Vij D.R. Handbook of Applied Solid State Spectroscopy
Подождите немного. Документ загружается.

1. Nuclear Magnetic Resonance Spectroscopy
24
equation (1.30), but it also depends on the time scale and geometry of the
molecular motion [50]. In the limit where many frequency changes occur
during the signal acquisition, W
c
<< 2S/Z, (the fast exchange limit) [49] a fully
averaged powder pattern with the shape defined by the characteristics of the
molecular motion is observed. The characteristic shape of the spectrum
obtained in the fast exchange regime is dictated by the amplitude and
geometry of the motion. In general, the NMR line shape in the fast exchange
limit can be obtained simply by calculating the effective precession frequency
(average frequency) under the anisotropic spin interaction [7, 49, 51]. This
effective precession frequency is calculated according to an average tensor
that depends on the motion geometry. Because the average tensor is also a
second rank tensor, its anisotropy can also be characterized by two
parameters, namely
Ș
and į .
The dependence of
Ș
and į on the geometry of the motion is
demonstrated in Figures 1.6a and b, for the cases of chemical shift anisotropy
(I = 1/2 nuclei) and first order quadrupolar (I = 1 nuclei) interactions,
respectively.
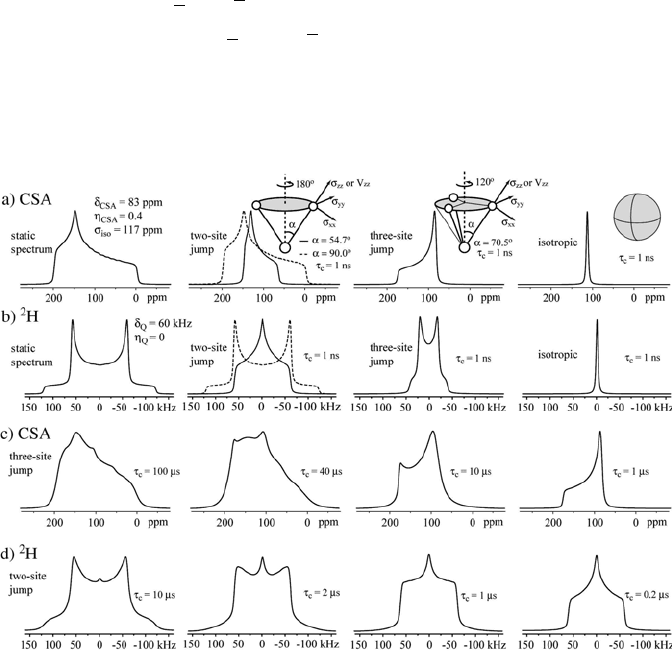
Figure 1.6 Series of powder pattern line shapes simulated for different kinds of molecular
motions: a) I = ½ (CSA) and b) I = 1 (
2
H quadrupolar). c) Chemical shift anisotropy powder
pattern for three-site jump motion with D = 70.5q (methyl rotations) and different correlation
2
different correlation
times.
times. d) H powder pattern for two-site jump motion with D = 54.7q and

1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
25
Different spectra are observed according to the motional geometry and
amplitude. Two general features are: i) for any uniaxial motion, such as the
three-site jump shown in Figure 1.6, the resulting average tensor will be
axially symmetric because the rotationally averaged x and y principal values
are equal; ii) for isotropic motions all the three principal values are averaged
out and a single isotropic line is observed in the fast limit [7].
For spin 1/2 systems under the influence of the Zeeman and chemical shift
interactions, the density matrix of the spin system behaves exactly like
macroscopic magnetizations. Hence, if many molecular orientations can exist
simultaneously, the concept of magnetization isochromats can be used for
describing the set of different nuclear spins with the same precession
frequency, i.e., under identical chemical shielding. If we consider the same
kind of chemical species, the spin system in an amorphous or polycrystalline
material can be described by a set of magnetization isochromats, M
i
, each one
corresponding to a given molecular orientation. Under the influence of
molecular motion during the precession of the transverse magnetization, the
molecular orientations, and consequently the precession frequencies, change
stochastically, producing significant modifications in the resulting NMR
spectrum.
Thus, the time evolution of the transverse magnetization isochromats
depends not only on their individual precession frequencies but also on the
probability of exchange between different isochromats. Assuming a stationary
Markov process, the time evolution of each transverse magnetization
isochromat can be calculated with the following equation [7]:
Ȧ
dt
it
dt
3
m
m
G
G
,
(1.74)
^`
1exp Ȧ 0Mt i t
ªº
3
¬¼
m
G
G
G
.
(1.75)
where
()tm
G
is a vector where each component is a complex number
representing the isochromat magnetization vector in the xy-plane, M
i
(t). Ȧ
is a
diagonal matrix, which is composed of the elements
ȦįȦ
jl jl j
= , where Z
j
is
the precession frequency of the jth magnetization isochromat and depends on
the molecular orientation according to equation (1.30). The matrix
3
is the
so-called exchange matrix, composed of off-diagonal elements 3
jl
that
represent the transition rate k from the state l (one given orientation) to the
state j (another orientation), and diagonal elements 3
jj
that represent the total
transition rate from a state j to any other state with a negative sign. Note that
the transition rates are directly associated with the correlation time of the
motion (for example, for a two-site jump k = 1/(2W
c
)). To obtain the total
magnetization it is necessary to sum over all isochromats. This is done by
multiplying the complex magnetization vector
()tm
G
by the row vector
1 (1, 1, 1, 1, . .., 1)=
G
. Using such procedure, from the formal solution of equation
(1.74), the total magnetization vector can be written as [7]
1. Nuclear Magnetic Resonance Spectroscopy
26
Thus, the resulting magnetization depends on the number of accessible
frequencies and on the exchange rates (correlation times) through the
exchange matrix. To include the initial distribution of magnetization
isochromats, the NMR signal under the influence of the motion is calculated
as the powder averaging of equation (1.75), i.e. [7, 50],
^`
1exp Ȧ 0St i t
ªº
3
¬¼
m
G
G
.
(1.76)
Figure 1.6c shows a set of simulated CSA powder patterns as a function of
the correlation time, considering jumps between three molecular sites. For
motional rates, k = 1/3W
c
, of the order of the chemical shift anisotropy
parameter, G
CSA
, the powder spectrum changes considerably with W
c
, making it
possible to estimate this parameter from the line shapes. In the fast exchange
regime, W
c
<< 2S/G
CSA
, the spectrum also has a powder pattern, but with
distinct CSA parameters (
CSA CSA
Ș and į ). Nevertheless, there are some
intrinsic difficulties in obtaining such information from CSA line shapes: i) a
small deviation in the size and orientation of the CSA tensor can produce
significant changes in the average spectrum; ii) different types of motion can
produce identical line shapes; iii) for the case of the
13
C NMR, which is the
most common nucleus for studying organic systems, line shape analysis can
only be done for systems with one or few different chemical sites, or requires
13
C isotopic labeling at selected molecular sites. This can actually be
overcome by special 2D NMR techniques that produce 2D spectra containing
high resolution MAS spectra in the first dimension and the corresponding
powder line shapes in the second dimension [52, 53]; and iv) the use of high
power broadband
1
H decoupling may also be problematic because the
molecular motion can also interfere with the proton decoupling, inducing
spectral line broadening and, sometimes, even precluding the acquisition of
CSA powder line shapes in the intermediate motional regime.
Other NMR interactions, such as quadrupolar or dipolar, can also be used
for probing molecular motions. In fact, in the case of the dipolar interaction,
more traditional NMR methods, e.g., second moment calculations [6, 49] and
studies of the line width as a function of temperature, allow one to obtain
information about the rates and activation energies of molecular motions.
However, site-specific information such as the reorientation angles and
geometry of the motion cannot be obtained by these methods.
This can be done using deuterium (
2
H) NMR.
2
H has nuclear spin I = 1, a
gyromagnetic ratio. Because
of
these reasons, line shape and relaxation
properties of
2
H resonances are
determined almost exclusively by the first
order quadrupolar interaction.
Besides, X-
2
H bonds can be arranged to replace
X-
1
H bonds (X is a low J
nucleus), and
2
H is a good spin label because its molecular properties are
similar
to
1
H. For
2
H in a X-
2
H bond the electric field
gradient tensor
is
almost axially symmetric, K
Q
= 0, and the z-axis of its PAS
roximately along the direction of the X-
2
H bond. Thus, the spectral
large quadrupolar moment, and a relatively small
points app-
shape

1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
27
produced by single transition between two adjacent
2
H energy levels is
the same as for the case of spin ½ nuclei with axially symmetric chemical
shift tensor. As a result, the full quadrupolar powder pattern can be calculated
by superimposing two mirror-symmetric powder patterns (one for each
2
H
transition) calculated in the same way as for the chemical shift interaction.
However, the effects of the NMR interaction during the rf pulses, which may
be significant for
2
H NMR [50], have been disregarded in equation (1.76), and
some care must be taken with finite pulse effects when using this
approximation. Figure 1.6d shows a set of simulated powder patterns as a
function of the motional rates for two-site jump motions. In the fast exchange
limit, W
c
<< 2S/G
Q
, an averaged powder pattern characterized by
QQ
Ș and į is
observed. Note that the axial symmetry of the
2
H quadrupolar interaction was
broken and a motionally averaged spectrum characteristic of a non-axial
interaction was observed.
An experimental problem in the acquisition of
2
H line shapes is that the
lines are very broad and, consequently, the decay time of the NMR signal is
very short (~ Ps). This makes it very difficult to acquire the first points of the
FID, because it is necessary to wait a few Ps to start the acquisition after the
application of an intense rf pulse (dead-time problem). Thus, to acquire the
2
H
signal, it is necessary to use a special pulse sequence, known as solid- or
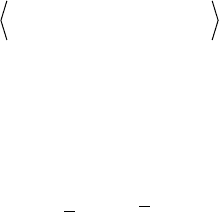
quadrupolar-echo sequence [50, 54]. The solid-echo pulse sequence is shown
in Figure 1.7a. It produces an echo with maximum intensity at 2W, making it
possible to displace the acquisition to the echo maximum, and thus avoiding
the dead time problem. Typical W values are of the order of 10s of Ps, and
molecular motion occurring with correlation times within this time scale
interferes with the refocusing of the echo, producing a decrease in the signal
intensity. Hence, motions in the intermediate exchange regime can be studied
by accompanying the W dependence of the echo intensity. This is shown in
Figure 1.7b, where the echo intensity, normalized by its rigid limit value
(reduction factor), is plotted as a function of the jump frequency (1/
C
) for
two-site jump motions occurring with several jump angles. Besides the
reduction in the signal intensity, molecular motions in the intermediate
exchange regime also affect the observed line shapes, and it is crucial to take
these effects into account when performing line shape analysis. This can be
done by adding two extra evolution periods W in equation (1.76):
ȦIJ ȦIJ Ȧ
solid-echo
10
iiit
St e e e
3r 3 3
½
®¾
¯¿
m
B
G
G
.
(1.77)
Figure 1.7c presents a set of experimental
2
H spectra for methyl deuterated
poly(methyl acrylate) (PMA) acquired with the solid-echo pulse sequence
using distinct W values. The effect of the molecular motion in the line shape is
clearly observed.
W
1. Nuclear Magnetic Resonance Spectroscopy
28
Figure 1.7 a) Molecular structure of PMA and solid-echo pulse sequence. b) Theoretical
reduction factor of the solid-echo amplitude for a two-site jump between equally populated
sites as a function of the jump frequency and for different jump angles. c) Effect of the solid-
echo delay W in the spectrum of deutered PMA. (Adapted with permission from references [55]
and [56] . Copyright 2005 American Chemical Society)
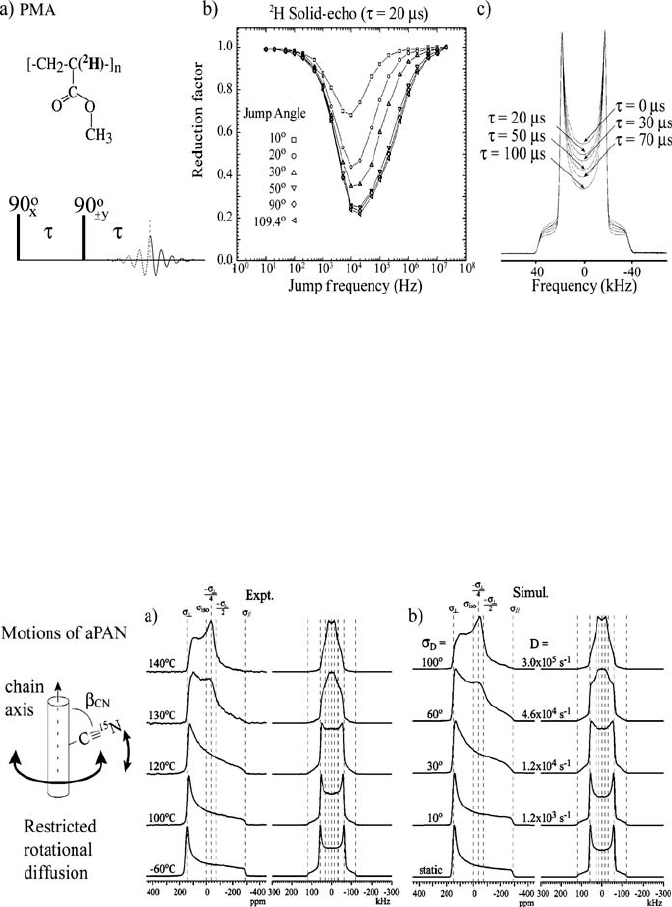
Figure 1.8 shows an experimental example of the use of
2
H and CSA line
shapes for obtaining information on the molecular motions in solids.
2
H and
15
N static spectra were acquired as a function of temperature to elucidate the
molecular mechanism of the relaxation processes observed by dielectric
relaxation in atactic poly(acrylonitrile) ([C
3
H
2
N]
n
-aPAN) [57].
Figure 1.8 a) Experimental 1D
15
N CSA (left) and
2
H (right) spectra of aPAN as a function of
temperature. b) Best-fit simulations of the experimental spectra based on the model of uniaxial
rotational diffusion with distributed restricted amplitudes (see left). The width V
D
of the
amplitude distribution and the diffusion coefficients D are shown for each case. (Adapted with
permission from reference [57]. Copyright 2005 American Chemical Society)
The experimental spectra are well reproduced by simulations considering
D
C, the standard deviation V of the
110°
uniaxial rotation around the main chain with a restricted distribution
of amplitudes. Below
Gaussian
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
29
distribution of motional amplitudes increases with temperature up to
~30 °C. The small-angle fluctuations rapidly change to larger-amplitude
motions above this temperature; V
D
increases from 30 to 100° in the
temperature range of 120–140 °C, which correlates with the intensity increase
observed in dielectric measurements.
Experiments involving line shape analysis under magic-angle spinning
have also been performed for
2
H. These experiments explore the dependence
of the spinning sidebands on the molecular motions. In these cases, the effects
of both molecular and mechanical rotation must be put together in the
simulation of the spectra, which can be done by using appropriated theories
[58]. Similar approaches have also been used for spin ½ systems under the
effect of the chemical shift interaction [32]. However, molecular motions can
also be studied in sideband-free MAS NMR spectra, which is particularly
useful for
13
C NMR, where multiline spectra are generally observed. This is
so because molecular rotations also interfere with the MAS averaging,
introducing characteristic line broadenings in the MAS line shapes [59, 60].
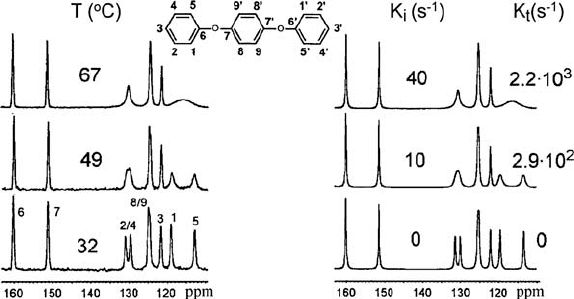
Figure 1.9 shows high resolution
13
C NMR spectra of 1,4-diphenoxy
benzene (DPB) obtained at different temperatures. A clear broadening of the
2,4,1,5 lines (see assignments in the inset) as a function of temperature is
observed.
Figure 1.9 Experimental (left) and simulated (right)
13
C MAS spectra of DPB (see molecular
structure in the inset) as a function of temperature at Q
R
= 5.2 kHz. The simulated spectra were
calculated considering S ring flips with the indicated rate. (Adapted from reference [60].
Copyright 2005 with permission from Elsevier)
Since carbons 2,4,1,5 belong to the terminal rings of DPB, this broadening
can be associated to 180q rotations S-flips around the 1,4 axis of the rings. A
slight broadening is also observed for the line attributed to carbons 8 and 9,
showing that a fraction of the inner rings also executes
S
-flips. The
temperature dependence of the experimental MAS NMR spectra is nicely
reproduced considering S-flips occurring with appropriate motional rates
1. Nuclear Magnetic Resonance Spectroscopy
30
k = 1/W
c
, k
i
for inner rings and k
t
for terminal rings. From these data the
temperature dependence of the motional rates was obtained and the S-flips
activation energies of the inner and terminal rings of DPB were estimated to
be 90 and 73 kJ/mol, respectively.
The use of
1
H line shapes is also one of the most traditional methods for
studying molecular dynamics. The
1
H NMR spectrum of rigid materials is
characterized by broad lines, usually with Gaussian or Lorentzian shapes due
to the strong coupling between the
1
H nuclei. As in the case of any other
anisotropic interaction, molecular motion can induce the averaging of the
1
H-
1
H dipolar interaction, reducing the line widths. Thus, monitoring the line
width as a function of temperature and using appropriate models, information
about correlation time and activation energy of molecular motions can be
obtained [61, 62]. These studies, which can also be performed for other
nuclei, are useful for the characterization of materials like solid polymer
electrolytes, where the line widths and T
1
relaxation of the charge carriers
(usually Li, Be, Ag, or Cs), provide information about their mobilities [37,
38]. However, due to the lack of spectral resolution in the static or regular
MAS
1
H spectra, these studies have been limited to cases where no local
NMR methods that are generally called separated-local-field (SFL)
experiments overcomes this limitation.
The simplest version of SLF experiments, the so-called wideline
separation (WISE) [63], is derived from the conventional CP-MAS method
by adding an extra time period t
1
in the CP pulse sequence, as shown in
Figure 1.10a. After the
1
H S/2 pulse, the
1
H magnetization evolves under the
action of the chemical shift and homonuclear dipolar interactions and then is
transferred to a low natural abundance X nucleus (usually a spin 1/2 nucleus
such as
13
C,
29
Si, or
15
N). Then, after the CP transfer, the magnetization of the
X nucleus depends upon the evolution of the
1
H magnetization during t
1
.
Incrementing t
1
according to the usual rules of signal sampling, a 2D time
matrix, S(t
1
,t
2
), can be obtained and its 2D Fourier transform provides a 2D
spectrum, S(Z
1
,Z
2
), that contains the high resolution MAS spectra of the X
nuclei along Z
2
, and the corresponding
1
H wideline spectra along Z
1
. This
experiment is very convenient for distinguishing regions of the material with
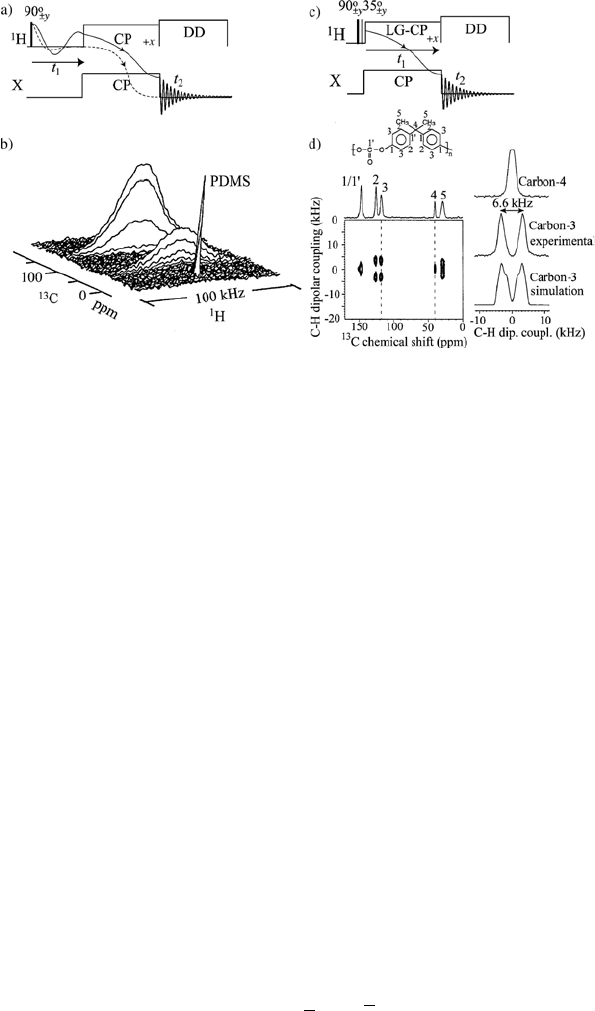
different mobilities. Figure 1.10b shows an example where the WISE
experiment was applied to study the phase separation in the block copolymer
polystyrene (PS)-b-poly(methyl siloxane) (PDMS). It is possible to observe
that while the PS phase of the material is rigid (broad lines) the PDMS phase
is mobile (narrow line), despite the high chemical connectivity and spatial
proximity (~20 nm) between the phases. The WISE experiment was also
modified to obtain information about the domain sizes of each phase [63].
information about molecular motions were necessary. A class of two-dimensional
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
31
Figure 1.10 a) Pulse sequence for 2D WISE experiments. b) Application of the WISE method
for identifying rigid and mobile phases in the block copolymer PS-b-PDMS. c) Pulse sequence
for 2D LG-CP experiments. The extra 35q pulse is applied because the precession plane of the
1
H magnetization under LG irradiation is 35q tilted relative to the transverse plane. (Adapted
with permission from references [43] and [63]. Copyright 2005 American Chemical Society)
The main problem of using WISE experiments for probing localized
motions is related to the so-called spin-diffusion phenomenom [6, 49]. Strong
homonuclear dipolar couplings induce transfer of z magnetization between the
coupled spins (spin-diffusion or spin-exchange) with or without rf irradiation
[6, 7, 49]. Hence, due to the high natural abundance and the strong
1
H-
1
H
dipolar coupling, the
1
H magnetization diffusion (
1
H spin-diffusion) during
the cross-polarization period is very effective [63, 64]. Some approaches were
used to avoid this problem for the WISE experiments [65]. However, a similar
experiment, but much less prone to spin-diffusion, was also proposed [43]. In
this experiment, the effect of spin-diffusion is minimized by replacing the CW
spin-lock pulse by the Lee-Goldburg homonuclear decoupling sequence. In
addition, instead of using an extra evolution period t
1
in the pulse sequence,
the CP period is incremented to produce the 2D signal (2D LG-CP
experiment); see Figure 1.10c. The result is a 2D spectrum that contains the
correlation between the X nucleus chemical shift, and the X-
1
H dipolar
interaction scaled by a factor of 0.577 due to the LG irradiation [66, 67].
Specifically for
13
C, the Z
2
spectral dimension contains the high resolution
13
C
NMR spectrum, and the corresponding scaled
13
C-
1
H dipolar powder
spectrum appears in the Z
1
dimension. As in the case of
2
H line shape, the
molecular motion breaks the axial symmetry of the dipolar interaction and in
the fast exchange limit the average
DD
Ș and į reflect the geometry of the
motion [43]. An example of local motion studied by 2D LG-CP is the S ring-
flip of polycarbonate. The two phenylene rings in each repeat unit undergo
S-flips superimposed with wobbling motions with a distribution of amplitudes
1. Nuclear Magnetic Resonance Spectroscopy
32
around the equilibrium positions. Figure 1.10d shows the 2D
13
C-
1
H LG-CP
spectrum of polycarbonate and several cross sections along the Z
1
axis. A
13
C
1D LG-CP MAS spectrum is shown at the top of the 2D spectrum, where the
five resolved
13
C signals are assigned. The C-H cross-section of the Carbon 4
signal, which results from the aliphatic quaternary carbon, does not exhibit
any resolved splitting, as expected for a carbon site that is not directly bonded
to any protons. In contrast, the aromatic Carbon-3 exhibits a splitting of 6.6
kHz and a smooth line shape devoid of sharp features. The spectral simulation
for this group is also shown [43]. The experimental spectrum is reproduced
using a model where both aromatic rings undergo S-ringflips and wobble
around the equilibrium ring positions with an average amplitude of ~ 20q.
Exchange NMR [7, 68] has become one of the premier tools for
characterizing segmental motions in organic solids. In these experiments
molecular reorientations on the milliseconds-to-seconds time scale (that do
not change the powder spectrum) are observed in terms of changes of
orientation-dependent NMR frequencies. They permit obtaining correlation
times and their distributions [69, 70], reorientation angle distributions [69,71],
orienta-tional memory and rate memory [72], verifying the existence of
dynamic heterogeneities [73], and determining their sizes [74]. Due to the
variety of exchange experiments that can be performed [75–82], some general
ideas and applications of standard 2D-exchange experiments will be discussed
first and then some extensions will be addressed together with the
corresponding experimental examples. For the sake of simplicity, the simpler
case of spin I = ½ will be discussed first and then extended for the case of
spin I = 1.
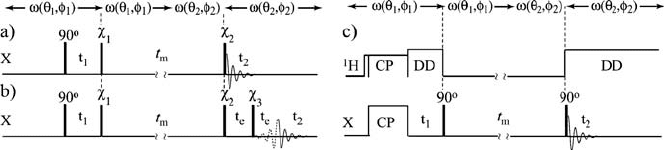
Figure 1.11a shows a general pulse sequence for the static 2D-exchange
experiment. The first S/2 pulse (or cross-polarization) excites the transverse
magnetization, which evolves under the chemical shift interaction during the
first period t
1
. After t
1
, a second S/2 pulse stores one component of the
magnetization along the z direction, where it remains during the long mixing
time (t
m
).
Figure 1.11 Pulse sequence used for 2D-exchange NMR experiment. a) Basic scheme using a
single 90q excitation pulse. b) With single 90q excitation pulse and echo acquisition. c) Using a
cross-polarization and
1
H decoupling.
1
Ȥ
and
2
Ȥ
represent rf pulses with general flip angles.
1.7.2 Two-Dimensional Exchange NMR Experiments

1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
33
The remaining transverse magnetization during t
m
is eliminated by cycling
the phases of the S/2 pulses. A second S/2 (read-out) pulse is applied after t
m
and then the magnetization evolves under the influence of the chemical shift
interaction during t
2
. Hence, the detected signal intensity S(t
1
,t
2
,t
m
) depends on
both evolution times, t
1
and t
2
, and on the mixing time t
m
. The 2D Fourier
transformation [28, 29] of the data produces a 2D spectrum that contains the
intensity distribution as a function of the NMR frequencies experienced by the
magnetization during t
1
(Z
1
) and t
2
(Z
2
), and also on the molecular motion that
occurred during t
m
, S(Z
1
,Z
2
,t
m
).
If no motions have occurred during t
m
, the orientation-dependent
frequencies are equal before and after the mixing time and the resulting 2D
spectrum is purely diagonal. In contrast, if segmental reorientation occurs
during t
m
, the orientation-dependent frequencies change and off-diagonal
intensities are observed. Moreover, the change in the NMR frequency for each
molecular site is dictated by the molecular motion occurring during t
m
and the
spectral intensity S(Z
1
,Z
2
,t
m
) depends on the time scale and geometry of the
molecular reorientations. While small-angle motions induce little
modifications in the NMR frequencies and only near diagonal intensity is
observed, high angle rotations produce large frequency changes and the
corresponding 2D spectrum contains intensities in a broad spectral range.
Actually, if the correlation time of the motion is much longer than the
evolution times t
1
and t
2
(slow motion regime), the magnetization that gives
rise to a certain intensity at a frequency point (Z
j
,Z
i
) depends on the fractional
population of spins that precess with Z
j
during t
1
, P
j
(0), and on the conditional
probability that it changes its orientation in such a way that it precesses with
Z
i
during t
2
, P
ij
(t
m
) [7, 69, 83]:
12
ȦȦ
12m m
,, 0
jj
it it
ij ij j
Mttt PtP e e .
(1.78)
Transverse relaxation during t
2
and longitudinal relaxation during t
1
were
disregarded. Because molecular motion can be generally considered a
stationary Markov process, P
i
(0) and P
ij
(t
m
) can be properly calculated from a
master equation [7, 69, 83], resulting in a 2D NMR signal given by
N
N
N
21
ȦȦ
12m
detect mix evolve
,, 1 01
m
t
it it
T
St t t e e e P
3
GG
G
.
(1.79)
The elements of
0P
G
represent the a priori probabilities P
j
of finding a
segment in a given orientation, i.e.,
0P
G
is another representation of the
initial magnetization vector
(0)m
G
, which accounts for the initial distribution of
magnetization isochromats. The exchange matrix
3
and the frequency matrix
Ȧ
have the same definitions as before.
The 2D spectrum can be interpreted as a map of reorientation probabilities,
i.e., it represents the probability density of finding a segment with frequency
Z
1
before t
m
and Z
2
after t
m
. Thus, because these probabilities are dictated by
the correlation time and the geometry of the motions, the 2D-exchange pattern
