Vlak J.M., de Gooijer C.D., Tramper J., Miltenburger H.G. (Eds.) Insect Cell Cultures: Fundamental and Applied Aspects
Подождите немного. Документ загружается.

192
exposed to any additional shear stress. Fraune et al.
(1991) have used a static PTFE hollow fiber membrane
module for the perfusion culture of various animal cell
lines including Spodoptera frugiperda but no further
details are given in their paper. An identical module
has been used by Klöppinger et al. (1990, 1991) for
the cultivation of Sf9 cells in a two stage process. A
maximum cell density of was achieved
in a silicone membrane-aerated 1.5-liter stirred tank
reactor for the production of wild-type Autographa
californica MNPV or recombinant A
different bioreactor system with membrane aeration via
microporous polypropylene tubings and an additional
hydrophilized polypropylene membrane for perfusion,
both mounted on a tumbling stirrer has been used for
the cultivation of IPLB-SF–21 AE cells. Cell densi-
ties of (Deutschmann & Jäger, 1991)
or (Deutschmann & Jäger, 1994) were
reached in a 1.2-liter stirred tank reactor with perfusion
rates of 3 and 4 reactor volumes per day, respective-
ly. Infection with recombinant Baculovirus and conse-
quent recombinant protein production was performed
at a cell density of up to (Jäger et al.,
1992) in bioreactors with 1.4 to 6 liter of working vol-
ume. Klöppinger et al. (1991) used a relatively high
multiplicity of infection (MOI) of 5 to 10, which pre-
supposes high titered virus stocks or large volumes of
virus suspension for infection. Jäger et al. (1992) used
an extremely low MOI of 0.015, which requires an
almost complete retention of virus within the bioreac-
tor in order to facilitate efficiently secondary infection
of all noninfected cells irrespective of the perfusion
rate. A retention of more than 99.9% was achieved
despite of the fact that the membrane pore size of
0.2 should theoretically allow a complete pen-
etration of the virus. More recently, an even lower
MOI of 0.001 has been used successfully for recom-
binant protein production with this perfusion system
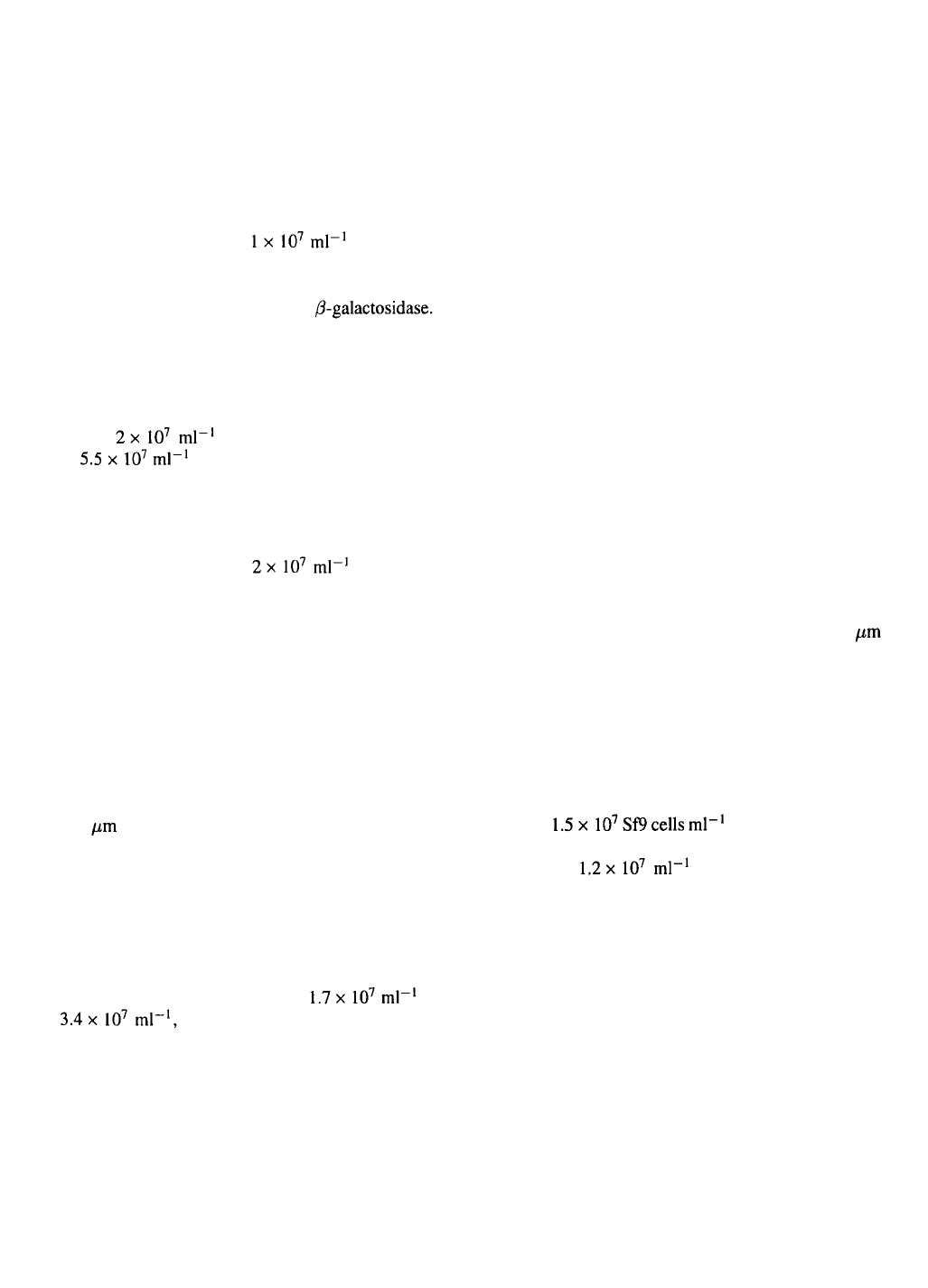
(Jäger, unpublished results) (Figure 1). Schütz et al.
(1991) cultivated the Drosophila cell lines Kc and
Schneider–2, which have been used for stable transfec-
tion, in a 1.4-liter bioreactor of the same type result-
ing in maximum cell densities of and
respectively. Data of bioreactor cul-
tivations with internal membrane perfusion are sum-
marized in Table 1. The most significant drawback of
internal membrane perfusion is the limited potential
for scale-up. However, a scale-up to more than 200
liters of working volume appears to be feasible.
External membrane perfusion
In contrast to the special reactor configuration required
for internal membrane perfusion almost any type of
stirred tank or airlift bioreactor can be combined with
external membrane perfusion provided that the reac-
tor system has sufficient capacity to aerate higher
cell densities without increasing shear forces (intro-
duced by vigorous mixing or sparging) to a critical
extent. In addition, external membrane perfusion offers
the advantage that the microfiltration module can be
exchanged if the need arises from membrane blocking.
However, pumping of cells and circulation through
crossflow microfiltration systems is able to generate
substantial laminar shear stress to insect cells (Maiorel-
la et al., 1991). Therefore, choice of the crossflow sys-
tem and the circulation pump as well as adjustment of
flow rates are of particular importance.
First attempts to use external membrane modules
for medium exchange were based on a two stage sys-
tem for the production of wild-type virus (Klöppinger
et al., 1990). Propagation of Sf9 cells as well as virus
propagation were performed in silicone membrane-
aerated 1.5-liter stirred tank bioreactors. The reactor
used for virus propagation was perfused by means
of an internal microporous tubing system with 1
pore size, whereas the reactor for cell propagation was
equipped with an external Microgon MiniKros hollow
fiber crossflow filtration system. However, this system
was not used continuously but in a batchwise mode to
allow medium exchange prior to infection.
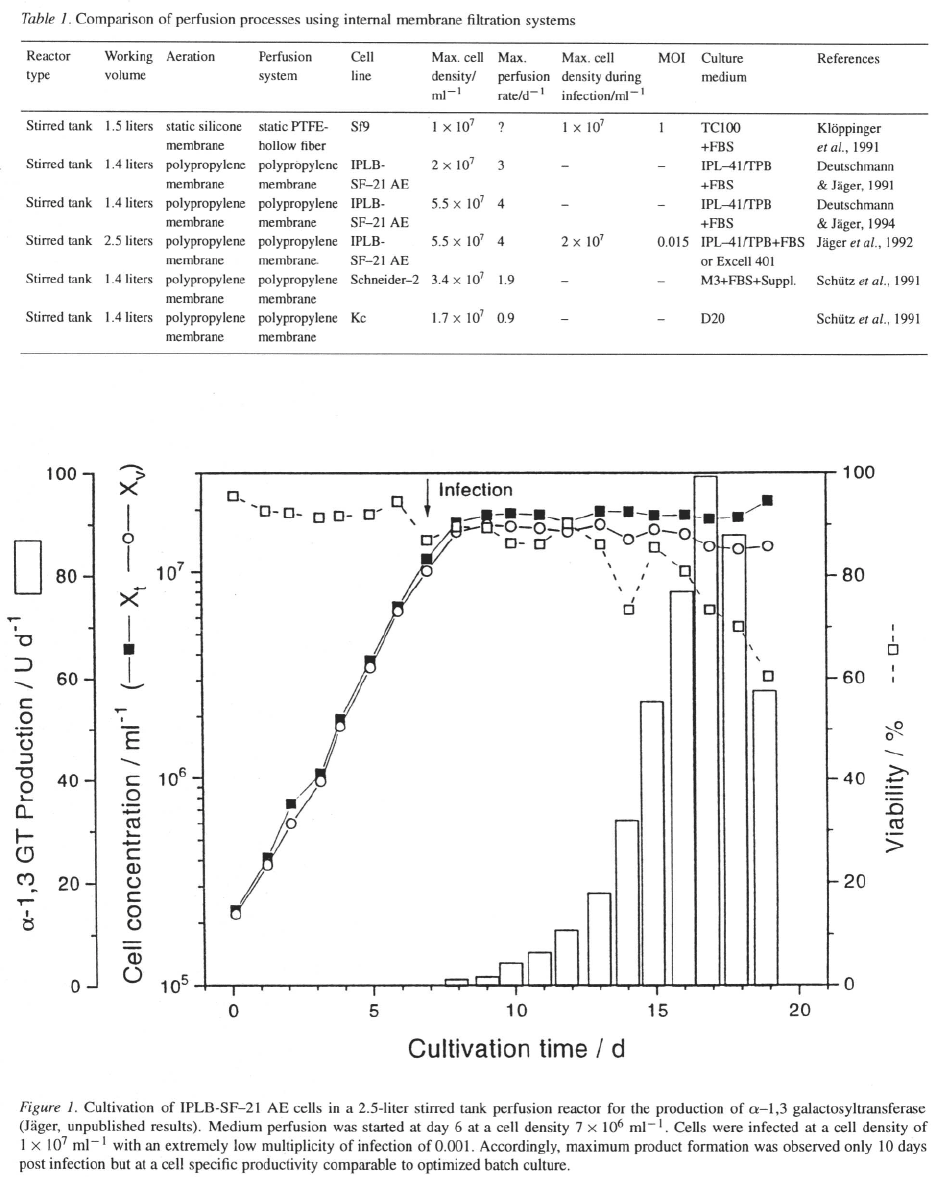
Continuous perfusion in a 4-liter Celligen reactor
combined with a home- designed tangential flow filtra-
tion system (de la Broise et al., 1991) resulted in den-
sities of (Massie et al., 1992).
However, the product yield, obtained when cells were
infected at was reduced to 50–75%
when compared to batch culture. This process was
further optimized by introduction of different spiral-
like tangential flow systems with or without integrated
membrane aeration (Caron et al., 1994). Perfusion at
rates between 1 and 4 reactor volumes per day was used
in the cell propagation phase as well as in the recombi-
nant protein production phase. 45 minutes after infec-
tion the tangential flow system was also used to con-
centrate cells before the initial culture volume was
reconstituted. Again, a reduced product yield of 75%
compared to spinner flasks was observed. In contrast,
Cavegn & Bernard (1992) obtained a normalized yield
varying from 0.5–3.5 compared to batch culture and a
significantly increased cell specific productivity (1.5–8
193
194
195
fold) in a 4-liter stirred tank reactor aerated by direct
pulse sparging and equipped with a Microgon Cellflo
hollow fiber crossflow microfiltration system for perfu-
sion (Cavegn et al., 1992). Densities of approximately
could be reached at perfusion
rates of up to 5 reactor volumes per day. Usually cells
were infected with a MOI of 5 at a density of
Using a 5-liter BioFlow III stirred tank reactor, aer-
ated by direct sparging and an A/G Technology hollow
fiber crossflow system Sf9 cells were grown to a den-
sity of at relatively low perfusion rates
of 0.4 to 0.6 reactor volumes per day (Reuveny et al.,
1994). The same crossflow system was also used for
high density cultures with medium replacement some-
times combined with fed-batch. The latter cultivation
method was suggested to be combined with a second
reactor for viral infection in order to obtain maximum
protein yields in minimum time.
Using a directly sparged 1.2-liter Cytoflow stirred
tank reactor combined with a hollow fiber crossflow
microfiltration system Sf9 and IPLB-SF–21 AE cells
were grown to maximum densities of
(Guillaume et al., 1992). Infections with a MOI of 1
were performed at The cell specific
productivity was comparable to batch cultures. The
same bioreactor system has also been used for the cul-
tivation of SPC-Mb 92 cells which were grown to a
maximum density of (Deramoudt et al.,
1994). Infected at a cell density of with
a MOI of 1 an 8.6 fold increase of the cell specific
productivity was observed.
IPLB-SF–21 AE were cultivated in a 10-liter air-
lift reactor equipped with a Prostak crossflow micro-
filtration system for medium perfusion (Hellenbroich,
1995). A maximum cell density of was
reached at a perfusion rate of 3 reactor volumes per
day. Cells were infected at a density of
with a MOI of 0.44. Due to the relatively small virus
196
inoculum cells continued to grow to
The yield of a recombinant interleukin–2 variant was
comparable to that obtained in experiments utilizing
membrane-aerated stirred tank reactors. In contrast,
Sf9 cells grown in a 23-liter airlift reactor with medi-
um perfusion showed a markedly reduced recombi-
nant protein production when compared to low density
batch cultures in the same reactor (Schlaeger et al.,
1992).
Perfusion processes using external membrane fil-
tration systems are summarized in Table 2. A wide
range of culture media with significantly differing
nutrient contents has been used for the cultivation of
insect cells with medium perfusion. Whereas IPLB-
SF-21 AE cells had to be perfused with 3 reactor vol-
umes per day to reach a cell density of
(Hellenbroich, 1995) the same cell density could be
achieved in batch culture by improved media formula-
tions (Ackermann et al., 1994). Due to this fact it is very
difficult to compare the different perfusion processes
and to extrapolate the potential of the reactor configu-
ration.
In general, external membrane perfusion has
proved to be a vital alternative to batch and fed-batch
culture. The most critical point appears to be a scale-
down of such bioreactor systems. In this case, the
circulation frequency of cells becomes too high or the
membrane channels become too small and thus gener-
ate intolerable shear stress.
Cell immobilization
Different approaches have been used to immobilize
insect cells for the production of virus and recombinant
proteins. One method is to encapsulate the cells by
alginate or other polymers (King et al., 1988, 1989).
This procedure is reviewed in more detail in a separate
chapter of this issue.
Another method is to grow cells by making use of
their potential to adhere to suitable carrier matrices.
Cells attached on these carriers can be cultivated in
various bioreactor systems depending on the properties
of the carriers with respect to size, shape and specif-
ic weight. Most frequently used are stationary (fixed,
packed) bed bioreactors and fluidized bed bioreactors.
Kompier et al. (1991a,b) cultivated IPLB-SF–21 AE
cells on non-woven fabric discs in a stationary bed reac-
tor for the production of recombinant
This small reactor consisted of a 50 ml column contain-
ing the stationary bed and a 350 ml membrane-aerated
medium reservoir. The medium was circulated with
a peristaltic pump. Even smaller columns of approxi-
mately 20 ml and filled with glass spheres were used
for the cultivation of BTI-Tn 5BI–4 cells (Shuler et
al., 1990). This column was connected to a small bub-
ble column. The culture medium was circulated by
means of a peristaltic pump or by the airlift itself.
More recently, this combination of two vessels was
replaced by introducing the bed directly into a split
flow airlift bioreactor of 210 ml volume (Chung et al.,
1993a). Although it is theoretically possible that all of
these reactor configurations can be run continuously
without complications, all systems were run in batch
mode at cell densities of or less. All
reactor systems contained solid particles without pores
as matrices which allow growth of cells only on the
surface. However, the question remains unanswered
whether insect cells show identical growth and pro-
ductivity at increased densities especially when limited
surface areas force cells to grow in multiple layers.
As an alternative, cells can be immobilized on
spherical macroporous carriers made of borosilicate
glass (SIRAN® , Schott) or hydrophobic polyethylene
(Cytoline–1® , Pharmacia) and cultivated in fluidized
bed bioreactors (Jäger, unpublished results). IPLB-SF–
21 AE and SPC-Bm36 were cultivated in a 0.25-liter-
fluidized-bed reactor described for the cultivation of
mammalian cells (Lüllau et al., 1994). Cell densities
of approx. were achieved based on the
total reactor volume or based on the
available volume inside of the macroporous carriers.
As shown in Figure 2 for the cultivation of IPLB-SF–
21 AE cells the growth rate as well as the produc-
tion rate of the recombinant protein are significantly
reduced when compared to perfused suspension culture
in stirred tank bioreactors (Jäger et al., 1992). It can
be assumed that in contrast to the surrounding culture
medium there might be insufficient oxygen concen-
trations inside the carriers with almost tissue-like cell
densities. The unsatisfactory results in the fluidized bed
could be a result of the fact that insect cell growth and
baculovirus-directed protein production are strongly
dependent on sufficient oxygen supply (Deutschmann
& Jäger, 1994; Jäger & Kobold, 1995).
Conclusion
High density perfusion culture of insect cells for the
production of recombinant proteins has proved to be an
attractive alternative to batch and fed-batch processes.

197
A comparison of the different production processes
is summarized in Table 3. Internal membrane per-
fusion has a limited scale-up potential but appears
to the method of choice in smaller lab-scale produc-
tion systems. External membrane perfusion results in
increased shear stress generated by pumping of cells
and passing through microfiltration modules at high
velocity. However, using optimized perfusion strate-
gies this shear stress can be minimized such that it is
tolerated by the cells. In these cases, perfusion cul-
ture has proven to be superior to batch production with
respect to product yields and cell specific productivity.
Although insect cells could be successfully cultivat-
ed by immobilization and perfusion in stationary bed
bioreactors, this method has not yet been used in con-
tinuous processes. In fluidized bed bioreactors with
continuous medium exchange cells showed reduced
growth and protein production rates.
For the cultivation of insect cells in batch and fed-
batch processes numerous efforts have been made to
optimize the culture medium in order to allow growth
and production at higher cell densities. These improved
media could be used in combination with a perfusion
process, thus allowing substantially increased cell den-
sities without raising the medium exchange rate. How-
ever, sufficient oxygen supply has to be guaranteed
during fermentation in order to ensure optimal produc-
tivity.
Acknowledgment
I am grateful to S. Grammatikos for critical reading of
the manuscript.
References
Ackermann M, Hellenbroich DHJ & Jäger V (1994) Improvement
of the performance of commercially available insect cell culture
media for the Baculovirus-directed production of recombinant
proteins in bioreactors. Cytotechnology 14, Suppl. 1: 2.8.
Bédard C, Perret S & Kamen A (1995) Fed-batch culture supports
Presented at the ‘Baculovirus and
insect cell gene expression conference’, Pinehurst, NC, March
26–30, 1995.
Caron AW, Tom RL, Kamen AA & Massie B (1994) Baculovirus
expression system scaleup by perfusion of high-density Sf–9 cell
cultures. Biotechnol. Bioeng. 43: 881–891.
Cavegn C, Blasey HD, Payton MA, Allet B, Li J & Bernard AR
(1992) Expression of recombinant proteins in high density insect
cell cultures. In: Spier RE, Griffiths JB & MacDonald C (eds)
Animal cell technology: Developments, processes and products
(pp. 569–578) Butterworth-Heinemann, Oxford.
Cavegn C & Bernard AR (1992) A perfusion process for high density
insect cell cultures. In: Vlak JM, Schlaeger E-J & Bernard AR
(eds) Baculovirus and recombinant protein production processes
(pp. 262–273) Editiones Roche, Basel.
Chung IS, Taticek RA & Shuler ML (1993) Production of human
alkaline phosphatase, a secreted, glycosylated protein, from a
Baculovirus expression system and the attachment-dependent cell
line Trichoplusia ni BTI-Tn 5B1–4 using a split-flow, air-lift
bioreactor. Biotechnol. Prog. 9: 675–678.
Chung IS & Shuler ML (1993) Effect of Trichoplusia ni BTI-Tn
5B1–4 cell density on human secreted alkaline phosphatase pro-
duction. Biotechnol. Lett. 15: 1007–1012.
de la Broise D, Noiseux M, Lemieux R & Massie B (1991) Long-
term perfusion culture of hybridoma: A ‘grow or die’ cell cycle
system. Biotechnol. Bioeng. 38: 781–787.
Deramoudt F-X, Monnet S, Rabaud J-N, Quiot J-M, Cerutti M,
Devauchelle G & Kaczorek M (1994) Production of a recombi-
nant protein in a high density insect cell Cytoflow reactor. In:
Spier RE, Griffiths JB & Berthold W (eds) Animal cell technol-
ogy: Products of today, prospects for tomorrow (pp. 222–226)
Butterworth-Heinemann, Oxford.
Deutschmann S & Jäger V (1991) High density suspension culture
of insect cells in a stirred bioreactor. In: Sasaki R & Ikura K (eds)
Animal cell culture and production of biologicals (pp. 151–158)
Kluwer, Dordrecht.
Deutschmann SM & Jäger V (1994) Optimization of the growth
conditions of Sf21 insect cells for high-density perfusion culture
in stirred-tank bioreactors. Enzyme Microb. Technol. 16: 506–
512.
Fraune E, Fenge C, Kuhlmann W & Broly H (1991) Development
of perfusion bioreactors for high density cultures. In: White MD,
Reuveny S & Shafferman A (eds) Biologicals from recombinant
microorganisms and animal cells (pp. 159–164) VCH, Weinheim.
Guillaume JM, Couteault N, Hurwitz DR & Crespo A (1992) High
density insect cell homogenous perfusion culture for recombinant
proteins production. In: Vlak JM, Schlaeger E-J & Bernard AR
(eds) Baculovirus and recombinant protein production processes
(pp. 285–296) Editiones Roche, Basel.
Hellenbroich DHJ (1995) Einsatz von Airlift-Reaktoren für Pro-
duktionsverfahren mit tierischen Zellkulturen. Ph.D. Thesis,
Brunswick Technical University.
Jäger V, Grabenhorst E, Kobold A, Deutschmann SM & Conradt
HS (1992) High density perfusion culture of insect cells for the
production of recombinant glycoproteins. In: Vlak JM, Schlaeger
E-J & Bernard AR (eds) Baculovirus and recombinant protein
production processes (pp. 274–284) Editiones Roche, Basel.
Jäger V & Kobold A (1995) Propagation of Spodoptera frugiperda
cells (Sf9) and production of recombinant proteins with the Bac-
ulovirus expression system using improved spinner flasks with
membrane aeration. Biotechnol. Techniques 9: 435–440.
King GA, Daugulis AJ, Faulkner P, Bayly D & Goosen MFA (1988)
Growth of Baculovirus-infected insect cells in microcapsules to
a high cell and virus density. Biotechnol. Lett. 10: 683–688.
King GA, Daugulis AJ, Goosen MFA, Faulkner P & Bayly D
(1989) Alginate Concentration: A key factor in growth of
temperature-sensitive Baculovirus-infected insect cells in micro-
capsules. Biotechnol. Bioeng. 34: 1085–1091.
Klöppinger M, Fertig G, Fraune E & Miltenburger HG (1990) Multi-
stage production of Autographa californica Nuclear Polyhedrosis
Virus in insect cultures. Cytotechnology 4: 271–278.
Klöppinger M, Fertig G, Fraune E & Miltenburger HG (1991) High
density perfusion culture of insect cells for production of Bac-
ulovirus and recombinant protein. In: Spier RE, Griffiths JB &

198
Meignier B (eds) Production of biologicals from animal cells in
culture (pp. 470–476) Butterworth-Heinemann, Oxford.
Kompier R, Tramper J & Vlak JM (1988) A continuous process for
the production of Baculovirus using insect-cell cultures. Biotech-
nol. Lett. 10: 849–854.
Kompier R, Kislev N, Segal I & Kadouri A (199la) Use of a sta-
tionary bed reactor and serum-free medium for the production of
recombinant proteins in insect cells. Enzyme Microb. Technol.
13: 822–827.
Kompier R, Kislev N, Segal I & Kadouri A (1991b) The use of a
non-woven fabric carrier for the production of
from insect cells. In: White MD, Reuveny S & Shafferman A
(eds) Biologicals from recombinant microorganisms and animal
cells (pp. 271–274) VCH, Weinheim.
Lüllau E, Biselli M & Wandrey C (1994) Growth and metabolism
of CHO-cells in porous glass carriers. In: Spier RE, Griffiths JB
& Berthold W (eds) Animal cell technology: Products of today,
prospects for tomorrow (pp. 252–255) Butterworth-Heinemann,
Oxford.
Maiorella B, Dorin G, Carion A & Harano D (1991) Crossflow
microfiltration of animal cells. Biotechnol. Bioeng. 37: 121–126.
Massie B, Tom R & Caron AW (1992) Scale-up of a Baculovirus
expression system: Production of recombinant protein in per-
fused, high-density Sf9 cell cultures. In: Vlak JM, Schlaeger
E-J & Bernard AR (eds) Baculovirus and recombinant protein
production processes (p. 234) Editiones Roche, Basel.
Reuveny S, Kemp CW & Shiloach (1994) High cell density in insect
culture. In: Spier RE, Griffiths JB & Berthold W (eds) Animal cell
technology: Products of today, prospects for tomorrow (pp. 494–
503) Butterworth-Heinemann, Oxford.
Schlaeger E-J, Loetscher H & Gentz R (1992) Production of recom-
binant soluble human TNF receptor using the Baculovirus-insect
cell expression system. In: Spier RE, Griffiths JB & MacDon-
ald C (eds) Animal cell technology: Developments, processes &
products (pp. 562–568) Butterworth-Heinemann, Oxford.
Schütz C, Jäger V, Driesel AJ & Wagner R (1991) Cultivation of
insect cell lines in stirred membrane reactors. In: Spier RE, Grif-
fiths JB & Meignier B (eds) Production of biologicals from animal
cells in culture (pp. 460–466) Butterworth-Heinemann, Oxford.
Shuler ML, Cho T, Wickham T, Ogonah O, Kool M, Hammer DA,
Granados RR & Wood HA (1990) Bioreactor development for
production of viral pesticides or heterologous proteins in insect
cell cultures. Ann. N.Y. Acad. Sci. 589: 399–422.
Tramper J, van den End EJ, de Gooijer CD, Kompier R, van Lier
FLJ, Usmany M & Vlak JM (1990) Production of baculovirus
in a continuous insect-cell culture. Bioreactor design, operation,
and modeling. Ann. N.Y. Acad. Sci. 589: 423–430.
van Lier FLJ, van den End EJ, de Gooijer CD, Vlak JM & Tramper
J (1990) Continuous production of Baculovirus in a cascade of
insect-cell reactors. Appl. Microbiol. Biotechnol. 33: 43–47.
van Lier FLJ, van der Meijs WCJ, Grobben NG, Olie RA, Vlak JM
& Tramper J (1992) Continuous production with
a recombinant Baculovirus insect-cell system in bioreactors. J.
Biotechnol. 22: 291–298.
van Lier FLJ, van Duijnhoven GCF, de Vaan MMJACM, Vlak JM
& Tramper J (1994) Continuous production in
insect cells with a p10 gene based Baculovirus vector in a two-
stage bioreactor system. Biotechnol. Prog. 10: 60–64.
Wood HA, Johnston LB & Burand JP (1982) Inhibition of Auto-
grapha californica Nuclear Polyhedrosis Virus replication in
high-density Trichoplusia ni cell cultures. Virology 119: 245–
254.
Address for correspondence: Volker Jäger, Abteilung Zellkul-
turtechnik, Gesellschaft für Biotechnologische Forschung mbH,
Mascheroder Weg 1, D–38124 Braunschweig, Germany. E-mail:
vja@gbf-braunschweig.de

Cytotechnology 20: 199–208, 1996. 199
© 1996 Kluwer Academic Publishers. Printed in the Netherlands.
Immobilization of insect cells
Jian-Yong Wu
1,3
& Mattheus F. A. Goosen
1,2
*
1
Department of Chemical Engineering, Queen’s University, Kingston, Ontario, K7L 3N6, Canada;
2
Sultan
Qaboos University, College of Agriculture, P.O. Box 34, Al-Khod 123, Muscat, Sultanate of Oman;
3
Current
Address: Department of Applied Biology & Chemical Technology, Hong Kong Polytechnic, Kowloon, Hong Kong
Key words: immobilized insect cell culture
Introduction
The production of baculoviruses and recombinant pro-
teins in insect cell culture has been mostly carried out
in culture systems with freely-suspended cells. Sus-
pended culture systems usually suffer from a number
of disadvantages, such as the fluid mechanical cell
damage from agitation and air-sparging, low cell and
product concentrations, and low productivity. Another
limitation is that the method is only suitable for the
growth of non anchorage-dependent cell (e.g., Sf–9
cells). These drawbacks can be eliminated with the
exploitation of cell immobilization techniques, such
as the attachment of cells on solid surfaces, entrap-
ment of cells in polymer gels, and encapsulation of
cells in artificial membranes. With immobilized cells,
a 10-fold or more increase in the cell and produc-
tion concentrations can be achieved (e.g., Dean et al
.,
1987; Reiter et al
.,
1991; and Park & Stephanopoulos,
1992). The technology will also allow for simpler cell-
medium separation and product purification processes.
In addition, immobilization of cells in porous supports
and microencapsules could also prevent the cell from
mechanical damage due to mechanical agitation and
gas sparging.
There have been extensive studies on immobiliza-
tion of animal cells and its application in large-scale
production of mammalian cells and associated prod-
ucts, such as hybridoma cells in the production of
monoclonal antibodies (Reuveny, 1985; Heath and
Belfort, 1987). The development of immobilized insect
* To whom correspondence should be addressed.
cell culture, however, is still in its infant stage. This
is not surprising since it is only recently that insect
cell culture has been recognized as an important tool
in the biotechnology industry (Luckow and Summers,
1988). Other factors responsible for the fewer studies
on insect cell immobilization may be related to the type
of insect cells used for large-scale production and the
mechanism of virus and recombinant protein produc-
tion. The cell lines used predominantly in baculovirus
and recombinant protein production, i.e., those derived
from Spodoptera frugiperda insect, Sf–21 and Sf–9,
grow well in suspension, which undermines the advan-
tages of immobilized culture. On the other hand, pro-
duction of cell products involves viral infection and
subsequent lysis of the cell. Immobilized cell culture
can thus only be used for one batch operation. With
the development of anchorage-dependent insect cells
for virus and protein production, and processes of pro-
ducing secreted cell products without cell lysis, immo-
bilization technology will become more attractive for
large-scale production.
In this chapter, we will primarily focus on tech-
niques for animal cell immobilization in particulate
supports, including microcarrier beads and microcap-
sules, with emphasis on their applications in insect cell
culture. Typical immobilization materials and meth-
ods, immobilized cell bioreactors and the major con-
cerns associated with each technique will be addressed.
The review will conclude with an outline of the studies
conducted in our laboratory on insect cell immobiliza-
tion techniques.

200
Cell immobilization systems
A variety of systems can be employed for cell and
enzyme immobilization. These include, for example,
microcarriers (van Wezel, 1967), gel entrapment (Guo
et al., 1990), hollow fibers (Knazek et al., 1972), and
encapsulation (Lim and Moss, 1981). In principle,
these methods fall into one of two categories: entrap-
ment in a three dimensional polymer matrix or cap-
ture behind a semipermeable membrane. Among these
methods, microcarriers and gel entrapment are proba-
bly the most popular. However, one common problem
with both is the leakage of the immobilized biocata-
lyst. Hollow fibers and microencapsulation overcome
this problem by separating the cells from converted
medium with a semipermeable membrane. Our atten-
tion in this section will be on the immobilization of
animal cells in particulate carriers, including micro-
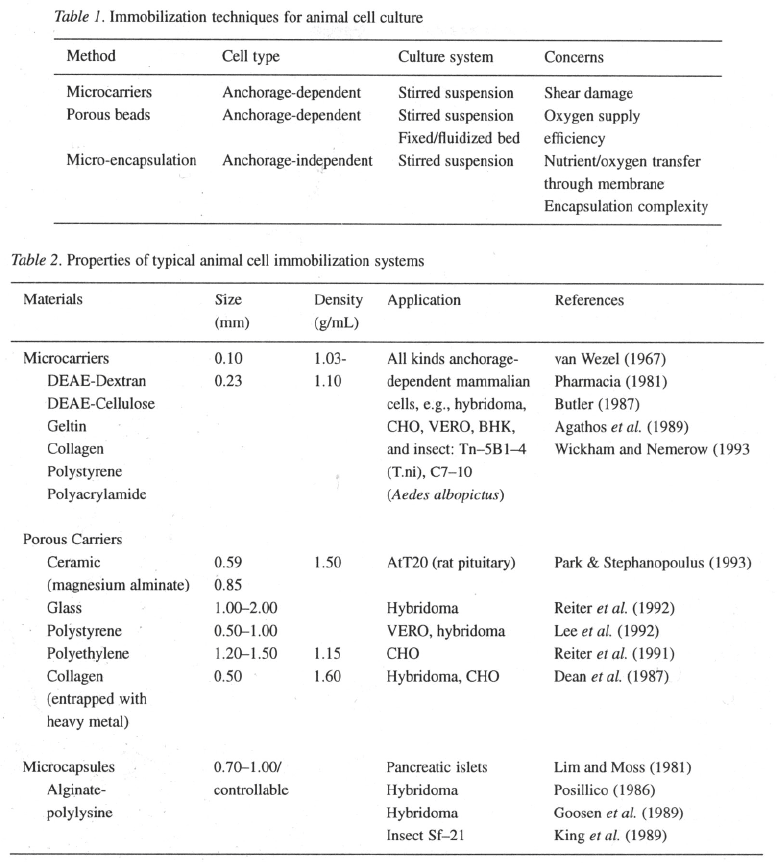
carriers, porous beads and microcapsules (Table 1).
Microcarriers
The introduction of microcarriers to animal cell cul-
ture is a major breakthrough in the development of
anchorage-dependent animal and mammalian cell cul-
ture processes. The traditional method for large-scale
production of anchorage-dependent animal cells was
to grow the cells on the inside surfaces of rotating bot-
tles, or roller bottles. Major disadvantages of the roller
bottle system include a very low surface-to-volume
ratio, variable quality of the cell products and exten-
sive labor costs required for processing large numbers
of bottles: In a microcarrier culture, on the other hand,
cells are attached on the surface of small carrier beads
suspended in the medium. The small carrier beads,
with a diameter of a few hundred microns, provide
a large surface area for cell attachment. For instance,
the surface-to-volume ratio of a microcarrier culture
is about at 5 g/l particle concentration, com-
pared to 1.2 cm
–1
for a roller bottle (Butler, 1987).
Apart from a significant increase in the surface-to-
volume ratio, use of microcarriers has made it possible
to cultivate anchorage-dependent cells in a homoge-
neously suspended condition, which greatly facilitates
large-scale production.
Microcarrier culture technology was first report-
ed by van Wezel (1967), nearly three decades ago.
He cultivated mammalian cells adhering on DEAE-
Sephadex
®
A–50 beads in suspension culture. The
microcarrier chosen by van Wezel (1967), i.e., DEAE-
Sephadex A–50, was designed primarily for ion
exchange chromatography. These microcarrier beads
consist of a dextran matrix with a net positive surface
charge which is considered necessary for cell attach-
ment. The microcarriers were found suitable for cell
growth at particle concentrations no greater than 1 g/l.
Exceeding that level of beads loading was found to
cause inhibitory effects on cell growth. This adverse
effect was believed to be related to the charge density
on the bead surface. By reducing the surface charge
of the microcarriers, Levine et al. (1977) were able
to eliminate this toxic effect, achieving cell attach-
ment and growth at particle concentrations up to 5 g/l.
This breakthrough made it feasible to grow anchorage-
dependent cells to high cell densities to suspension
culture bioreactors.
Polymeric products, such as dextran (polysaccha-
ride), cellulose, Gelatin (polysaccharide) and plastic
(polystyrene) have been primary matrix materials for
cell immobilization (Table 2). Particularly, the dextran-
based microcarriers, Cytodex 1, 2, 3 (Pharmacia Fine
Chemicals, Uppsala, Sweden), have been most widely
used in large-scale cell culture as well as laboratory
research. The most important physical and chemical
properties of microcarriers particles may include sur-
face charge, density and size. Surface charge, as men-
tioned earlier, is crucial for cell attachment and cell
growth on the microcarriers. The optimal charge den-
sity is 2.0 meg/g (Butler, 1987). Usually the density
of the microcarrier beads is relatively low (typical-
ly 1.03 g/l, slightly higher than the liquid medium)
to facilitate suspension with gentle agitation. Heavier
materials requiring high stirring speeds to suspend may
cause cell damage. The diameter of the microcarriers
should conform to a high surface-to-volume ratio, there
should be sufficient surface area on each microcarri-
er for a single cell to divide for several generations,
the manufacture process should also be inexpensive.
Diameters in the range of 150–220 have been found
to be desirable. Other properties, such as microcarri-
er transparency, size distribution and rigidity are also
important concerns for cell attachment, growth in sus-
pension, visual observation, and cell growth and viabil-
ity measurement. In addition, non-toxicity is essential
for cell survival as well as for medical applications of
cell products.
A major drawback with current microcarriers is that
they cannot be reused (i.e., cleaning and sterilization).
This results in considerable increase in the cost of the
culture process (e.g., $50/l with 5 g/l beads loading,
Glacken et al., 1983). In addition, one of the major
concerns with microcarrier culture is mechanical dam-
201
age of the cell attached on the carrier surface due to
fluid shear stresses and possibly collision between the
carrier beads (Croughan et al., 1988).
The kinetics of animal cell growth on microcarriers
was found to be essentially the same as in monolayer
culture, but much higher cell densities can be achieved
if the medium is replenished (e.g., medium perfusion)
(van Wezel, 1972). The choice of a suitable micro-
carrier depends on the type of cells (e.g., plating effi-
ciency and morphology) and the purpose of the culture
(product harvest or cell biology studies). This will in
turn affect the choice of the culture vessels. Although
in principle microcarrier culture can be contained in
virtually any type of cell culture vessel (static mono-
layer or suspension), its advantages are best realized
in stirred suspension culture. To avoid cell detachment
and fluid mechanical damage, the stirring action should
be gentle, and evenly distributed, with minimum agi-
tation.
Porous carriers
Although microcarrier technology has many advan-
tages for animal cell culture, it still has some draw-
backs. First of all, the technique is only suitable for
anchorage-dependent cells. Secondly, cells attached
