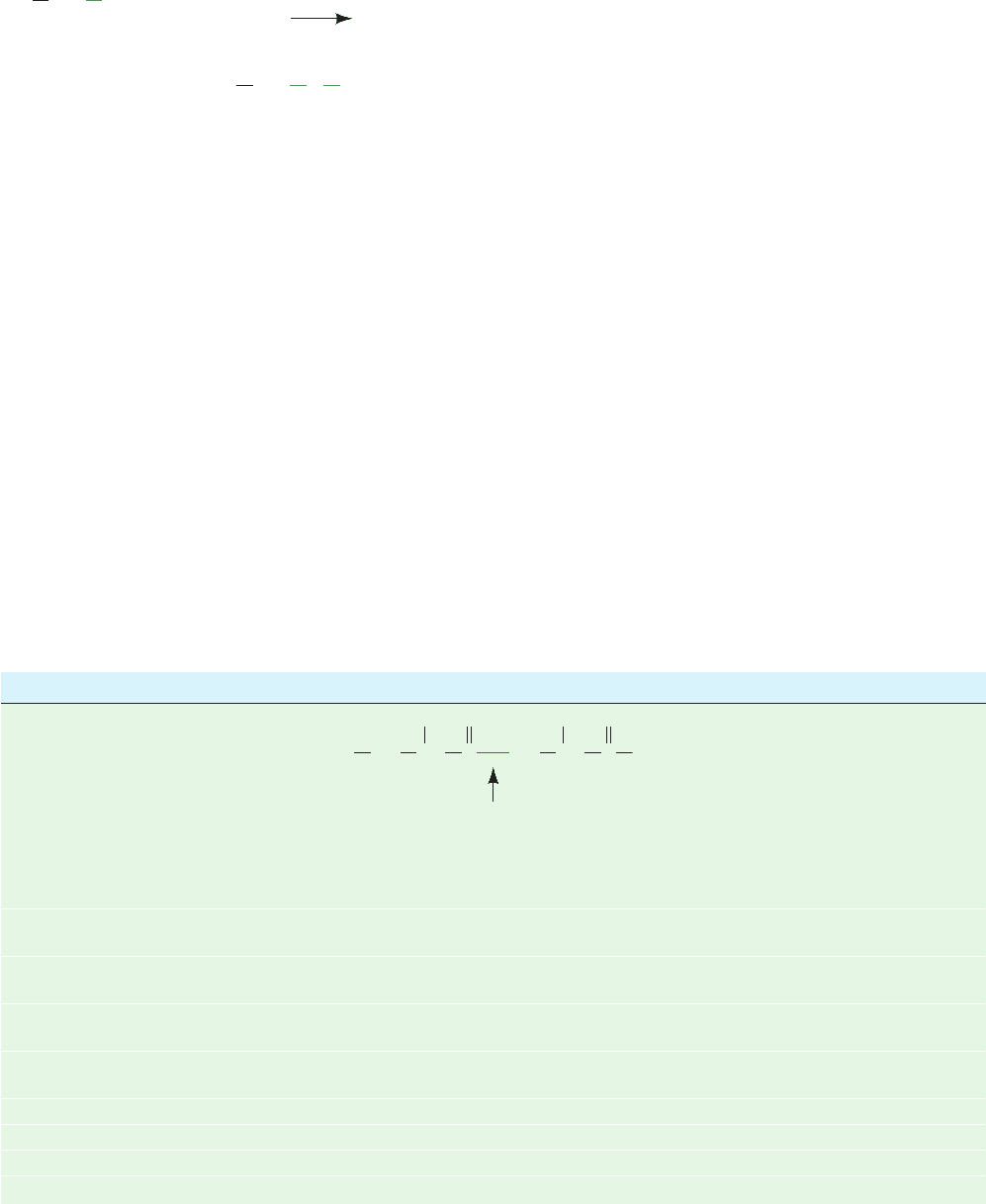
Disulfide bonds are most often cleaved reductively by
treatment with sulfhydryl-containing compounds such as
2-mercaptoethanol:
In order to expose all disulfide groups to the reducing
agent, the reaction is usually carried out under conditions
that denature the protein. The resulting free sulfhydryl
groups are alkylated, usually by treatment with iodoacetic
acid, to prevent the reformation of disulfide bonds through
CH
2
CH
2
NH C
...
CH
O
NH C
...
CH
O
+
Cystine
CH
Cysteine
2-Mercaptoethanol
...
...
S
S
2 HSCH
2
CH
2
OH
+
CH
2
NH C
...
O
...
SH
CH
2
NH C
...
CH
O
...
SH
+
SCH
2
CH
2
OH
SCH
2
CH
2
OH
168 Chapter 7. Covalent Structures of Proteins and Nucleic Acids
Table 7-1 Specificities of Various Exopeptidases
Enzyme Source Specificity
a
Carboxypeptidase A Bovine pancreas R
n
Arg, Lys, Pro; R
n1
Pro
Carboxypeptidase B Bovine pancreas R
n
Arg, Lys, R
n1
Pro
Carboxypeptidase C Citrus leaves All free C-terminal residues; pH optimum 3.5
Carboxypeptidase Y Yeast All free C-terminal residues, but slowly with R
n
Gly
Leucine aminopeptidase Porcine kidney R
1
Pro
Aminopeptidase M Porcine kidney All free N-terminal residues
a
R
1
the N-terminal residue; R
n
the C-terminal residue.
JWCL281_c07_163-220.qxd 2/22/10 9:11 PM Page 168
oxidation by O
2
. S-Alkyl derivatives are stable in air and
under the conditions used for the subsequent cleavage of
peptide bonds.
C. Separation, Purification, and Characterization of
the Polypeptide Chains
A protein’s nonidentical polypeptides must be separated
and purified in preparation for their amino acid sequence
determination. Subunit dissociation, as well as denatura-
tion, occurs under acidic or basic conditions, at low salt
concentrations, at elevated temperatures, or through the
use of denaturing agents such as urea, guanidinium ion
(Section 5-5G), or detergents such as sodium dodecyl sul-
fate (SDS; Section 6-4C).The dissociated subunits can then
be separated by methods described in Chapter 6 that capi-
talize on small differences in polypeptide size and polarity.
Ion exchange and gel filtration chromatography, usually by
HPLC (Section 6-3Dg), are most often used.
It is, of course,desirable to know the number of residues
in the polypeptide to be sequenced, which can be estimated
from its molecular mass (⬃110 D/residue). Molecular mass
can be measured with an accuracy of no better than 5 to
+
Cys
CH
2
Cys
SH
S
CH
2
Iodoacetate
S-Carboxymethylcysteine
I
CH
2
COO
–
CH
2
COO
–
Cysteine
+ HI
10% by the more traditional laboratory techniques of gel
filtration chromatography and SDS–PAGE (Sections 6-3Ba
and 6-4C). In recent years, however, mass spectrometry
(Section 7-1I) has provided a faster and far more accurate
means to determine the molecular masses of macromole-
cules. Mass spectrometry can determine the molecular
masses of picomolar amounts of 100 kD polypeptides
with accuracies of ⬃0.01%.
D. Specific Peptide Cleavage Reactions
Polypeptides that are longer than 40 to 100 residues cannot
be directly sequenced (Section 7-1F). Polypeptides of greater
length must therefore be cleaved, either enzymatically or
chemically, to fragments small enough to be sequenced (the
polypeptides produced by E. coli and humans have broad
distributions of lengths that average ⬃320 and ⬃470
residues, respectively). In either case, the cleavage process
must be complete and highly specific so that the aggregate
sequence of a subunit’s peptide fragments, when correctly
ordered, is that of the intact subunit.
a. Trypsin Specifically Cleaves Peptide Bonds after
Positively Charged Residues
Endopeptidases (enzymes that catalyze the hydrolysis
of internal peptide bonds), like exopeptidases, have side
chain requirements for the residues flanking the scissile
(to be cleaved) peptide bond. The side chain specificities
of the endopeptidases most commonly used to fragment
polypeptides are listed in Table 7-2.The digestive enzyme
trypsin has the greatest specificity and is therefore the
Section 7-1. Primary Structure Determination of Proteins 169
Table 7-2 Specificities of Various Endopeptidases
Enzyme Source Specificity Comments
Trypsin Bovine pancreas R
n–1
positively charged Highly specific
residues:Arg, Lys; R
n
Pro
Chymotrypsin Bovine pancreas R
n–1
bulky hydrophobic residues: Cleaves more slowly for
Phe,Trp,Tyr;R
n
Pro R
n–1
Asn, His, Met, Leu
Elastase Bovine pancreas R
n–1
small neutral residues:
Ala, Gly, Ser,Val; R
n
Pro
Thermolysin Bacillus thermoproteolyticus R
n
Ile, Met, Phe,Trp,Tyr, Val; Occasionally cleaves at R
n
Ala,
R
n–1
Pro Asp, His, Thr; heat stable
Pepsin Bovine gastric mucosa R
n
Leu, Phe,Trp,Tyr; Also others; quite nonspecific;
R
n–1
Pro pH optimum 2
Endopeptidase Arg-C Mouse submaxillary gland R
n–1
Arg May cleave at R
n–1
Lys
Endopeptidase Asp-N Pseudomonas fragi R
n
Asp May cleave at R
n
Glu
Endopeptidase Glu-C Staphylococcus aureus R
n–1
Glu May cleave at R
n–1
Gly
Endopeptidase Lys-C Lysobacter enzymogenes R
n–1
Lys May cleave at R
n–1
Asn
NH CH C
OR
n1
NH
Scissile
peptide bond
CH
C
OR
n
JWCL281_c07_163-220.qxd 2/22/10 9:11 PM Page 169
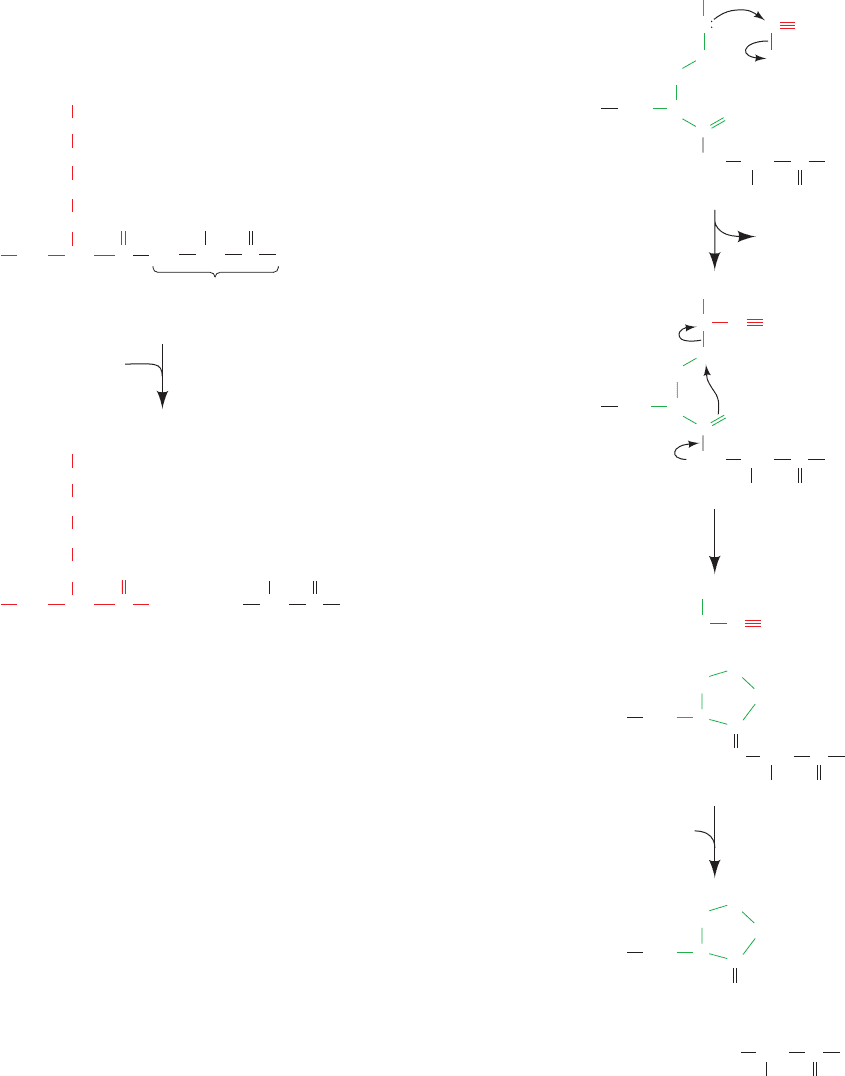
most valuable member of the arsenal of peptidases used
to fragment polypeptides. It cleaves peptide bonds on the
C-side (toward the carboxyl terminus) of the positively
charged residues Arg and Lys if the next residue is not
Pro:
The other endopeptidases listed in Table 7-2 exhibit
broader side chain specificities than trypsin and often yield
a series of peptide fragments with overlapping sequences.
However, through limited proteolysis, that is, by adjusting
reaction conditions and limiting reaction times, these less
specific endopeptidases can yield useful peptide fragments.
This is because the complex native structure of a protein
(subunit) buries many otherwise enzymatically susceptible
peptide bonds beneath the surface of the protein molecule.
With proper conditions and reaction times, only those pep-
tide bonds in the native protein that are initially accessible
to the peptidase will be hydrolyzed. Limited proteolysis is
often employed to generate peptide fragments of useful
size from subunits that have too many or too few Arg and
Lys residues to do so with trypsin (although if too many are
present, limited proteolysis with trypsin may also yield use-
ful fragments).
b. Cyanogen Bromide Specifically Cleaves Peptide
Bonds after Met Residues
Several chemical reagents promote peptide bond cleav-
age at specific residues. The most useful of these, cyanogen
bromide (CNBr), causes specific and quantitative cleavage
CH
2
CH
2
NH C
...
CH
O
NH
3
NH CCH
O
...
Any amino acid
residue but Pro
R
CH
2
CH
2
H
2
O
CH
2
CH
2
NH C
...
CH
O
NH
3
O
_
CH
2
CH
2
H
3
NCCH
O
...
R
+
+
trypsin
Lys
(or Arg)
on the C-side of Met residues to form a peptidyl homoser-
ine lactone:
The reaction is performed in an acidic solvent (0.1M HCl
or 70% formic acid) that denatures most proteins so that
cleavage normally occurs at all Met residues.
S
Br
Br
Cyanogen
bromide
Methyl
thiocyanate
Peptidyl
homoserine
lactone
Aminoacyl
peptide
NC
CH
3
CH
3
H
2
O
S
H
3
N
O
CH
NH
...
NH
...
C
NH
...
NH
...
:
NH CH
RO
C
...
:
NH CH
RO
C
...
NCH
RO
C
...
CH
RO
C
...
S NC
S NC
CH
2
C
O
CH
2
C
H
CH
2
CH
C
O
CH
2
CH
2
C
O
CH
2
C
CH
2
C
O
CH
2
C
H
170 Chapter 7. Covalent Structures of Proteins and Nucleic Acids
JWCL281_c07_163-220.qxd 2/22/10 9:11 PM Page 170
