Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


7.6 Molecular Chaperones: Biology's Effort to Overcome Improper Insolubility
305
and result in an unfolding. Within the cage sur-
rounded by polar species, the Tt-divide, or the
cusp of insolubility for the hydrophobic associ-
ations for correct protein folding, is substan-
tially above the operating temperature. Then
the cusp of insolubility must be lowered in a
carefully controlled manner to sort out the
correct hydrophobically folded structure. Thus,
by the consilient mechanism, ATP binding to
the chaperonin raises the cusp of insolubility for
protein folding above physiological temperature
to give solubility, and the subsequent stepwise
removal of Pi lowers the cusp of insolubility step
by step, allowing for a controlled refolding of
the spatially localized hydrophobic domains as
AGapy
the apolar-polar repulsive free energy of
hydration between wall and protein substrate,
abates.
7.6.1 Molecular Structure
of Chaperonins
7.6.1.1
5frwcmre o/Escherichia coli
GroEL'.A Homo-oligomer with
14 Subunits of 57,000 Da Each
The 14 subunits arrange as two heptameric
rings,
that is, with 7 units having a C7 symme-
try axis, related to the second 7 units by a
twofold dyad axis. This results in two water-
filled cavities of 85,000 A^ each.^^ Each of the 14
subunits consists of three domains—an equato-
rial domain, an intermediate domain, and an
apical domain. Seven ATP molecules bind to
the 7 equatorial domains at a position near the
intermediate domain and, in our view by means
of AGap, propagate dissociations of hydropho-
bic domains (including their intrinsic ion pairs)
and domain rotations in the intermediate and
apical domains due to apolar-polar repulsions.
The result is a water-filled cavity with its size
doubled to 175,000 Al'^
7.6.1.2
Structure ofE. coli GroES
Seven GroES, 10,000 Da protein molecules,
bind coincident with ATP binding at one end of
the D7 GroEL structure to cap the structure
and enclose a water-filled cavity of 175,000 A^
with the capacity to contain protein or peptide
substrate inside of a size up to 70kDa.
7.6.1.3
Crystal Structure of One State of
the GroEL/GroES Chaperonin
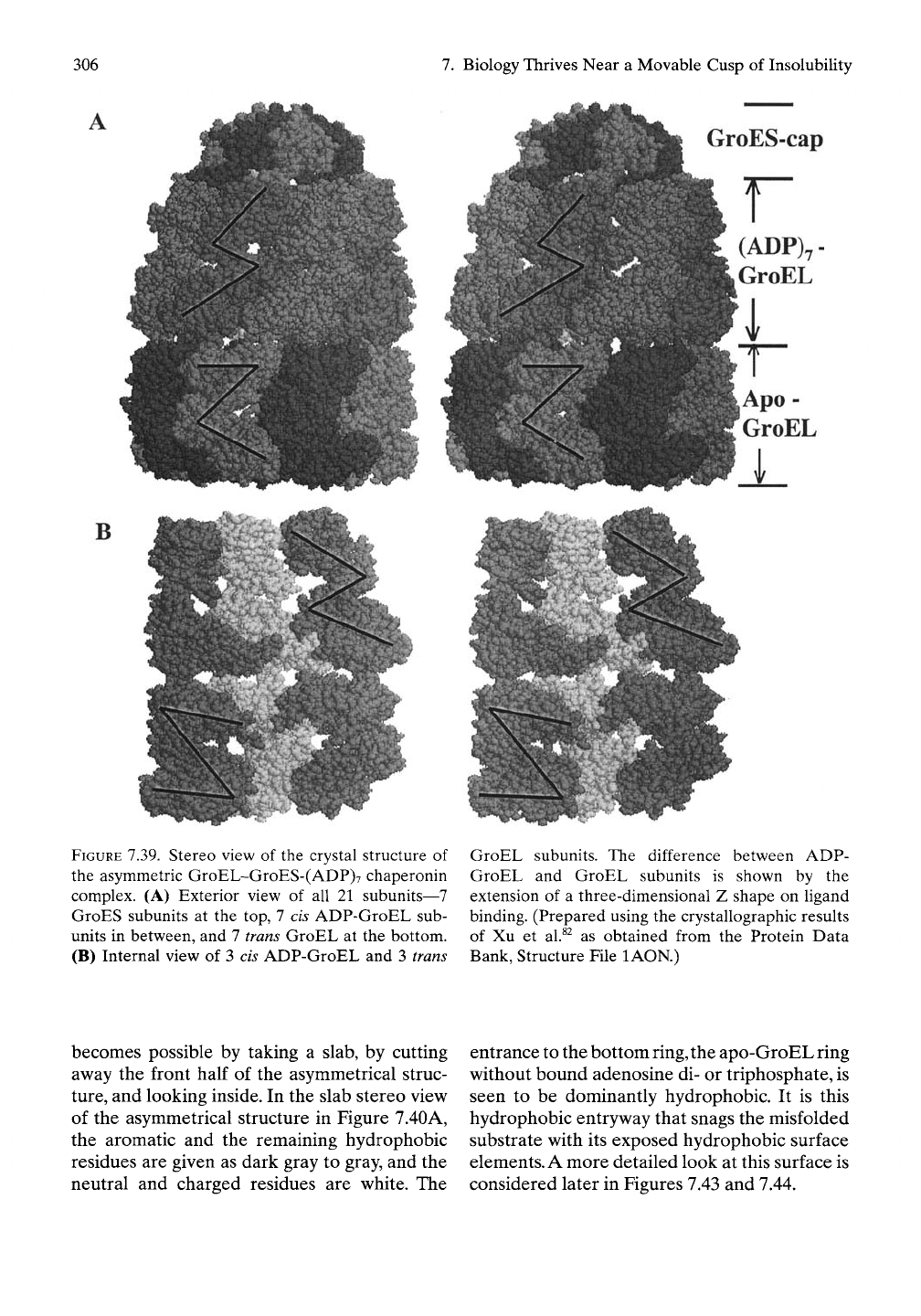
Figure 7.39A shows a space-filling representa-
tion of the crystal structure of one state of
the GroEL/GroES chaperonin with a ring of 7
subunits of apo-GroEL (without hgand) at the
bottom, a second ring of 7 subunits of
(ADP)7-GroEL in the middle, plus a cap of 7
subunits of GroES.^^This single structure exem-
plifies the change in structure that attends
nucleotide binding (the middle ring) as the
bottom ring of 7 GroEL subunits is without any
bound nucleotide. The purpose of the added Z-
shaped lines is discussed below.
7.6.2 Substrate Considerations
7.6.2.1
Substrates
for Chaperonin
Substrates of improperly folded protein are
characterized by having exposed hydrophobic
surfaces that bind in the groove between the H
and I helices of the apical portion of GroEL.
GroES subunits displace such snagged sub-
strates inward in a concerted process with
ATP binding. At this entrance to the internal
chamber of apo-GroEL, there are nine
residues, eight hydrophobic and one Ser avail-
able for hydrophobic association with the
non-native polypeptide. In general, any protein
state with significant exposure of hydrophobic
surface could be expected to bind to chaper-
onin at this apical site. A common substrate for
GroEL is protein containing an aP fold where
a hydrophobic side of an a-helix associates with
a hydrophobic side of a P-sheet. This is remi-
niscent of the types of protein structures that
on partial hydrolysis to remove a-helix give rise
to amyloidosis, a P-sheet of hydrophobically
associated protein chains common to prions, as
discussed above.
7.6.2.2
A Structure for Snagging
the Substrate
The asymmetrical structure in Figure 7.39A pro-
vides an opportunity to visualize how a ring of
seven nonliganded GroEL, that is, an apo-
GroEL, subunits snags its substrate. This
306
7.
Biology Thrives Near a Movable Cusp of Insolubility
GroES-cap
r
(ADP)7-
GroEL
Apo -
GroEL
i
FIGURE 7.39. Stereo view of the crystal structure of
the asymmetric GroEL-GroES-(ADP)7 chaperonin
complex. (A) Exterior view of all 21 subunits—7
GroES subunits at the top, 7 cis ADP-GroEL sub-
units in between, and 7 trans GroEL at the bottom.
(B) Internal view of 3 cis ADP-GroEL and 3 trans
GroEL subunits. The difference between ADP-
GroEL and GroEL subunits is shown by the
extension of a three-dimensional Z shape on ligand
binding. (Prepared using the crystallographic results
of Xu et al.^^ as obtained from the Protein Data
Bank, Structure File lAON.)
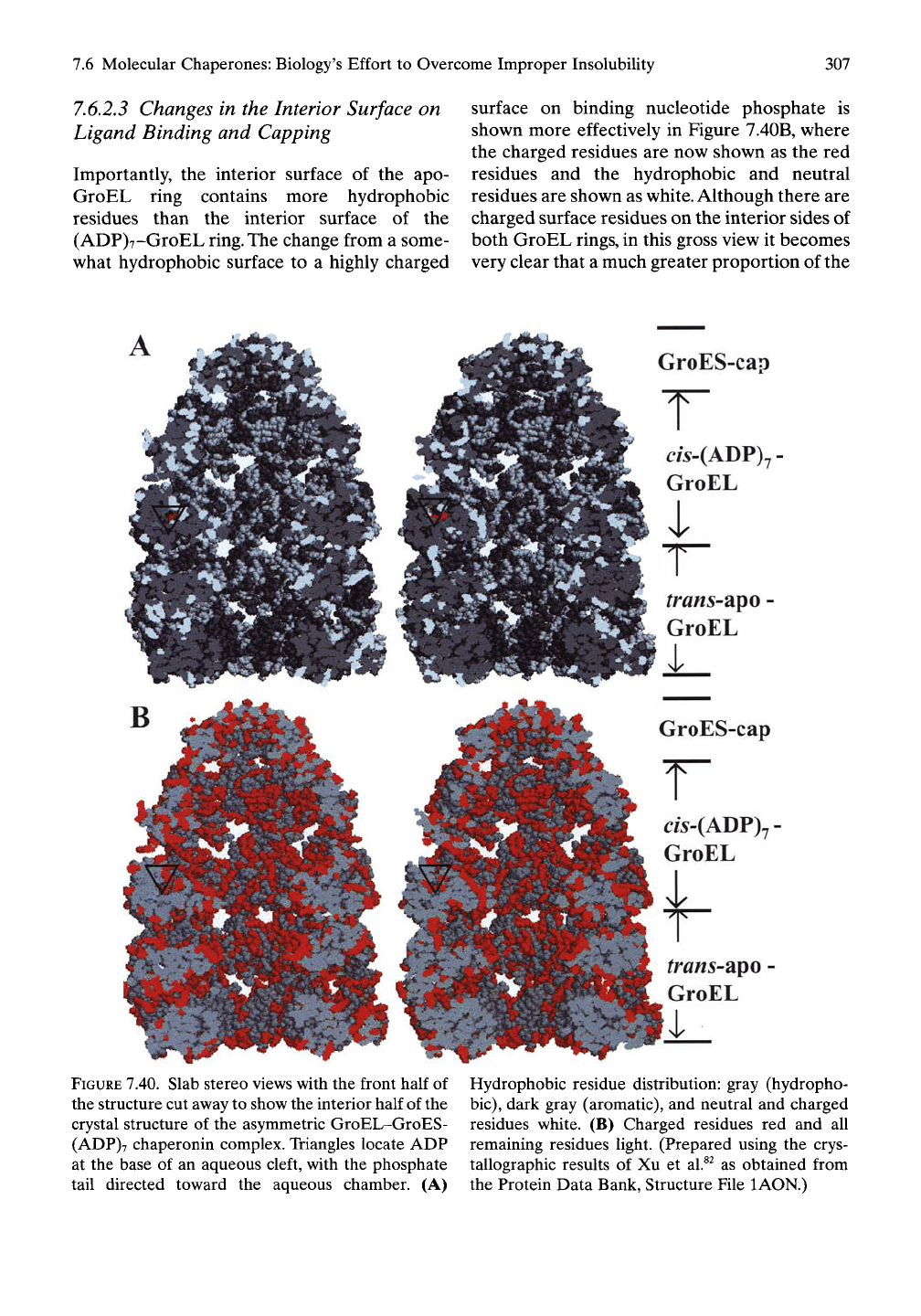
becomes possible by taking a slab, by cutting
aw^ay the front half of the asymmetrical struc-
ture,
and looking inside. In the slab stereo view
of the asymmetrical structure in Figure 7.40A,
the aromatic and the remaining hydrophobic
residues are given as dark gray to gray, and the
neutral and charged residues are white. The
entrance to the bottom ring, the apo-GroEL ring
without bound adenosine di- or triphosphate, is
seen to be dominantly hydrophobic. It is this
hydrophobic entryway that snags the misfolded
substrate with its exposed hydrophobic surface
elements. A more detailed look at this surface is
considered later in Figures 7.43 and 7.44.
7.6 Molecular Chaperones: Biology's Effort to Overcome Improper Insolubility
307
7.6,23
Changes in the Interior Surface on
Ligand Binding and Capping
Importantly, the interior surface of the apo-
GroEL ring contains more hydrophobic
residues than the interior surface of the
(ADP)7-GroEL ring. The change from a some-
what hydrophobic surface to a highly charged
surface on binding nucleotide phosphate is
shown more effectively in Figure 7.40B, where
the charged residues are now shown as the red
residues and the hydrophobic and neutral
residues are shown as
white.
Although there are
charged surface residues on the interior sides of
both GroEL rings, in this gross view it becomes
very clear that a much greater proportion of the
GroES-cap
T
cw-(ADP)7
GroEL
^ra«s-apo
GroEL
i_
GroES-cap
r
ds-(ADP)7-
GroEL
/ra«5-apo
GroEL
i
FIGURE 7.40. Slab stereo views with the front half
of
the structure cut away to show the interior half of the
crystal structure of the asymmetric GroEL-GroES-
(ADP)7 chaperonin complex. Triangles locate ADP
at the base of an aqueous cleft, with the phosphate
tail directed toward the aqueous chamber. (A)
Hydrophobic residue distribution: gray (hydropho-
bic),
dark gray (aromatic), and neutral and charged
residues white. (B) Charged residues red and all
remaining residues light. (Prepared using the crys-
tallographic results of Xu et al.^^ as obtained from
the Protein Data Bank, Structure File lAON.)

308
7.
Biology Thrives Near
a
Movable Cusp
of
Insolubility
surface contains charged residues once bound
to nucleotide phosphate
and
capped with
GroES.
After reviewing
the
mechanical steps
of
this two-cycle unfolding-refolding machine
immediately below,
the
physical basis
as
seen
through
the
perspective
of the
consilient mech-
anism will
be
considered
at
further molecular
detail.
7.62.4
Summary
of
Domain Mechanics
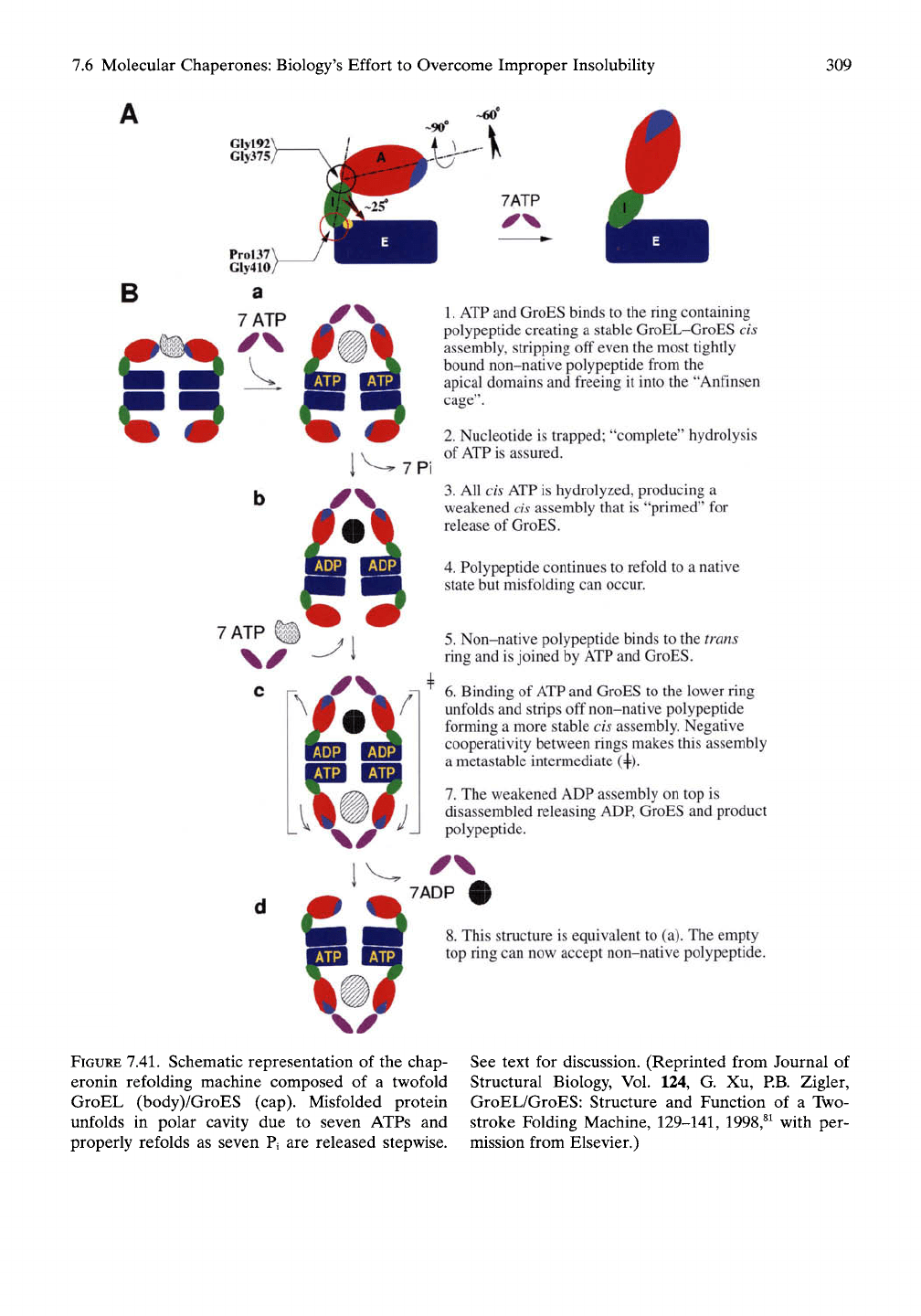
During
an
ATP-mediated Unfolding-
Folding Cycle
(see
Figure
7.41)
Step
1.
Substrate
of
misfolded protein binds
at
the
cis
apical region
by
hydrophobic associa-
tion
to the H and I
helices
and the
loop
region.
Step
2. ATP and
GroES bind with positive
cooperativity
to the cis
side
of the
ring con-
taining
the
misfolded protein, displacing
it
from
the
apical binding site
and
releasing
it
into
an
aqueous cage that, during
the
process,
increases
in
size from 85,000 A^
to
175,000
A^
(see Figure 7.41).^^
In the
resulting large
polar cavity, called
the
Anfinsen cage,^^
the
caged protein unfolds,
and the
nucleotide
is
trapped
in a way
that ensures complete
hydrolysis
of
ATP.
Step
3, The
seven
cis ATP
molecules
are
hydrolyzed
to ADP and
inorganic phosphate.
Pi with stepwise release
of Pj.
Step 4. The unfolded polypeptide refolds
to the
native
or a
more nearly native state during
stepwise release
of Pi, but
does
not
always
complete proper refolding
in a
single cycle.
Step
5.
Misfolded polypeptide attaches
to the
trans side
and is
joined
by ATP and
GroES
as
in
step
2
above,
but
this
ATP
binding
exhibits negative cooperativity
and
causes
the release
of
seven ADP, GroES,
and
prop-
erly folded polypeptide
on the cis
side.
The
trans side then goes through steps
2
through
4.
If
proper refolding
has not
occurred
on the
cis side,
the
exposed hydrophobic groups
of
the
as yet
improperly folded protein will
again
be
snagged
at the
apical region
on the
cis side
for a
repeat cycle.
If
proper folding
has occurred,
the
native protein will escape,
and
a new
misfolded protein with exposed
hydrophobic patches will again bind
on the
cis side followed
by
steps
2
through
4 on the
cis side.
7.6.2.5
Recap
of
Cooperativity
The binding
of ATP to the
first side
of the D7
(C7
-I- C2
symmetry) symmetrical structure
of
GroEL exhibits positive cooperativity, that
is,
the first
ATP
binds weakly
and
then each sub-
sequent ATP binds more tightly. The binding
of
ATP
to the
trans side, while there remains
ADP
in
the
seven sites
and
GroES
on the cis
side,
shows negative cooperativity, that
is, the
first
ATP binds more tightly,
and
each subsequently
bound
ATP
binds more weakly
in the
process
of displacing
the ADP and
GroES
of the cis
side.
7.6.3 Insights Provided
by the
ConsiUent Mechanism into
Chaperonin-Directed Hydrophobic
Unfolding
and
Refolding
The consilient mechanism
was
born
out of
controlling
the
hydrophobic association-
dissociation
of
elastic-contractile model pro-
teins
to
achieve
the
possibility
of
some
18
classes
of
pairwise energy conversions
(see
Chapter
5,
section
5.6). In the
process
a set of
five Axioms became
the
phenomenology
out of
which
the
consihent mechanism arose.
For the
first time
"a
common groundwork
of
explana-
tion"^"^
was
able
to
perform
the
diverse energy
conversions
of
biology.
Application
of the
consilient mechanism
to
chaperonins, therefore, seems most fitting,
because
the
role
of
chaperonin
is to
hydro-
phobically unfold
and
refold proteins that
have hydrophobically misfolded.
The
problem
divides into
two
parts functioning under
the same dominant interaction, changes
in
hydrophobic association within
the
chaperonin
itself
and the
hydrophobic unfolding
and
refolding
of the
substrate.
By the
consihent
mechanism, phosphate
is the
most effective
chemical grouping known
for
disrupting
hydrophobic association.
In the
GroEL chap-
eronin seven phosphate groupings,
as
seven
ATPs,
ring
the
protein
to be
unfolded
and
7.6 Molecular Chaperones: Biology's Effort to Overcome Improper Insolubility
309
-60'
GlylWX
GIy375/
l^^-^7Pi
7 ATP
1.
ATP and GroES binds to the ring containing
polypeptide creating a stable GroEL-GroES cis
assembly, stripping off even the most tightly
bound non-native polypeptide from the
apical domains and freeing it into the "Anfinsen
cage".
2.
Nucleotide is trapped; "complete" hydrolysis
of ATP is assured.
3.
All cis ATP is hydrolyzed, producing a
weakened
cis
assembly that is "primed" for
release of GroES.
4.
Polypeptide continues to refold to a native
state but misfolding can occur.
5.
Non-native polypeptide binds to the trans
ring and is joined by ATP and GroES.
6. Binding of ATP and GroES to the lower ring
unfolds and strips off non-native polypeptide
forming a more stable cis assembly. Negative
cooperativity between rings makes this assembly
a metastable intermediate (+).
7.
The weakened ADP assembly on top is
disassembled releasing ADP, GroES and product
polypeptide.
7ADP
8. This structure is equivalent to (a). The empty
top ring can now accept non-native polypeptide.
FIGURE
7.41. Schematic representation of the chap-
eronin refolding machine composed of a twofold
GroEL (body)/GroES (cap). Misfolded protein
unfolds in polar cavity due to seven ATPs and
properly refolds as seven Pi are released stepwise.
See text for discussion. (Reprinted from Journal of
Structural Biology, Vol. 124, G. Xu, RB. Zigler,
GroEL/GroES: Structure and Function of a Two-
stroke Folding Machine,
129-141,
1998,^^ with per-
mission from Elsevier.)

310
7.
Biology Thrives Near a Movable Cusp of Insolubility
subsequently refolded. Obviously, the stage is
set. The apolar-polar repulsive free energy of
hydration, AGap, becomes the specific physical
process of focus (see Chapter
5,
sections 5.1.7.4,
5.3.3,
and 5.7.9). However, do the molecular
details support the contention that
AGap
drives
the changes in GroEL structure and dominates
the interaction of the GroEL/GroES structure
with hydrophobically misfolded substrate?
7.63.1
ATP Binds with its Triphosphate
Tail at an Internal Aqueous Cleft
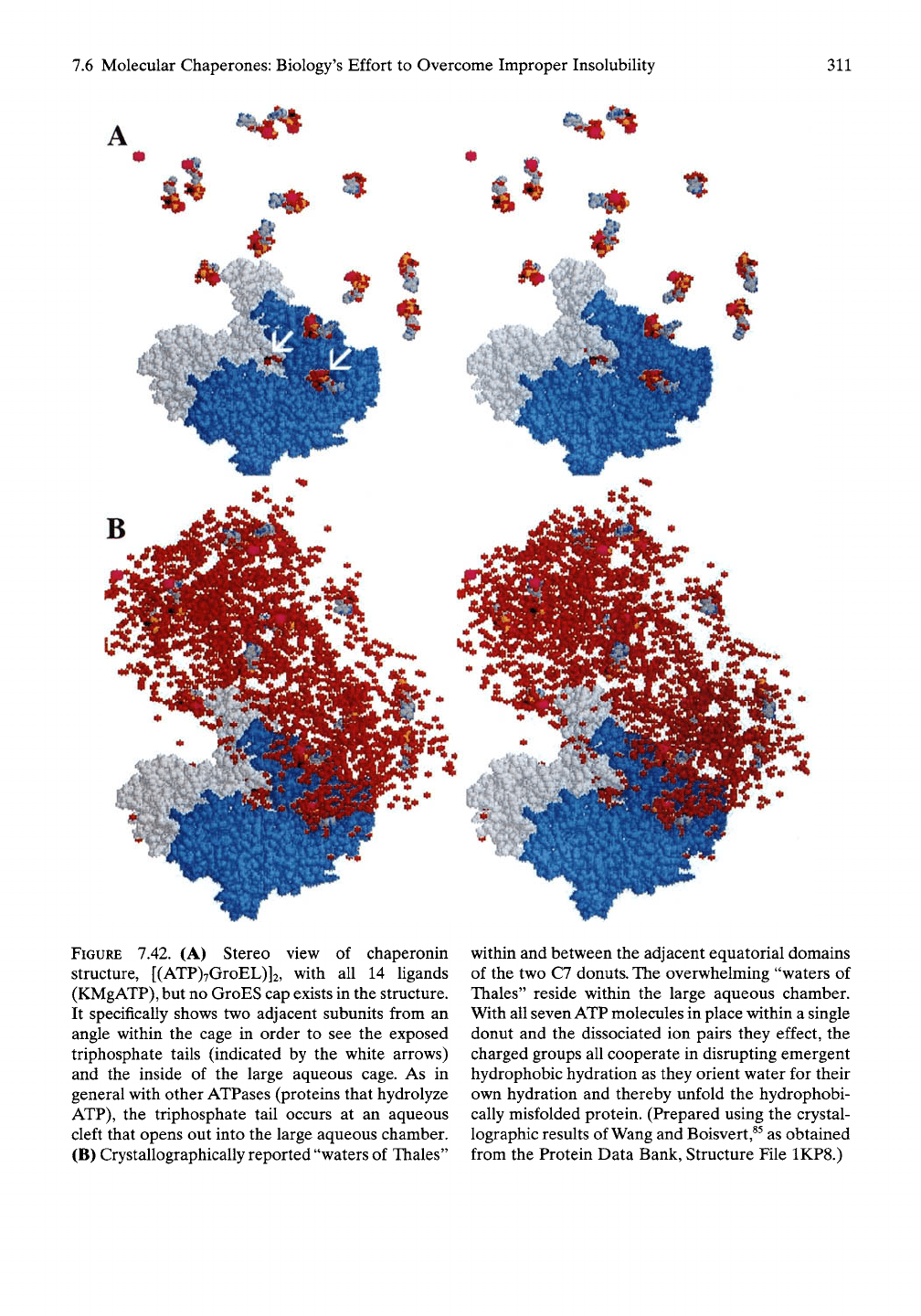
As has become classic for ATPases, which are
the proteins that hydrolyze ATP during func-
tion,
ATP
binds at the base of an aqueous cleft.
Even more to the point, ATP binds with its
triphosphate tail specifically directed toward
the protein-aqueous interface. As shown in
Figure 7.42A, the triphosphate tail can be seen
when looking at the wall from within the
aqueous chamber.^^ The triphosphate tail in the
equatorial domain of GroEL is positioned to
obtain hydration from within the aqueous
chamber. In so doing the polar phosphate tail
orients the water molecules in the cleft and
beyond in a manner unsuited for hydrophobic
hydration. Accordingly, while many water
molecules are seen in the crystal structure (see
Figure 7.42B) and may be called "waters of
Thales,"
most of the waters involved in function
are those within the enclosed large aqueous
chamber of some 175,000 A^. The water mole-
cules of the large aqueous chamber, however,
are too mobile to be located by crystallographic
means.
By the apolar-polar repulsive free energy of
hydration,
AGap,
which results from the compe-
tition for hydration between hydrophobic and
charged species, the water molecules within the
chamber, nonetheless, take on a preferred ori-
entation in which they are properly oriented
toward the charges that develop over the inte-
rior surface during the concerted process of
nucleotide binding and capping with
GroES.
As
explained below on the basis of the consilient
mechanism, such interior water does not readily
form hydrophobic hydration as the trapped and
misfolded protein substrate begins to unfold.
The result is that hydrophobic dissociation
within the substrate dominates as long as the
nucleotide binding sites are filled with ATP and
the chamber is capped with GroES.
7.6.3.2
Recap of the Thermodynamic
Basis for Hydrophobic Association/
Dissociation in Water
The key aspect to keep in mind for hydrophobic
association in water is that it is equivalent to
insolubihty of hydrophobic groups in water.
Remarkably, the
1937
report of Butler^^ contains
the fundamental insight. Methanol (CH3-OH)
is miscible with water in all proportions, and the
process of dissolving methanol in water is
exothermic. Heat is released, and the com-
bination of water and methanol results in a
favorable state of lower free
energy.
Dissolution
of ethanol (CH3-CH2-OH) is even more
exothermic, releasing approximately
1.4kcal
more heat per mole on dissolution than does
methanol. So too for n-propanol (CH3-CH2-
CH2-OH); its dissolution releases some
1.4kcal
more heat per mole on being dissolved in water
than does ethanol. The same holds for each CH2
group added, according to the Butler data, up to
n-pentanol (CH3-CH2-CH2-CH2-CH2-OH).
For the methanol to pentanol series as each CH2
group
is
added, the average change in heat (AH)
is -1.4kcal/mole-CH2. (The negative sign indi-
cates the release of heat.) Therefore, as far as
the release of heat is concerned, formation of
hydrophobic hydration is a favorable reaction.
Indeed, hydrophobic hydration occurs, it
is a
fact
and has been seen in crystal structures (see, for
example. Figure 2.8). However, we also know
that n-octanol, CH3-CH2-CH2-CH2-CH2 CH2-
CH2-CH2-OH,
is
insoluble in water, despite the
fact that the heat released increases for each
CH2 group added.
The governing thermodynamic expression
for solubility is AG(solubility) = AH -
TAS,
and
as long as AG(solubility) is negative, solubility
occurs. Therefore, the reason for decreased
solubility and ultimate loss of solubility as the
number of CH2 groups increases must be due
to a positive (-TAS) term. The formation of
the ordered hydrophobic hydration from less
ordered bulk water results in a negative
AS
due
to formation of water more ordered than bulk
7.6 Molecular Chaperones: Biology's Effort to Overcome Improper Insolubility
311
FIGURE 7.42. (A) Stereo view of chaperonin
structure, [(ATP)7GroEL)]2, with all 14 ligands
(KMgATP),
but no GroES cap exists in the structure.
It specifically shows two adjacent subunits from an
angle within the cage in order to see the exposed
triphosphate tails (indicated by the white arrows)
and the inside of the large aqueous cage. As in
general with other ATPases (proteins that hydrolyze
ATP),
the triphosphate tail occurs at an aqueous
cleft that opens out into the large aqueous chamber.
(B) Crystallographically reported "waters of Thales"
within and between the adjacent equatorial domains
of the two C7 donuts. The overwhelming "waters of
Thales" reside within the large aqueous chamber.
With all seven ATP molecules in place within a single
donut and the dissociated ion pairs they effect, the
charged groups all cooperate in disrupting emergent
hydrophobic hydration as they orient water for their
own hydration and thereby unfold the hydrophobi-
cally misfolded protein. (Prepared using the crystal-
lographic results of Wang and Boisvert,^^ as obtained
from the Protein Data Bank, Structure File 1KP8.)

312
7.
Biology Thrives Near a Movable Cusp of Insolubility
water, such that the (-TAS) term is indeed pos-
itive.
Now, for a pair of associated hydrophobic
domains, a transient opening fluctuation causes
the build up of hydrophobic hydration, but
when so much hydrophobic hydration has
occurred that the positive (-TAS) term
becomes larger than the negative AH term,
AG(solubiUty) becomes positive, which means
insolubiUty. During a transient dissociation of a
pair of hydrophobic domains hydrophobic
association recurs, when too much hydrophobic
hydration forms.
On the other hand, by the consilient mecha-
nism, if a proximal charged group should
appear during the opening fluctuation, then it
recruits the water of hydrophobic hydration
for its own hydration. With the development
of insufficient hydrophobic hydration due to
the presence of proximal charge during the
opening fluctuation, hydrophobic dissociation
stands. Thus the task of the chaperonin is to
develop sufficient proximal charge to allow
dissolution of the hydrophobically misfolded
protein substrate. Then proper hydrophobic
refolding would take place during careful with-
drawal of the charge, as would occur on hydrol-
ysis of ATP to form ADP and Pi and release of
the highly charged inorganic phosphate. Does
the structure of the GroEL/GroES chaperonin
accommodate such a mechanism?
7.6.3.3
In the Absence of Ligand
Binding, Charges Can Be Buried as
Ion Pairs Within Complementary
Hydrophobic Domains
Some of the unseen charges in the interior of
the lower ring, the rra/i^-apo-GroEL ring of
Figure 7.40B, occur as ion-pairs. Due to the
influence of proximal hydrophobic domains the
separated ions lack adequate hydration and
lower their free energy by forming ion pairs.
Wang and Boisvert^^ have noted some ion-pair
separations, namely.
Upon binding of
ATP,
the hydrophobic property of
the apical domain surface is reduced. In the apo
structure^^^'^^] many charged residues (K207, K226,
R231,
K272, E216, E252, E255, and E257) are
involved in inter-subunit interactions and are inter-
locked at the apical-apical domain interface. In this
structure, many of these interactions are substan-
tially weakened.... This change weakens the elec-
trostatic interactions among a large number of
charged residues (K207, K226, R231, K272, E216,
E252,
E255, and E257) at the interface.
The ion pairs often constitute complemen-
tary hydrophobic surfaces, where one hydro-
phobic surface contains a negative charge and
the other contains a positive charge, and the
hydrophobic surfaces structurally fit such that
the ions pair on association. When a proximal
charge causes dissociation of the complemen-
tary hydrophobic surfaces, the separated
charges that emerge from the previous ion pairs
now contribute as separated charged species to
propagate hydrophobic dissociation to greater
distances from the initial source of charge at the
ATP binding site. In this way, ATP binding
propagates ion-pair separation, extending the
reach of AGap to greater distances.
7.6.3.4
Repulsion Between Bound ATP
and the Hydrophobic Domain
Responsible for Positive Cooperativity
and for Apical Domain Rotation
A more direct use of AGap brings in abundant
charge. AGap results from the competition for
hydration between hydrophobic and charged
groups. Therefore, charged groups and
hydrophobic groups achieve the state of lowest
free energy when they each access water un-
disturbed by the other. This translates into an
apolar(hydrophobic)-polar(charge) repulsive
free energy of hydration. Thus, if ATP were to
bind at a site where the ATP must compete with
a hydrophobic domain for hydration, there
would occur a repulsion that makes binding less
favorable and that applies a force for moving
the domain.^^ Because the location of the ATP
binding site occurs at one side of its subunit,
as shown in Figures 7.42A and 7.43B, binding
of the first ATP must also contend with
the hydrophobic domain of the neighboring
subunit. Binding of the second ATP in that
neighboring subunit would contend with less
repulsion so that it binds with a higher affinity.
The work of binding the second ATP has
already been done, in part, by the binding of the
7.6 Molecular Chaperones: Biology's Effort to Overcome Improper Insolubility
313
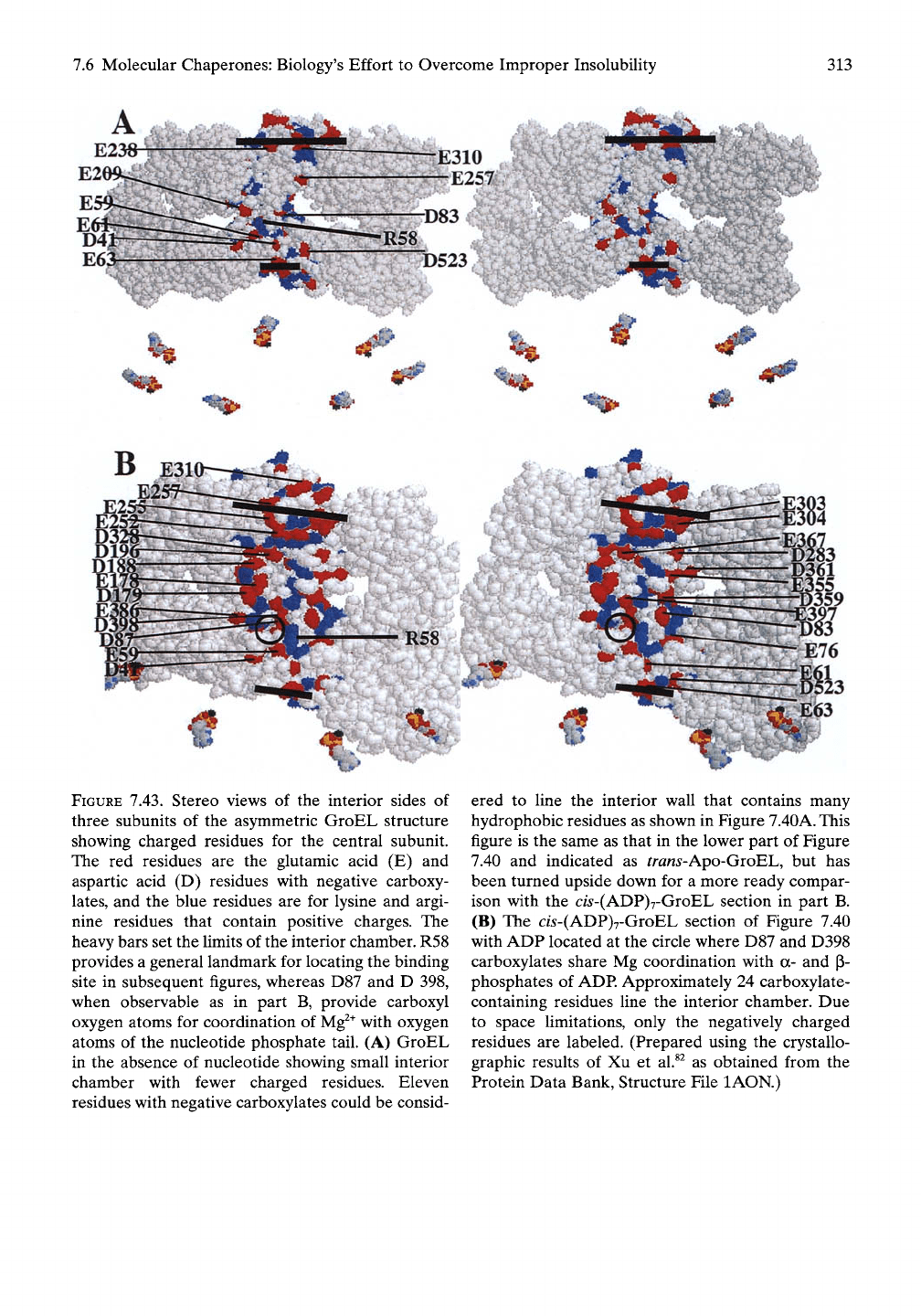
FIGURE 7.43. Stereo views of the interior sides of
three subunits of the asymmetric GroEL structure
showing charged residues for the central subunit.
The red residues are the glutamic acid (E) and
aspartic acid (D) residues with negative carboxy-
lates,
and the blue residues are for lysine and argi-
nine residues that contain positive charges. The
heavy bars set the limits of the interior chamber. R58
provides a general landmark for locating the binding
site in subsequent figures, whereas D87 and D 398,
when observable as in part B, provide carboxyl
oxygen atoms for coordination of
Mg^^
with oxygen
atoms of the nucleotide phosphate tail. (A) GroEL
in the absence of nucleotide showing small interior
chamber with fewer charged residues. Eleven
residues with negative carboxylates could be consid-
ered to fine the interior wall that contains many
hydrophobic residues as shown in Figure
7.40A.
This
figure is the same as that in the lower part of Figure
7.40 and indicated as trans-Apo-GroBh, but has
been turned upside down for a more ready compar-
ison with the c/5-(ADP)7-GroEL section in part B.
(B) The cw-(ADP)7-GroEL section of Figure 7.40
with ADP located at the circle where D87 and D398
carboxylates share Mg coordination with a- and p-
phosphates of
ADP.
Approximately 24 carboxylate-
containing residues line the interior chamber. Due
to space limitations, only the negatively charged
residues are labeled. (Prepared using the crystallo-
graphic results of Xu et al.^^ as obtained from the
Protein Data Bank, Structure File lAON.)

314
7.
Biology Thrives Near a Movable Cusp of Insolubility
first
ATP.
This provides the physical basis for
positive cooperativity.
Repulsion between hydrophobic surfaces of
the apical domain and ATP molecules in their
binding sites within each subunit also provides
a driving force for rotation of the apical
domains. The repulsion between ATP in the
binding site of its subunit and the hydrophobic
domain of the adjacent subunit would drive the
rotation in a clockwise rotation as seen looking
from the end of the apical domain toward the
intermediate and equatorial domains. The rota-
tion moves the hydrophobic residues into posi-
tion for intersubunit hydrophobic association
and, at the same time, brings charged residues
from the sides of aqueous pores into the
chamber formed by seven GroEL subunits.
From the structure and the perspective of the
consilient mechanism, the AGap developed on
ATP binding would appear to contribute to
both the apical and intermediate domain
rotations.
Thus,
the apolar-polar repulsive free energy
of hydration drives both domain rotation and
the separation of ion pairs during the separa-
tion of complementary hydrophobic surfaces.
The result becomes apparent on comparison of
the two interior surfaces in Figure 7.43. The
most effective residues contributing to AGap are
the carboxylates. Because of this and in order
to keep the comparisons in Figure 7.43 from
being too cluttered, only the carboxylate-con-
taining residues are labeled. In short, there are
more than twice as many carboxylates attribut-
able to the surface of the aqueous chamber
after the nucleotide and GroES have bound.
Also,
the magnitude of the rotation of the
apical domain becomes apparent on noting the
positions of E257 and E310 in Figure 7.43A
before rotation with their locations in Figure
7.43B after rotation.
7.6.3.5
Estimate of Change in
AGHA
of
Inner Wall on Nucleotide Binding and
GroES Capping
Differential scanning calorimetry data of the
inverse temperature transition for hydrophobic
association of a series of model proteins,
reported in Chapter 5, allowed calculation of
the relative values of the Gibbs free energy of
hydrophobic association, AGHA, for the side
chain of each amino acid residue. Table 5.3 lists
the values of AGHA with the glycine (Gly, G)
residue taken as zero. When the residues seen
from the central aqueous chamber of the apo-
GroEL ring are fisted, as labeled in Figure 7.44,
the summation over AGHA for the labeled
residues yields a relatively hydrophobic value
of approximately -lOkcal/mole-subunit of
internal surface as seen from the aqueous
chamber. On the other hand, when the summa-
tion is taken over the residues labeled in Figure
7.45,
which is the surface of the large aqueous
chamber after nucleotide binding and GroES
capping, that is, the interior surface of
(ADP)7-GroEL/GroES, the summation over
AGHA reports a very polar surface of greater
than +50kcal/mole-subunit of internal surface
as seen from the aqueous chamber. Clearly,
nucleotide binding and GroES capping create
a very polar surface.
7.6.3.6
View of the ATP Binding Site
Along Inner Wall from the Top of
the Apical Domain
Figure 7.46 demonstrates continuity from
bound nucleotide to the full length of the polar
surface. Looking from the top of the apical
domain downward along the inner face of the
chamber exposes the a- and (i-phosphates of
ADP at the base of the classic ATPase cleft.
Thus,
ATP binds the equatorial domain at the
aqueous interface of an aqueous cleft. From this
position, by means of the apolar-polar repul-
sive free energy of hydration, AGap, seven ATP
molecules working in a positively cooperative
manner transform the uniquely poised struc-
ture to create a remarkably polar face that
reaches far into the large chamber for adequate
hydration. How then can such a structure
perform the task of unfolding and properly
refolding hydrophobically misfolded proteins?
7.6.4 Chaperonin Design Exemplifies
the Consilient Mechanism
The design of the GroEL/GroES molecular
chaperone presents a clear statement of the
