Vij D.R. Handbook of Applied Solid State Spectroscopy
Подождите немного. Документ загружается.

1. Nuclear Magnetic Resonance Spectroscopy
44
An example of motion that produces changes in the isotropic chemical
shifts is the ring S-ringflips in poly(p-vinylene phenylene) (PPV). Figure
1.17b shows the 2D-MAS exchange of PPV acquired at 258 K with a
spinning frequency of 7.5 kHz. The presence of hetero-cross-peaks linking the
2,5 and the 3,6 lines is remarkable, indicating the occurrence of an exchange
process where the isotropic chemical shifts of the 2,5 and 3,6 carbons are
interchanged. Since the isotropic chemical shift of the 2,5 and 3,6 carbons
depends on the conformation of these segments relative to the vinylene
carbon, the interchange in the isotropic chemical shift can be attributed to a
180q rotation of the phenylene rings around the 1,4 axis. In the particular case
of PPV, slow conformational dynamics are directly associated with chain
disorder, which affects its photoluminescence behavior [109]. Some other
examples of applications of rotor synchronized 2D MAS exchange NMR are
the study of conformational dynamics in amorphous and semicrystalline
polymers, liquid crystals and the study of tautomerism, and molecular
dynamics in molecular crystals [110–112].
2D exchange NMR experiments have also been used for studying local
structure of amorphous solids. This is possible because for dipolar coupled
spins 2D exchange NMR is capable of probing the relative orientation of the
principal axis of the individual chemical shift tensors, which can be translated
in terms of molecular torsion angles. In these cases the exchange process is
not governed by molecular motions, but by local magnetization exchange,
known as spin-diffusion, between directly bonded nuclei [113]. In fact, in
studies of molecular reorientation, spin-diffusion is a limiting factor, because
its effects can be confused with the exchange due to molecular motion. For
this reason, low abundant and low J nuclei such as
13
C or
2
H are preferable for
probing molecular rotations, but the mixing times have to be kept short
(usually smaller than 1 s) to avoid spin-diffusion effects [114]. However, in
systems of highly abundant nuclei the dominant exchange process will be
spin-diffusion. Since the NMR frequencies are orientation-dependent, if a
nucleus in a given molecular segment exchanges its magnetization with
another nucleus in a neighbor site during t
m
, it will experience different
frequencies before and after t
m
. The shape of the resulting 2D pattern will
depend on the relative orientation of the two molecular segments. However, it
is very important to guarantee that spin-diffusion will be the only exchange
mechanism, i.e., that no slow motion takes place during t
m
. 2D exchange has
been used for determining relative orientations between different carbon sites
in many organic materials [115–118]. For that, the use of a
13
C-
13
C or
15
N-
15
N
pair is necessary, which has been provided by specific
13
C or
15
N isotopic
labeling at the sites of interest. Other applications of the method not requiring
isotopic labeling are those where
31
P nuclei are used to probe relative
molecular orientations.
31
P is a spin 1/2, 100% abundant nucleus, found in
many organic and inorganic systems. An example of these studies is the
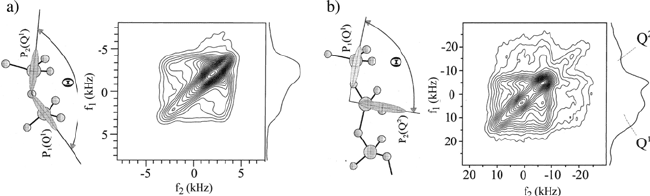
measurement of relative tensor orientations of different phosphate units (Q
1
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
45
and Q
2
units in NMR terminology [9]); see molecular structure in Figure 1.18
in crystalline diphosphate Ca
2
P
2
O
7
and in binary sodium phosphate glasses
[119].
Figure 1.18 a) 2D exchange spectra using slow MAS for measuring the relative tensor
orientations for a diphosphate Ca
2
P
2
O
7
. b) Same as a) for a binary sodium phosphate glass with
sodium to phosphorous ratio of 1:4. Molecular representation of the relative orientations of Q
1
-
Q
1
and Q
1
-Q
2
connectivities and their anisotropic powder patterns are also shown. (Adapted
from reference [119], Copyright 2005 with permission from Elsevier)
In this case it is advantageous to use a slightly different version of the 2D
exchange NMR, where slow MAS is applied together with radio frequency
pulses in such a way that the local transfer of magnetization is enhanced
during the mixing time (radio frequency–driven spin-diffusion — RFDR)
[113]. For crystalline diphosphate Ca
2
P
2
O
7
, which is composed of only Q
1
units, a single exchange pattern is obtained, Figure 1.18a. From this spectrum
the relative orientation of two neighbor phosphate units can be estimated. In
contrast, for the binary phosphate glass, where Q
1
and Q
2
units are present, the
2D exchange spectrum is a superposition of the individual spectra for Q
1
- Q
1
,
Q
1
- Q
2
, Q
2
- Q
2
, according to their statistical weights; see Figure 1.18b. In this
case, because of the spectral superposition, it is difficult to determine
unambiguously the relative tensor orientation for each connectivity, but the
spectrum clearly reveals the predominance of Q
1
-Q
1
connectivities in the
glass.
1.7.3 One-Dimensional Exchange NMR Experiments
Beyond the 2D exchange NMR methods, there is a class of one-dimensional
(1D) techniques that have been used for extracting two- and multiple-time
correlation functions of rotational molecular motions occurring on the
millisecond-to-second time-scale [39, 75, 77, 80, 81, 102, 120, 121]. In
particular, the stimulated-echo experiment (STE) [35, 39, 77] has been widely
employed for studying time scale, amplitude, and heterogeneity of slow
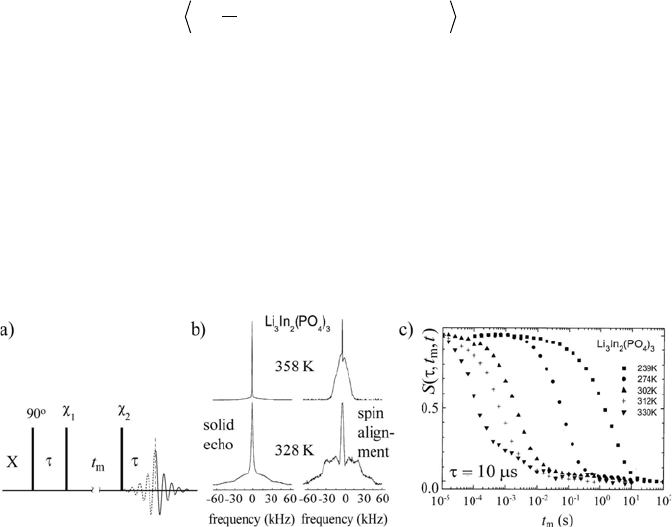
motions in organic and inorganic solids. The pulse sequence for the basic
stimulated-echo experiment is shown in Figure 1.19a. After the excitation
pulse, the quantum coherences (or magnetization components) evolve during
the evolution period W under the action of an anisotropic NMR interaction
(usually chemical shift or quadrupolar). A store pulse is then applied with

1. Nuclear Magnetic Resonance Spectroscopy
46
adequate phase and amplitude in order to convert a selected single-quantum
coherence that evolved during W into a term that does not evolve during the
long mixing time, because it commutes with the spin Hamiltonian (usually it
is represented by T
20
— spin-alignment , or I
z
— Zeeman order). Finally, a read-
out pulse is applied to the system to reconvert the stored coherence into an
observable magnetization. Using the pulse sequence shown in Figure 1.19a
and disregarding relaxation effects, the NMR signal can be written as
mm
IJ,, sinȦ 0 IJ sin ȦStt A tt ,
(1.87)
where
Ȧ 0 and
m
Ȧ t are the NMR frequencies before and after t
m
. The
amplitude A depends on the flip angles and phases of the store and read-out
pulses, which are chosen according to the nuclear spin interaction used for
probing the molecular motion [77]. The maximum value of
m
IJ,,Stt occurs
at t = W (echo maximum) and depends on the relation between
Ȧ 0 and
m
Ȧ t . The decrease of the echo amplitude as a function of t
m
provides the
two-time correlation function of the exchange process. The number of sites
involved in the exchange process also affects the stimulated-echo, in
particular its intensity at long mixing times. The longitudinal relaxation, T
1
,
also produces echo decay as a function of the mixing time, which may happen
in the same time scale of the decay due to molecular motion. Thus, to
guarantee that only motion effects are encoded in the stimulated-echoes, the
data must be normalized by the T
1
decay, which can be obtained by setting the
evolution time W = 0 and acquiring the echo intensities as a function of t
m
.
Corrections for T
2
relaxation during the W period are performed by
normalizing the echo intensity by its value at short mixing times. STE
experiments were extensively used to study molecular dynamics in organic
systems. Most of these applications used
2
H STE, where the anisotropic
2
H
quadrupolar interaction is employed to probe the molecular motion. In this
case spin-alignment echoes (the T
20
term is stored during t
m
) are produced and
the expression for the STE signal is
3
em 0 1 2 Q e Q m
4
,sin2Ȥ sin 2Ȥ sin Ȧ 0sinȦStt t M t t t ,
(1.88)
where it becomes clear that the pulse flip angles
1
Ȥ and
2
Ȥ that maximize the
signal are each equal to 55q.
2
H spin-alignment echoes have been used for many years in the study of D-
and E-relaxation in supercooled liquids [35, 97, 122, 123]. Important informa-
tion about the motional rates, motional geometry, and distribution of
correlation times have been obtained using this technique. New experiments
obtained by adding extra mixing times and evolution periods in the
stimulated-echo pulse sequence have also made it possible to obtain multiple
time correlation functions. These techniques were used to elucidate the origin
of the non-exponential behavior of the correlation functions that characterize
the molecular motions responsible for the glass transition of supercooled
systems [73, 74, 78, 95, 123, 124]. The results revealed that non-exponen-
,
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
47
tiality in the correlation functions are associated with dynamic hetero-
geneities with lifetimes comparable to that of the D-relaxation, and their sizes
were estimated [74, 124]. More recently, STE experiments were also applied
to study the slow exchange of the
7
Li and
29
Be cations in inorganic solid
electrolytes [39, 78, 125]. In these cases the experiment was adapted to spin
3/2 systems. With the appropriate phase cycling [125], the echo intensity can
be written as
9
m0Q Qm
20
IJ,, sinȦ 0 IJ sin ȦStt M tt
(1.89)
for best pulse flip angles F
1
and F
2
equal to 45q. The phase cycling employed
in reference [78] was used to provide pure quadrupolar order, stimulated-
echoes (spin-alignment echoes) as in the case of
2
H. However, homonuclear
dipolar coupling between the cations also contributes to the echo intensity.
This is shown in Figure 1.19b, where the regular solid-echo spectra of the
ionic conductor Li
3
In
2
(PO
4
)
3
are compared with the spin-alignment spectra
(Fourier transform of the STE signal) recorded with t
m
= 50 ms. It is evident
that the satellite contributions in the spin-alignment spectra are much more
prominent than in the solid-echo spectra. However, the presence of significant
central lines indicates that dipolar order is also produced.
Figure 1.19 a) Pulse sequence for stimulated-echo experiments. b) Solid-echo spectra of
Li
3
In
2
(PO
4
)
3
(left) as compared with
7
Li spin-alignment echo spectra (right) at two
temperatures. c) t
m
dependence of the
7
Li spin-alignment echoes amplitudes in the Li
3
In
2
(PO
4
)
3
ionic conductor. The activation energy barrier for the Li hopping was estimated as 11,300 K/k
B
.
(Adapted from reference [78], Copyright 2005 with permission from Elsevier)
Fortunately, at short evolution times W the echo intensity is dominated by
quadrupolar order, making it possible to select pure (or mostly pure)
quadrupolar order echoes [78]. Because the quadrupolar frequency depends
on the strength and orientation of the electric field gradient at the cation site,
any modification in these parameters’ changes Z
Q
. As a consequence, jumps
of the ions between electrically non-equivalent sites change the quadrupolar
frequency, making it possible to detect the cation motion in STE experiments.
Again, the measurement of the stimulated-echo amplitude as a function of the
mixing time provides the two-time correlation function of the ionic motions,
whose decay time is the average ion-hopping time. Figure 1.19c shows the
mixing time dependence of the spin-alignment echo amplitudes for different
1. Nuclear Magnetic Resonance Spectroscopy
48
temperatures in the Li
3
In
2
(PO
4
)
3
ionic conductor. From these measurements
the mean correlation times (decay times) as a function of the temperature and
the activation energy of the ionic motion were obtained [78]. Other STE
studies on ionic conductors have been carried out using
109
Ag (spin ½
nucleus) NMR. In this case two-time and three-time correlation functions
were obtained, which allowed one to conclude that the non-exponentiality
observed in the correlation function is due to a correlation time distribution,
i.e., dynamic heterogeneities, rather than to an intrinsic non-exponentiality
[126].
Concerning
13
C stimulated-echo experiments, these have been widely
employed for studying not only the time scale, but also the amplitude of slow
motions in organic solids. However, the use of this technique for studying
complex motions suffers from the same problem as the regular 2D exchange
experiment, i.e., there is no specific site resolution, and it is very difficult to
characterize the exchange intensity if only a small fraction of the molecular
segments takes part in the molecular motion. To overcome one of these
problems a 1D pure exchange version of the stimulated-echo technique has
also been developed. This technique, named 1D pure exchange NMR (1D-
PUREX), suppresses the signals of immobile molecules and thus provides
spectra selectively from segments that reorient during the mixing time. Thus,
the amplitude and time scale of slow motions can be determined even for a
small fraction of mobile segments [101, 102]. The pulse sequence for the 1D-
PUREX NMR experiment is obtained from the 2D-PUREX pulse sequence,
Figure 1.16, simply by removing the t
1
evolution and the first z-period (the
two 90q pulses flanking the t
z
period). Hence, after the excitation the spins
immediately start their evolution under the chemical shift anisotropy (CSA)
during the period W. Then, the magnetization is stored along the z-direction, so
that it does not precess or dephase during a long mixing time, t
m
. In the
absence of slow motions during t
m
, the CSA is refocused after a read-out
pulse and another evolution period W (total evolution time of W
CSA
= 2W). At
this point, the magnetization is stored again along the z direction during a
short period t
z
(<< t
m
) and then flipped back to the transverse plane for
detection. If segmental reorientations occur during t
m
, the orientation-
dependent frequency changes, and the CSA is not completely refocused. The
resulting dephasing of the stimulated-echo is observed as a decrease in the
detected spectral intensity. To remove effects of T
1
and T
2
relaxations during
t
m
and W
CSA
, respectively, a reference spectrum S
0
= S(t
z
, GW
CSA
)—which has
the same relaxation factors, but no motion effects during t
m
—is measured. S
0
is obtained by simply swapping t
m
and t
z
. To remove the contribution of
segments that do not move on the milliseconds time scale, the 1D-PUREX
intensity S = S(t
m
, GW
CSA
) is subtracted from the reference spectrum S
0
.
Dividing this difference by S
0
, one can obtain a normalized pure-exchange
intensity E(t
m
, GW
CSA
) = 'S/S
0
, which does not contain relaxation effects and
just accounts for the signals from the reorienting segments. For discrete jumps

1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
49
with correlation times W
C
in the range of milliseconds to seconds (slow-motion
regime), the normalized pure-exchange intensity E(t
m
, GW
CSA
) obtained by
integrating the spectra yields the same information as the standard stimulated-
echo experiment. In this motional regime, E(t
m
, GW
CSA
) can be approximated
by the following expression:
>@
>@
m
2
CSA
mCSA m 1 2
2
CSA
m12
1
IJ
,įIJ 12sin
4
IJ
12sin ,
4
fM
Et t
M
Et
f
§·
ªº
I ::
¨¸
¬¼
©¹
ªº
§·
ªº
I ::
«»
¨¸
¬¼
©¹
¬¼
(1.90)
where f
m
is the fraction of slow mobile segments (mobile fraction), M is the
number of magnetically non-equivalent sites accessible to the moving
segments, I(t
m
) is the correlation function of the molecular motion, and :
1
–
:
2
is the difference between the NMR frequencies before and after t
m
.
Therefore, the t
m
-dependence of E( t
m,
GW
CSA
) yields the time scale of the
motion, while its W
CSA
-dependence provides information on the amplitude of
the slow molecular reorientations that occurred during t
m
.
Figures 1.20a–b show, respectively, the t
m
- and the W
CSA
-dependence of
E(t
m
, GW
CSA
) curves for DMS at 293 K. A correlation time of 15 ms was
obtained from the t
m
-dependence of the E(t
m
, GW
CSA
) curves. Note that the final
intensity in both curves is 0.5, which is exactly what is expected for the DMS
two-site jump motion. Besides, as shown in the simulation for motions with
distinct reorientation angles, the 1D-PUREX experiment is very sensitive to
small-angle rotations. The best fit to the DMS experimental data occurs for a
reorientation angle of 70q. This is equivalent to 110q due to the symmetry
property of the axially symmetric CSA tensor of DMS that makes
reorientation by E
R
and 180q–E
R
equivalent. Figure 1.20c shows the reference
S
0
, the 1D-PUREX S, and the difference S
0
– S spectra obtained for non-
labeled PMMA at T = 295 K. The S
0
– S spectrum shows significant intensity
for all carbons confirming that the E-relaxation of PMMA involves both the
side group and main chain motions. The W
CSA
-dependence of E(t
m
, GW
CSA
) for
the carbonyl group in PMMA is shown in Figure 1.20d. The bimodal
character of the curve is promptly recognized, showing the presence of both
small (slow build-up) and high (fast build-up) angle motions. The best-fit
simulation of the E(t
m
, GW
CSA
) 1D-PUREX intensities as a function of W
CSA
is
also shown. In the simulations, the three-component model discussed above in
the context of Figure 1.13 was used. All small-angle rotations were simulated
using Gaussian distributions of reorientation angles centered at 0q with root
mean square widths V. The E(t
m
, GW
CSA
) vs. W
CSA
curves for each component
and the corresponding distribution of rotation angles for the small-angle
motion are shown in Figure 1.20d.
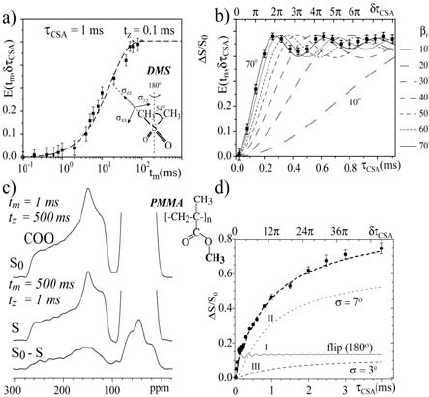
1. Nuclear Magnetic Resonance Spectroscopy
50
Figure 1.20 a) E(t
m
, GW
CSA
) vs. t
m
curve of DMS at 293 K. b) E(t
m
, GW
CSA
) vs. W
CSA
curve of
DMS at 293 K. c) Reference S
0
, exchange S, and difference S
0
– S 1D-PUREX spectra of
PMMA. d) E(t
m
, GW
CSA
) vs. W
CSA
build-up curve for the carbonyl groups of PMMA together
with simulated curves based on the model discussed in the text (components I, II, III). (Adapted
with permission from reference [89], Copyright 2005 Springer, and from reference [101],
Copyright 2005 American Institute of Physics)
Following the idea of the rotor synchronized 2D MAS exchange
experiments, there is also a series of 1D MAS experiments especially
designed for studying slow molecular reorientations using spinning sidebands
[80, 81]. Named one-dimensional exchange spectroscopy by sideband
alternation (ODESSA), this is an alternative version of the 2D MAS
exchange. In this method, the first evolution period in Figure 1.11c is set to
half of the rotor period, t
1
= t
r
/2. Then, after the rotor synchronized mixing
period, the signal is detected during the period t = t
2
. The magnetization stored
along the z direction during the mixing periods corresponds to distinct
polarizations of the spinning sidebands. During the mixing period, the
molecular reorientations redistribute the polarization among the various
spinning sidebands, which makes their intensities dependent on the molecular
motion that occurred during t
m
. From a series of spectra acquired with
different mixing periods, dynamic parameters such as the correlation time
and, in favorable cases, the amplitude and number of accessible sites for the
motion can be obtained. Other variants of these techniques were proposed in
order to obtain pure absorptive MAS spectra for systems with many non-
equivalent sites (time-reverse ODESSA) and to separate the sideband
intensities of different chemical groups (PATROS) [80, 81, 83]. In the
particular case of tr-ODESSA the correlation time of the motion can be
obtained either by the increase of the spinning sidebands intensities or the
decrease of the centerband intensity as a function of the mixing time.
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
51
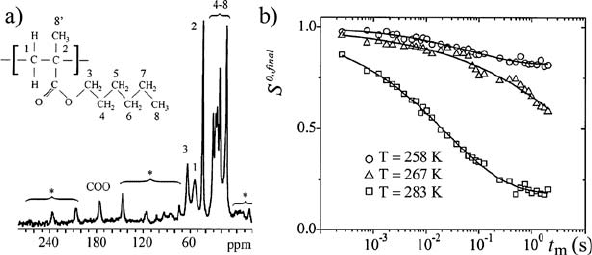
Figure 1.21 shows an example of the use of the tr-ODESSA pulse
sequence to study molecular motions responsible for the D- and E-relaxation
of the glassy polymer poly(n-hexyl methacrylate) (PHMA). Using tr-
ODESSA, the correlation times and their distribution were obtained as a
function of temperature for the side group and main chain motion [92]. This
made it possible to conclude that the alkyl side group contributes in the
dynamic window of the NMR exchange experiments mainly to the E-
relaxation, while the main chain and carboxyl groups contribute to both D-
and E-relaxations.
Figure 1.21 a)
13
C CP-MAS spectrum of poly(n-hexyl methacrylate) (PHMA) at Q
R
= 3 kHz.
COO spinning sidebands are indicated with asterisks. b) t
m
-dependence of tr-ODESSA
experiments for PHMA. (Adapted with permission from reference [92], Copyright 2005
American Chemical Society)
The main disadvantages of the above discussed MAS exchange methods is
the necessity of observing sidebands in the spectrum. This is particularly
problematic for
13
C studies in chemically complex systems where the overlap
between the sidebands of different chemical groups may preclude the accurate
intensity measurement. This was overcome by a class of methods called
centerband-only detection of exchange (CODEX), where slow molecular
motions are quantified on the basis of the line intensities of a high resolution
MAS spectrum, without the necessity of observing MAS sidebands. The
CODEX experiment is essentially the MAS version of 1D-PUREX NMR [75,
121]. The averaging of the chemical shift anisotropy under MAS provides
higher site resolution and sensitivity. However, MAS would make the pulse
sequence insensitive to segmental orientations and reorientations. Then, it is
necessary to reintroduce (recouple) the chemical shift anisotropy during the
periods used to codify the exchange process, i.e., the evolution periods, Nt
r
.
This is performed by using a train of rotor synchronized 180q (S) pulses
capable of reintroducing the chemical shift anisotropy even under high MAS
spinning frequencies. This scheme, known as recoupling of the CSA, was
originally developed for using in the so-called rotational echo double
resonance (REDOR) experiments, which are a very useful class of NMR
methods for measuring internuclear distances [47]. CSA recoupling during the
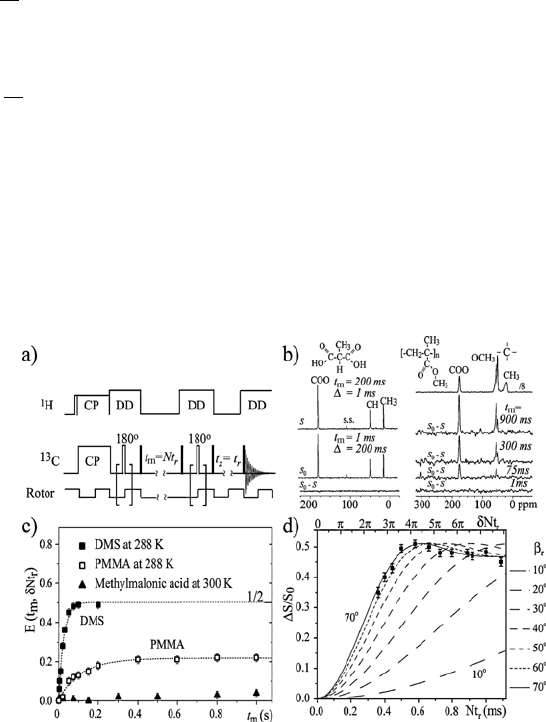
1. Nuclear Magnetic Resonance Spectroscopy
52
evolution periods is achieved by replacing each evolution period W in the 1D-
PUREX pulse sequence by a train of N/2 180q pulses spaced by t
r
/2, where t
r
is the sample rotation period; see Figure 1.22a. This leads to a total evolution-
and-refocusing period of Nt
r
for CODEX. With the CSA recoupling, the phase
accumulated during the first and second evolution periods before ()
1
) and
after ()
2
) the mixing time are given by
rr r
r
/2 /2
1 iso aniso,1 iso aniso,1 aniso,1
0/2 0
ȦȦ() ȦȦ() Ȧ ()
2
tt t
t
N
tdt tdt N tdt
§·
ªºªº
)
¨¸
¬¼¬¼
©¹
³³ ³
rr r
r
/2 /2
2 iso aniso,2 iso aniso,2 aniso,2
0/2 0
ȦȦ () ȦȦ () Ȧ ()
2
tt t
t
N
tdt tdt N tdt
§·
ªºªº
)
¨¸
¬¼¬¼
©¹
³³ ³
(1.91)
where
aniso,1
()tZ and
aniso,2
()tZ are the NMR frequencies due to the anisotropic
part of the chemical shift under MAS, equation (1.71), before and after t
m
,
respectively. Note that equation (1.73) was used to derive the expressions for
the phases. If Z
1
(t) = Z
2
(t), i.e., if no exchange occurs, equation (1.91) leads to
a total phase accumulation of )
1
+ )
2
= |)
2
| – |)
1
| = 0; in other words, the
magnetization is refocused in a stimulated-echo along its original direction.
Figure 1.22 a) Pulse sequence for CODEX experiments. b) CODEX S, reference S
0
, and the
difference S
0
– S, spectra obtained for the molecular crystal methylmalonic acid (left) and
difference S
0
– S, spectrum as a function of t
m
for PMMA (right) (see chemical structures in the
insets). c) t
m
-dependence of the pure-exchange CODEX intensity for PMMA, methylmalonic
acid, and DMS. d) Nt
r
-dependence of the E(t
m
, GNt
r
) intensity obtained for DMS using
t
m
= 50 ms. (Adapted with permission from reference [75], Copyright 2005 American Chemical
Society, and from reference [89], Copyright 2005 Springer)
1.7 Molecular Dynamics and Local Molecular Conformation in Solid Materials
53
Molecular motions during t
m
reduce the stimulated-echo and, as a result,
the observed line intensities. Therefore, the analysis of the motional amplitude
and time scale can be performed in the same way as for the 1D-PUREX
measurements. The CODEX intensity S = S(t
m
, GNt
r
) and the reference
intensity with no motion effects, S
0
= S(t
z
, GNt
r
), also obtained after swapping
t
m
and t
z
, are combined to produce the normalized pure exchange intensity,
E(t
m
, GNt
r
). E(t
m
, GNt
r
) plotted as a function of t
m
or Nt
r
provides information
on the motional rate and amplitude, respectively. The main advantage of
CODEX is the possibility of studying the molecular motion of each chemical
site independently, since a high resolution MAS spectrum is acquired. This
makes possible the probing of local motions at each individual site of
chemically complex systems. Figure 1.22b shows the CODEX S, the reference
S
0
, and the difference S
0
– S spectra obtained for the molecular crystal
methylmalonic acid and for the glassy polymer PMMA (see molecular
structures in the insets).
The absence of lines in the difference spectrum S
0
– S, of methylmalonic
acid indicates that the molecular segments of methylmalonic acid do not
undergo slow rotations. In contrast, molecular groups in both side groups and
main chain of PMMA execute molecular motions in the millisecond-to-second
time scale. Figure 1.22c shows the t
m
-dependence of the pure-exchange
CODEX intensity for PMMA, methylmalonic acid, and DMS. The resulting
curves are the correlation functions of these dynamics on the millisecond time
scale. For DMS, a single exponential curve is obtained, while PMMA shows
some non-exponentiality. Methylmalonic acid shows no exchange except for
13
C spin-diffusion (~5%). E(t
m
, GNt
r
) plotted as a function of Nt
r
for DMS is
shown in Figure 1.22d. Simulations with various reorientation angles are also
shown. As in the case of 1D-PUREX experiments, high sensitivity to small-
angle reorientations can be observed in these curves. As expected, the best fit
to the DMS experimental data is shown for a reorientation of 70q, i.e., 110q, as
explained before.
Many extensions of the CODEX technique have appeared, including an
extension to a four-time pulse sequence used for studying motional memory
[75, 127]; a triple-resonance CODEX applied to study protein dynamics
[128]; scaled-CODEX, used for measurements at low spinning rates [120];
multiple-quantum CODEX used for torsion angle determination in solids
[129]; and
19
F CODEX, was used to study the oligomerization in lipid bilayer
proteins [130]; and a CODEX variant to measure internuclear distances
between a spin ½ and a quadrupolar nucleus [131].
1.7.4
Conformation-NMR
The experimental elucidation of molecular conformations in non-
oriented solids, such as amorphous polymers, unoriented polypeptides, or
polycrystalline proteins, is a great challenge. Solidstate NMR provides a set of
