Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


7.5 Grave Medical Consequences
of
Protein Insolubilities
295
7.5 Grave Medical
Consequences
of
Protein
Insolubilities
7.5.1 Introductory Remarks
7.5JJ
Excursions
Too Far
into
the
Realm
of Protein Insolubility
Excursions
too far
into
the
realm
of
protein
insolubility present
a
variety
of
tragic
and
ulti-
mately fatal neurodegenerative diseases.
In
humans there
are two
causative proteins:
the
prion protein
of
transmissible spongiform
encephalopathies
and the
amyloid precursor
protein of Alzheimer's disease.
In
general, there
are some 15 proteins^^ that
by
mutations
or
pro-
cessing errors exhibit similar protein amyloid
deposits^^ that result
in
organ damage. Such
protein insolubilities occur
in
mammals, birds,
and even yeast.^^
7.5.1.2
The
Etiology
Is
Common
to the
Formation
of all
Amyloid Deposits
Very stable protein aggregates, originating
within
the
host
or
introduced into
the
host,
act
as "condensation nuclei"^^
on
which
the
natural
host protein grows until
the
resultant aggre-
gates destroy their host. Thus,
the
protein
aggregates constitute proteinaceous-only infec-
tious agents, named prions
by
Prusiner,^^'^^ that
destroy tissue
and
ultimately
the
host.
A common theme runs through
all of
these
injurious protein aggregates. They contain
a p-
structure composed
of
a
relatively hydrophobic
protein sequence.
7.5.1.3
General Comments
on
Our Proposed Physical Basis
for
Amyloid Formation
We believe that
an
understanding
of the
devel-
opment
of
amyloid deposits resides within
the development
in
Chapter
5 of the
physical
process underlying hydrophobic association
dissociation.
The
simplest statement
of the
physical process
is the
presence
of a
competi-
tion
for
water between oil-like (hydrophobic)
and polar (e.g., charged) groups
of
protein.
The
competition
was
expressed
by
Equation (5.13)
in Chapter
5 as an
apolar-polar repulsive free
energy
of
hydration, AGap,
on the
basis
of pKa
shifts.
The
physical process
of
hydrophobic
association/dissociation
was
also given expres-
sion
in
Equation
(5.8) of
Chapter
5 as a
Gibbs
free energy
of
hydrophobic association, AGHA,
which
can be
related
to the
shifts
in the
tem-
perature
at
which
the
inverse temperature
transition occurs.
The factors underlying protein insolubility
diseases
can be
understood
in
terms
of the
same
factors that control hydrophobic association.
Loss
of
charged groups favors hydrophobic
association, that
is,
favors protein insolubility.
Obviously
an
increase
in
hydrophobicity
is a
shift toward insolubility. However, increased
hydrophobicity makes unfavorable
the
pres-
ence
of
polar groups, that
is,
raises
the
free
energy
of
even
the
peptide groups
of the
protein backbone.
An increase
in
hydrophobicity provides
a
driving force
for
hydrogen-bonded association
of protein chains.
In our
view, this
can
occur
under conditions where
one
would have other-
wise thought that
the
peptide groups were
already quite satisfied
by
their exposure
to
water.
The
first clear insight into this perspec-
tive came from
the
stretch-induced
pKa
shifts
seen
in our
hydrophobically associated elastic
model proteins
(see
Chapter
5,
section 5.7.8.2).
In this case even though stretching with
increased exposure
of
hydrophobic groups
increased
the
amount
of
water within
the
elastic
matrix, carboxylates,
COO",
experienced less
available water; they became water thirsty even
though more water
was in the
elastic matrix.
Accordingly,
the
water surrounding hydropho-
bic groups must
be the
wrong kind
of
water
for
hydrating polar groups such
as
carboxylates
and,
to a
lesser
but
significant extent, peptide
groups.
7.5.1.4
Segue
to the
Magnitude
of the
Medical Problem
Before pondering specific structural examples,
however,
a
brief discussion
of the
medical sig-
nificance
of
protein insolubility sharpens inter-
est because
of the
increasingly common nature

296
7.
Biology Thrives Near
a
Movable Cusp
of
Insolubility
of
the
problem. Perhaps even more critically,
relevance
of the
issue
to our
everyday lives
becomes apparent
on
equating protein insolu-
bility with
the
growing problem
of
Alzheimer's
disease
and
current well-known victims,
notably former
U.S.
President Ronald Reagan
and most recently respected actor Charlton
Heston. The fearsome side
of
protein insolubil-
ity also equates
to the
recent scares
of mad cow
disease
and
reports
of the
insoluble protein
infecting humans
who
have eaten tainted
beef.
Then there
are
tragic inherited diseases
in
humans
and in
other animals
and
organisms
that enhance
the
process
of
insoluble protein
formation.
It is our
belief that
the
science devel-
oped
in
Chapter
5
provides
the
basis whereby
these disease processes
can be
explained.
7.5.2 Magnitude
of the
Medical Problem
of
Excessive
Protein Insolubility
7.5.2.1
Alzheimer's Disease
Currently
in the
United about
4
million people
suffer from Alzheimer's disease.
As the
popu-
lation demographics continue
to
shift with
the
percentage
of
people
in the
older groups
growing most rapidly,
the
medical care problem
becomes more demanding.
It is
estimated
that nearly
50% of
people over
85
years
of age
have Alzheimer's disease. Furthermore,
in the
western world Alzheimer's disease
is
consid-
ered
the
fourth leading cause
of
death.
The time course
of the
disease from diagno-
sis
to
death
is
from several years
to two
decades. Over that period, Alzheimer's disease
presents
a
steady progression toward decreased
mental faculties. Limited mental function com-
monly becomes apparent
in the
loss
of
ability
to think through even simple mathematical
problems.
The
debilitation then progresses
to
loss
of
recall
of
common numbers, like those
of
telephone numbers, dates,
and
addresses.
The
progression continues with loss
of
recogni-
tion
of
friends
and
family, with loss
of
coordi-
nation resulting
in
incapacity
for
personal care
and speech,
and
with loss
of
awareness
of
surroundings.
This progressive dementia results from
growing protein deposits, insoluble protein
plaques (amyloid plaques),
and
insoluble
protein neurofibrillary tangles
in the
brain that
cause death
of
brain cells
and
loss
of
brain
tissue.
7.5.2.2
Prion Diseases
Two decades
ago
Prusiner proposed protein
particles
as the
infectious agent
of
scrapie,^^
the neurodegenerative disease
of
sheep.^^
He
called
the
infectious protein
a
prion, standing
for protein infectious only. The infectious agent
was protein without involvement
of
pathogens,
such
as
bacteria
and
viruses,
and
devoid
of any
nucleic acid. During
the
same period
and
working among
the
Stone
Age
Fore Tribe
in
Papua
New
Guinea, Gajdusek identified
a
similar neurodegenerative disease process
in
humans, called kuru.^^
The
ritual
of
eating
the
brain tissue
of
deceased tribal members pro-
vided
for
transmission
of the
disease. This iden-
tified
the
occurrence
of an
infectious disease
in
humans with insoluble protein
as the
causative
agent
for
transmission.
Collectively known
as
transmissible spongi-
form encephalopathies
(TSEs),^"*
the
recognized
transmissible prion diseases
now
include
Creutzfeld-Jakob disease (CJD), Gerstmann-
Straussler-Scheinker disease (GSS), fatal famil-
ial insomnia (FFI), familial thalamic dementia,
and kuru. Furthermore, animal TSEs include
scrapies
in
sheep
and
goats,
mad cow
disease
(bovine spongiform encephalopathy,
BSE),
feline spongiform encephalopathy (FSE), trans-
missible mink encephalopathy,
and
chronic
wasting disease
(CWD) in
deer
and elk.
These transmissible diseases, with insoluble
protein
as the
infectious agent, would seem
to
have originated
as
mutations,
and
within this
context they became inherited prion diseases.
Creutzfeld-Jakob disease,
GSS,
and FFI
number
among those
now
recognized
as
inherited
prion diseases
in
humans.

7.5 Grave Medical Consequences
of
Protein Insolubilities
297
7.5.3 Phenomenology
of
Amyloid
(Insoluble Protein) Deposits
from
Mad Cow
Disease
to
Alzheimer's Disease
7.5.3.1 How Can a
Natural Protein
Become
a
Transmissible Infectious Agent?
A prion protein, designated
as PrP, has a
natural monomeric form, designated PrP^,
and
an insoluble, phase-separated
or
associated
form, designated
PrP^*',
where
the
superscript
C
stands
for the
normal cellular form
and the
superscript
Sc
stands
for the
infectious scrapie
form.
PrP^ is
degradable
by
proteolytic
enzymes, whereas
PrP^*^,
the smaller component
of PrP^, forms
a
proteolytic resistant core.
The prion protein, therefore, exhibits
a
phase
transition from
a
monomeric protein
to an
aggregated protein that bears analogy
in
fundamental respects
to the
phase transition
exhibited
by the
model proteins discussed
in
Chapter
5. For the
phase transition
of our
model proteins, association is relatively fast
and
dissociation
or
dissolution is quite slow. The
dif-
ference with
the
prion phase transition
is
only
qualitative
in
that
the
association step
of the
phase transition
of
prion protein
is
extremely
slow,
and
dissociation
is so
slow
as to be
irre-
versible.
The
slow relentless growth
of
insolu-
ble prion protein fibers continues until
it
destroys
the
cell
or
tissue with which
it may be
associated. This calls
to
mind sickle cell anemia
due
to an
inverse temperature transition
of
hemoglobin
S
wherein intracellular fiber
growth distorts
and
sickles
red
blood cells
and
leads
to
their destruction
(see
Figure 7.22).
A fundamental question
of
particular inter-
est here becomes,
is the
prion aggregation step
controlled
by the
same forces that control
hydrophobic association?
In
other words, is
the
prion protein solubility-insolubility phase tran-
sition
an
inverse temperature transition?
To
find the answer, we again look
for a
coherence
of phenomena characteristic
of
inverse temper-
ature transitions.
7.5.3.2 How
Infectious
Can a
Prion Protein
Be?
Infectiousness
of the
insoluble fiber form
of
prion protein, PrP^^ derives from its capacity
to
induce
the
normal
PrP^
form
to
change
its
three-dimensional shape
and
grow
on the
PrP^*^
template. Part
of the
severity
of the
medical
problem
is
that autoclaving
and
other possible
sterilization procedures
are
insufficient
to
destroy
the
fibrous PrP^' form.^^'^^ The stability
of
the
PrP^'' form
has
resulted
in
transmission
of CJD from contaminated, autoclaved surgical
instruments, from donor dura mater, from
donor corneas,
and
from human growth
hormone—all materials associated
in
some way
with neural tissue.
Transmission
of
prion diseases from
one
person
to
another through
use of
diseased
human tissues becomes
a
particularly difficult
problem, because
the
growth
of the
insoluble
Pfpsc
fQYxn
can
require years before symptoms
of the disease are apparent.The gestation period
for prion diseases
can be
years,
yet the
PrP^*'
would seem to be infectious during that period.^^
The epidemic
of "mad cow
disease" that
resulted
in the
loss
of
upwards
of
200,000 cattle
apparently arose
due to
feeding cattle
a
meal
that contained contaminated neural tissue.
Animal feed containing nervous tissue
is
considered
to be the
source
of
prion diseases
occurring
in
sheep
and
goats. There also
appears
to be
cross-infectivity between species,
as scores
of
humans have contracted
a
variant
form
of
Creutzfeld-Jakob disease (vCJD) from
eating contaminated
beef.^^"^^
Having quaUtatively noted
the
phenomenol-
ogy
of
prion-related diseases arising from
excursions into
the
realm
of
excess insolubility,
we
now
look
for
correlations
of
phenomena
that point
to a
consilient mechanism.
7.5.4 Mechanism
of
Amyloid
Fiber Formation
and
Relentless
Fiber Growth
7.5.4.1
Scenario
of
Hydrophobic
Associations Based
on
Inverse
Temperature Transitions
and on the
Apolar-Polar Repulsive Free Energy
of Hydration,
AGap
Whenever
an
opening fluctuation toward
hydrophobic dissociation
occurs,
progression
to
dissociation continues only
as
long
as too
much
hydrophobic hydration does
not
result. Should
298
7.
Biology Thrives Near a Movable Cusp of Insolubility
there be polar groups sufficiently proximal to
the site of hydrophobic dissociation, such as
charged groups able to compete for hydration
and in doing so to destructure enough nascent
hydrophobic hydration, then dissociation
results. If instead too much hydrophobic hydra-
tion results during an opening fluctuation, the
Tt-divide drops too far below the operating
temperature, and hydrophobic association
holds firm. This we believe is the origin of the
insoluble PrP^*^ form that gives rise to forma-
tion of insoluble protein of the fibrous aggre-
gates.
The fibrous aggregates utilize a particular
structural element in the aggregation process. It
is,
a structural element different from that of
hemoglobin S fibers. In our view and as a
consequence of the consilient mechanism,
the structural element is responsible for the
extremely slow but relentless growth.
7,5.4.2
^-Structures: The Common Theme
of Prion Protein Amyloid Fibers
7.5.4.2.1 The Infective Form of Prion Protein
Is Aggregated P-structure
The experimental findings are that PrP^*^ forms
insoluble aggregates with more p-structure and
less a-helix than occurs in the PrP^ monomeric
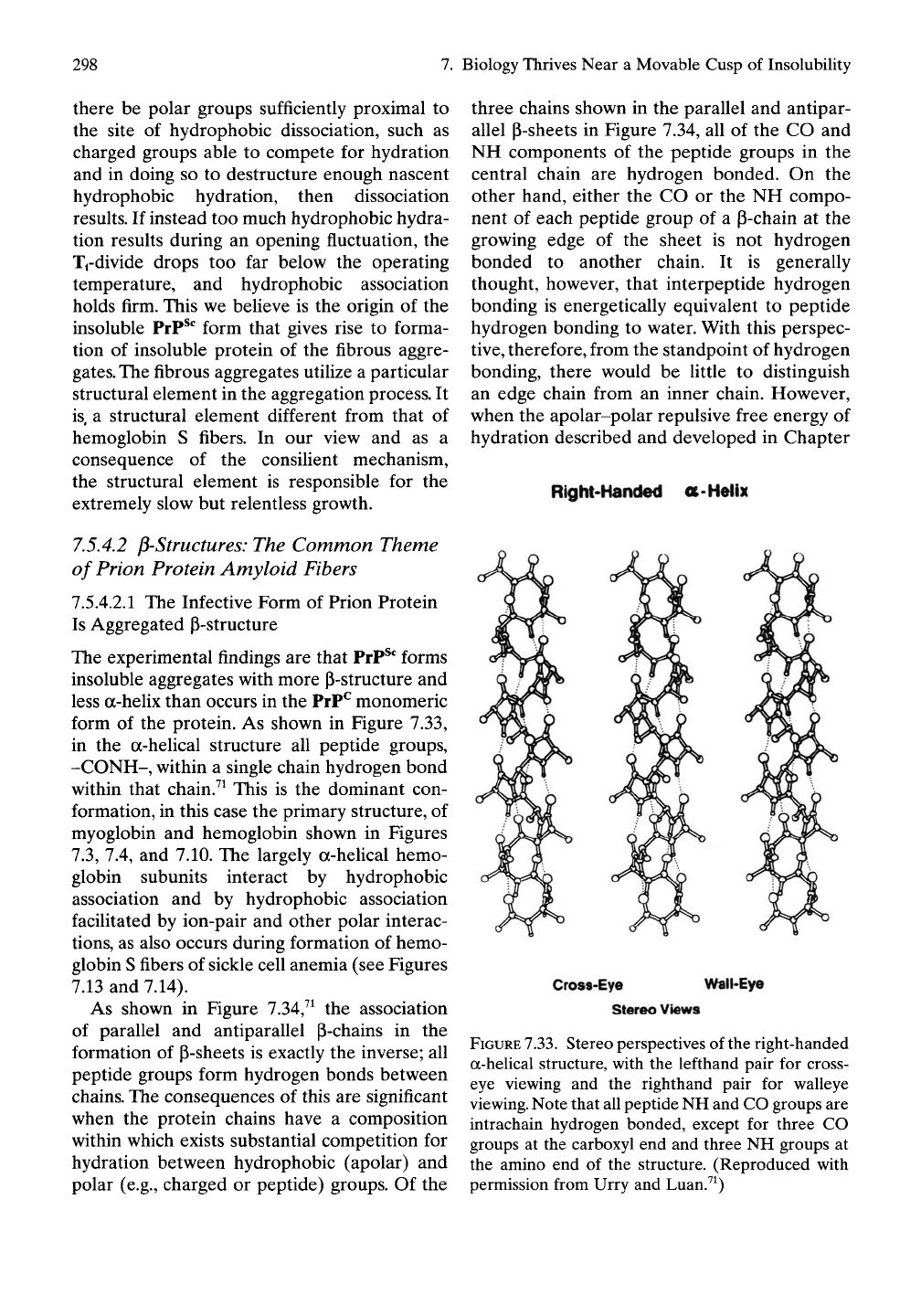
form of the protein. As shown in Figure 7.33,
in the a-helical structure all peptide groups,
-CONH-, within a single chain hydrogen bond
within that chain.^^ This is the dominant con-
formation, in this case the primary structure, of
myoglobin and hemoglobin shown in Figures
7.3,
7.4, and 7.10. The largely a-helical hemo-
globin subunits interact by hydrophobic
association and by hydrophobic association
facilitated by ion-pair and other polar interac-
tions,
as also occurs during formation of hemo-
globin S fibers of sickle cell anemia (see Figures
7.13 and 7.14).
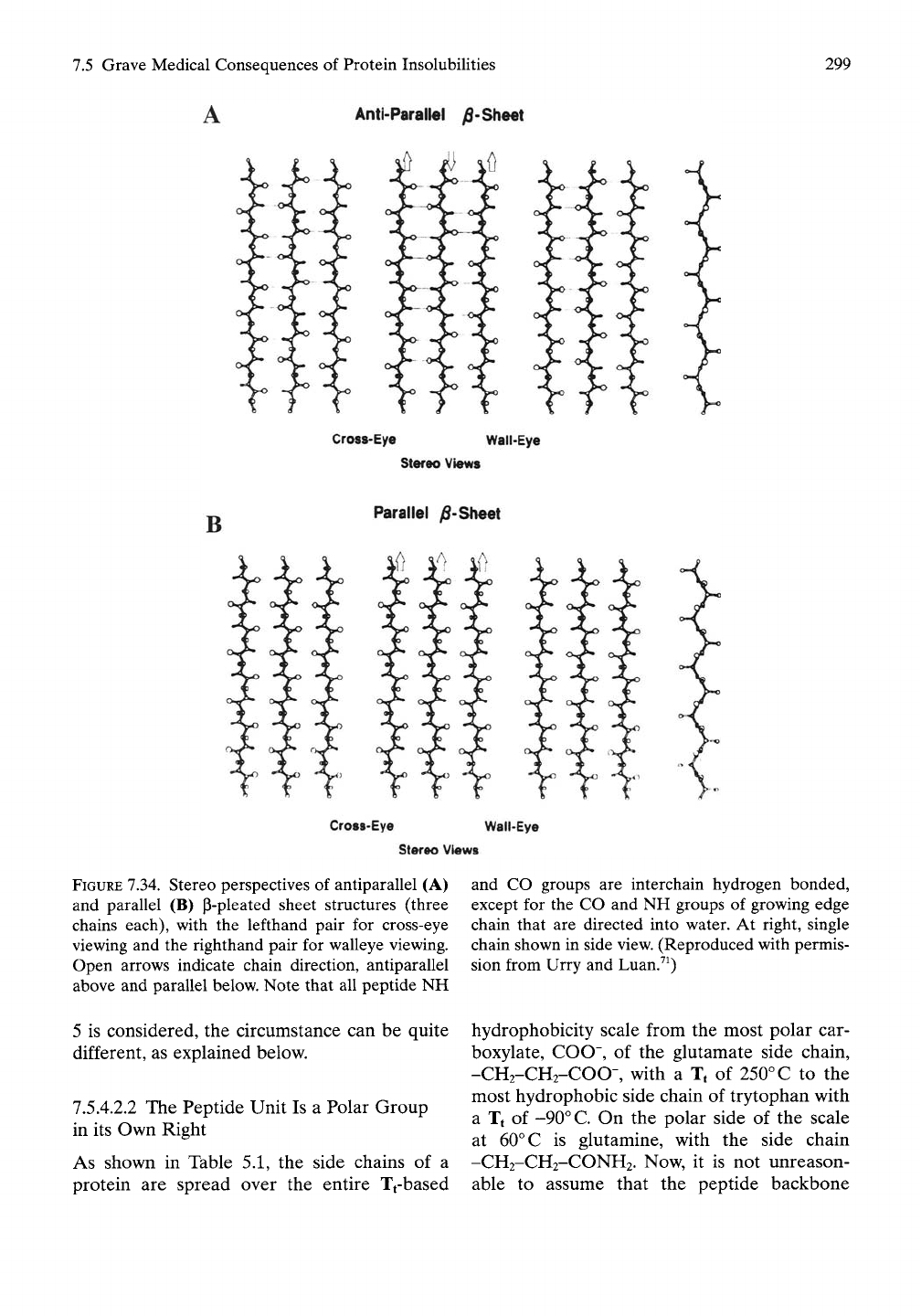
As shown in Figure 7.34,^^ the association
of parallel and antiparallel P-chains in the
formation of P-sheets is exactly the inverse; all
peptide groups form hydrogen bonds between
chains. The consequences of this are significant
when the protein chains have a composition
within which exists substantial competition for
hydration between hydrophobic (apolar) and
polar (e.g., charged or peptide) groups. Of the
three chains shown in the parallel and antipar-
allel P-sheets in Figure 7.34, all of the CO and
NH components of the peptide groups in the
central chain are hydrogen bonded. On the
other hand, either the CO or the NH compo-
nent of each peptide group of a P-chain at the
growing edge of the sheet is not hydrogen
bonded to another chain. It is generally
thought, however, that interpeptide hydrogen
bonding is energetically equivalent to peptide
hydrogen bonding to water. With this perspec-
tive,
therefore, from the standpoint of hydrogen
bonding, there would be little to distinguish
an edge chain from an inner chain. However,
when the apolar-polar repulsive free energy of
hydration described and developed in Chapter
Right-Handed o-Helix
Cross-Eye Wall-Eye
Stereo Views
FIGURE
7.33.
Stereo perspectives of the right-handed
a-helical structure, with the lefthand pair for cross-
eye viewing and the righthand pair for walleye
viewing.
Note that all peptide NH and CO groups are
intrachain hydrogen bonded, except for three CO
groups at the carboxyl end and three NH groups at
the amino end of the structure. (Reproduced with
permission from Urry and Luan.^^)
7.5 Grave Medical Consequences of Protein Insolubilities
A Anti-Parallel ^-ShMt
299
Crost-Eyo Wail-Eye
Stereo Views
Crost-Eye Wall-Eye
Stereo Views
FIGURE 7.34. Stereo perspectives of antiparallel (A)
and parallel (B) p-pleated sheet structures (three
chains each), with the lefthand pair for cross-eye
viewing and the righthand pair for walleye viewing.
Open arrows indicate chain direction, antiparallel
above and parallel below. Note that all peptide NH
5 is considered, the circumstance can be quite
different, as explained below.
7.5.4.2.2 The Peptide Unit Is a Polar Group
in its Own Right
As shown in Table 5.1, the side chains of a
protein are spread over the entire Tfbased
and CO groups are interchain hydrogen bonded,
except for the CO and NH groups of growing edge
chain that are directed into water. At right, single
chain shown in side view. (Reproduced with permis-
sion from Urry and Luan.^^)
hydrophobicity scale from the most polar car-
boxylate, COO", of the glutamate side chain,
-CH2-CH2-COO-, with a Tt of 250° C to the
most hydrophobic side chain of trytophan with
a Tt of -90°
C.
On the polar side of the scale
at
60°
C is glutamine, with the side chain
-CH2-CH2-CONH2. Now, it is not unreason-
able to assume that the peptide backbone

300
7.
Biology Thrives Near
a
Movable Cusp
of
Insolubility
group, -CONH-, would
be in the
same range
of polarity
as the
glutamine side chain. Using
the reference state
of
valine with
a
Tf value
of
25°
C,
the
difference
in
Tt-values, ATt,
for
gluta-
mine
is
35°
C (60°C-25°C),
and the ATt for the
most polar glutamate
is
225°
C.
In
this case
one might estimate that
a
half dozen (225/35)
peptide groups would
sum to be as
polar
as a
single carboxylate
of
glutamic acid7^
With either
the CO or NH of
each peptide
group
(but not
both)
of
p-chain hydrogen
bonded
on one
side
to a
second P-chain, about
12 peptides
of a
p-chain
at the
edge
of a
p-sheet
would
be
equivalent
to a
single carboxylate.
Regardless
of the
exact numerical equivalency,
in
our
view there exists
a
competition
for
hydration between hydrophobic groups
and a
nonhydrogen-bonded
CO or NH
component
of
a peptide group. Thus,
as
further explained
below,
the
nonhydrogen-bonded portion
of
a peptide group
at the
growing edge
of a
hydrophobic P-sheet could
be a
polar group
starving
for
hydrogen bonding even though
directed into what appears
to be a sea of
avail-
able water molecules.
7.5.4.3
How Can a
Natural Protein
in a
^sheet-containing Amyloid Fiber
Function
as a
Seed Crystal
to
Induce Fiber
Propagation That
Is
Ultimately Injurious
to
the
Host Site?
We have seen hydrophobic-induced
pKa
shifts
for carboxylates that were produced
by
sys-
tematically increasing hydrophobicity
and due
to stretching
of
hydrophobic elastic matrices
containing carboxylates
(see
Chapter
5,
section
5.6,
and
Figures 5.20B, 5.23,5.29,5.30,5.31,
and
5.34).
The pKa
shift arises
due to
inadequate
hydration
for the
carboxylate—due
to a
com-
petition
for
hydration between hydrophobic
groups
and
charged carboxylate
for
water
(see
Figures
5.24 and 5.25 of
Chapter
5).
This
has
been called
an
apolar-polar repulsive free
energy
of
hydration, i^G^^
(see
Equation [5.13]
of Chapter
5).
The peptide group also
is a
polar group,
and
although
it
does
not
have
an
ionizable function
to quantify
its
lack
of
adequate hydration, when
part
of a
hydrophobic peptide,
it is
nonetheless
at
an
unfavorable increase
in
free energy.^^
For
an edge chain
of a
P-sheet,
the
high energy
is relieved only
by
hydrogen bonding
to an
additional P-chain.
In
this
way the
dominantly
hydrophobic environs
of the
edge chain
of a
natural protein
in a
^-sheet-based fiber acts
as a
seed site
for
fiber propagation.
7.5.4.4
Propensity
of
Prion Protein
for
Fiber Propagation: Correlation with
the Consilient Mechanism
If
the
above mechanism, based
as it is on
an inverse temperature transition,
is
indeed
responsible
for the
formation
of
infectious
prion protein aggregates, then
the
variables that
lower
the
value
of Tt
would
be
expected
to
favor fiber formation
and
infectivity. Increase
in
hydrophobicity
and
decrease
in
charge either
by neutralization
or by
mutation would
be
expected
to
favor fiber formation
and
growth.
Also
as
described above, because
the
free
energy that favors
the
association
of
P-chains
is
described
as an
apolar-polar repulsive free
energy
of
hydration,
the
presence
of
Hmiting
hydration would lead
to an
increase
in
P-sheet
propensity.
7.5.4.5
The
Trbased Single Residue Plot
of
the
Human Prion Protein
7.5.4.5.1 Increase
in
Hydrophobicity
by
Replacement with
a
more Oil-like Residue,
P->L,A-^V
The effect
of
more hydrophobic mutants
demonstrates
an
increased propensity
for the
insolubility
of
fiber formation.
For
example,
"The substitution
of
amino acids
in the
mutants,
e.g.,3A ->
V (at
positions 113,115,
and 118) and
PIOIL, enhances
the
folding
of the
peptide into
compact structural units, significantly enhanc-
ing
the
formation
of the
extensive P-sheet
fibrils."^^ The
Pro (P) to Leu (L)
mutant''
both enhances hydrophobicity
and
removes
the kink
due to a
proline residue that would
interfere with
a
continuous p-chain structure.
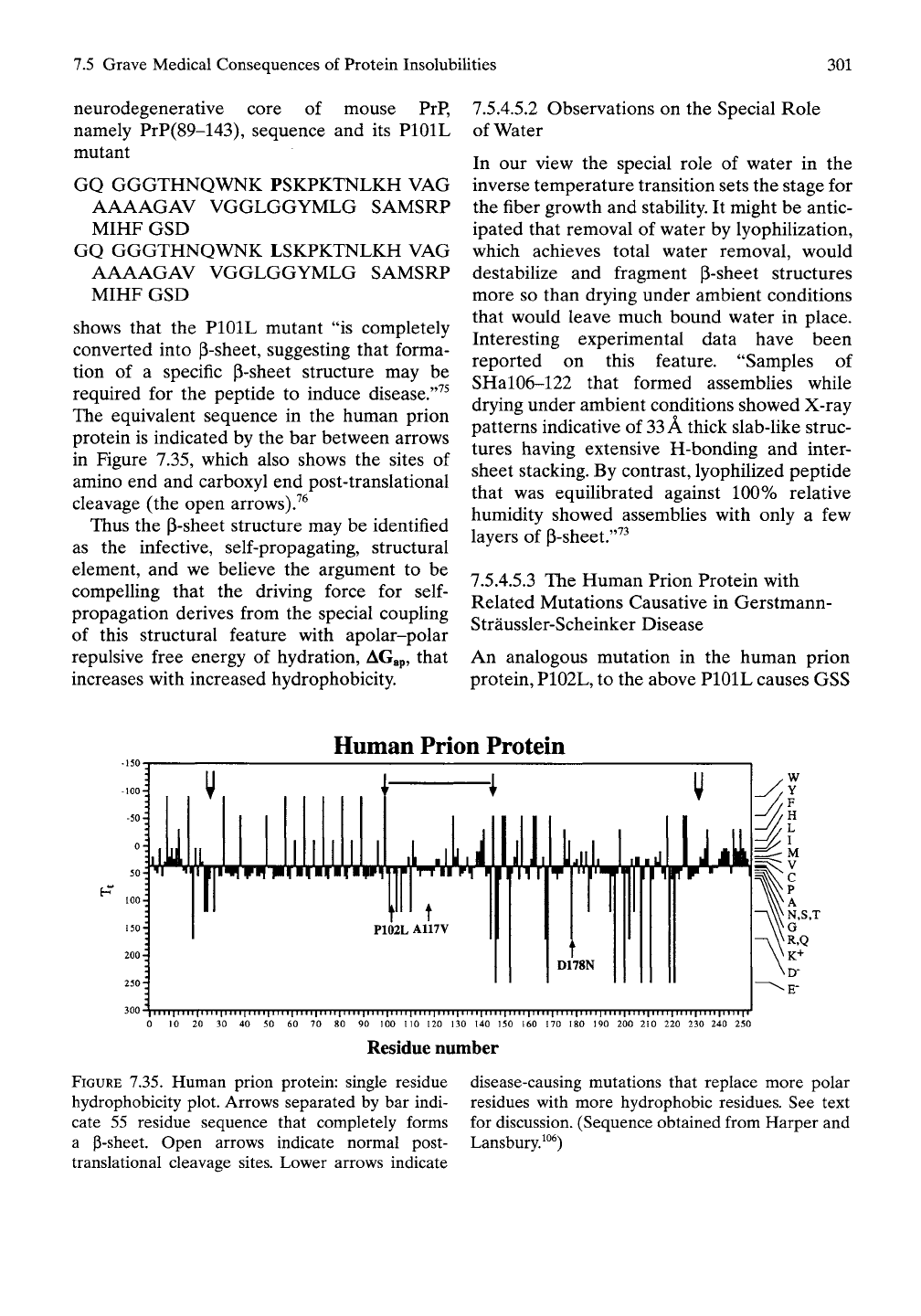
In particular, consideration
of the 55
residue
7.5 Grave Medical Consequences of Protein Insolubilities
301
neurodegenerative core of mouse PrP,
namely PrP(89-143), sequence and its PIOIL
mutant
GQ GGGTHNQWNK PSKPKTNLKH VAG
AAAAGAV VGGLGGYMLG SAMSRP
MIHF GSD
GQ GGGTHNQWNK LSKPKTNLKH VAG
AAAAGAV VGGLGGYMLG SAMSRP
MIHF GSD
shows that the PIOIL mutant "is completely
converted into P-sheet, suggesting that forma-
tion of a specific P-sheet structure may be
required for the peptide to induce disease."^^
The equivalent sequence in the human prion
protein is indicated by the bar between arrows
in Figure 7.35, which also shows the sites of
amino end and carboxyl end post-translational
cleavage (the open arrows)7^
Thus the P-sheet structure may be identified
as the infective, self-propagating, structural
element, and we believe the argument to be
compelling that the driving force for
self-
propagation derives from the special coupling
of this structural feature with apolar-polar
repulsive free energy of hydration, AGap, that
increases with increased hydrophobicity.
7.5.4.5.2 Observations on the Special Role
of Water
In our view the special role of water in the
inverse temperature transition sets the stage for
the fiber growth and stability. It might be antic-
ipated that removal of water by lyophilization,
which achieves total water removal, would
destabilize and fragment P-sheet structures
more so than drying under ambient conditions
that would leave much bound water in place.
Interesting experimental data have been
reported on this feature. "Samples of
SHal06-122 that formed assembUes while
drying under ambient conditions showed X-ray
patterns indicative of
33
A thick slab-like struc-
tures having extensive H-bonding and inter-
sheet stacking. By contrast, lyophilized peptide
that was equilibrated against 100% relative
humidity showed assembUes with only a few
layers of P-sheet."^^
7.5.4.5.3 The Human Prion Protein with
Related Mutations Causative in Gerstmann-
Straussler-Scheinker Disease
An analogous mutation in the human prion
protein, P102L, to the above PIOIL causes GSS
Human Prion Protein
300 )|M||IIII|IIII|IIII|
0 10 20 30 40
i|iiii|iiii|iiii|iiii|iiii|iiii|iiii|iin|iiii|iiii|im|iiii|ini|iiii|iiii|Mii|iiii|iiii|iiii|iiii|i'
50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250
Residue number
FIGURE 7.35. Human prion protein: single residue
hydrophobicity plot. Arrows separated by bar indi-
cate 55 residue sequence that completely forms
a p-sheet. Open arrows indicate normal post-
translational cleavage sites. Lower arrows indicate
disease-causing mutations that replace more polar
residues with more hydrophobic residues. See text
for discussion. (Sequence obtained from Harper and
Lansbury.^^^)
302
7.
Biology Thrives Near a Movable Cusp of Insolubility
disease as does the A117V mutation.^^'^^ The
positions of these mutations are indicated by
the upward-directed arrows in Figure 7.35 for
the human prion protein.
7.5.4.5.4 A Mutation Causative for Fatal
Familial Insomnia
The mutation responsible for the human prion
disease FFI, decreases charge on the human
prion protein in a sensitive sequence. The muta-
tion D178N converts a charged aspartic acid
residue to an uncharged asparagine. This too is
indicated in Figure 7.35. Again, the removal of
the D178 stand-alone carboxylate opens up the
second longest apolar sequence to add to (3-
sheet formation. If the mutation were instead
D202N, we would expect no significant effect
on amyloid formation. The remainder of polar
residues in the 196 to 221 sequence would dom-
inate and prevent assembly of hydrophobic P-
chains into a p-sheet.
7.5.4.5.5 Low pH and Ion Pairing Enhance
Insolubilization of Fiber Formation
Lowering the pH of the
PrP^*^
form in the range
of 4.4 to 6 favors amyloid formation.^^'^^ Low
pH,
however, appears to have no effect on the
conformation of the carboxyl-terminal portion
of the PrP^' form, but low pH does facilitate
incorporation of the amino-terminal portion
into amyloid. The few residues with
carboxylates in the 140 to 170 relatively
hydrophobic sequence could experience
hydrophobic-induced pKa shifts as demon-
strated in Figures 5.30 and 5.34 of Chapter
5 and become uncharged carboxyls.
7.5.4.5.6 Insight from Electron
Crystallography for a Working p-structure
of Scrapie Prion Protein
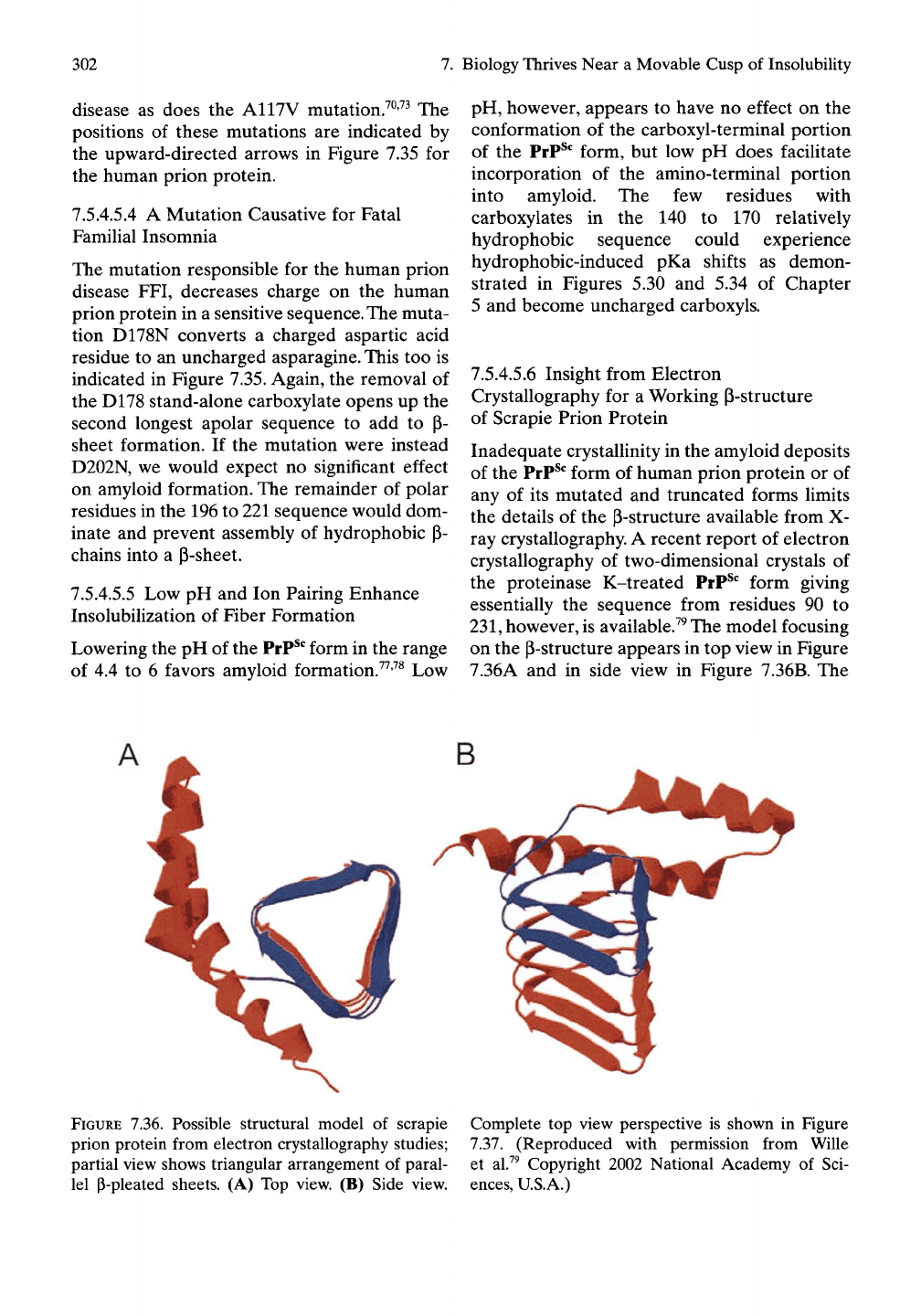
Inadequate crystallinity in the amyloid deposits
of the PrP^' form of human prion protein or of
any of its mutated and truncated forms limits
the details of the p-structure available from X-
ray crystallography. A recent report of electron
crystallography of two-dimensional crystals of
the proteinase K-treated PrP^*^ form giving
essentially the sequence from residues 90 to
231,
however, is available.^^ The model focusing
on the p-structure appears in top view in Figure
7.36A and in side view in Figure 7.36B. The
FIGURE 7.36. Possible structural model of scrapie Complete top view perspective is shown in Figure
prion protein from electron crystallography studies; 7.37. (Reproduced with permission from Wille
partial view shows triangular arrangement of paral- et al.^^ Copyright 2002 National Academy of Sci-
lel P-pleated sheets. (A) Top view. (B) Side view, ences, U.S.A.)
7.5 Grave Medical Consequences of Protein Insolubilities
303
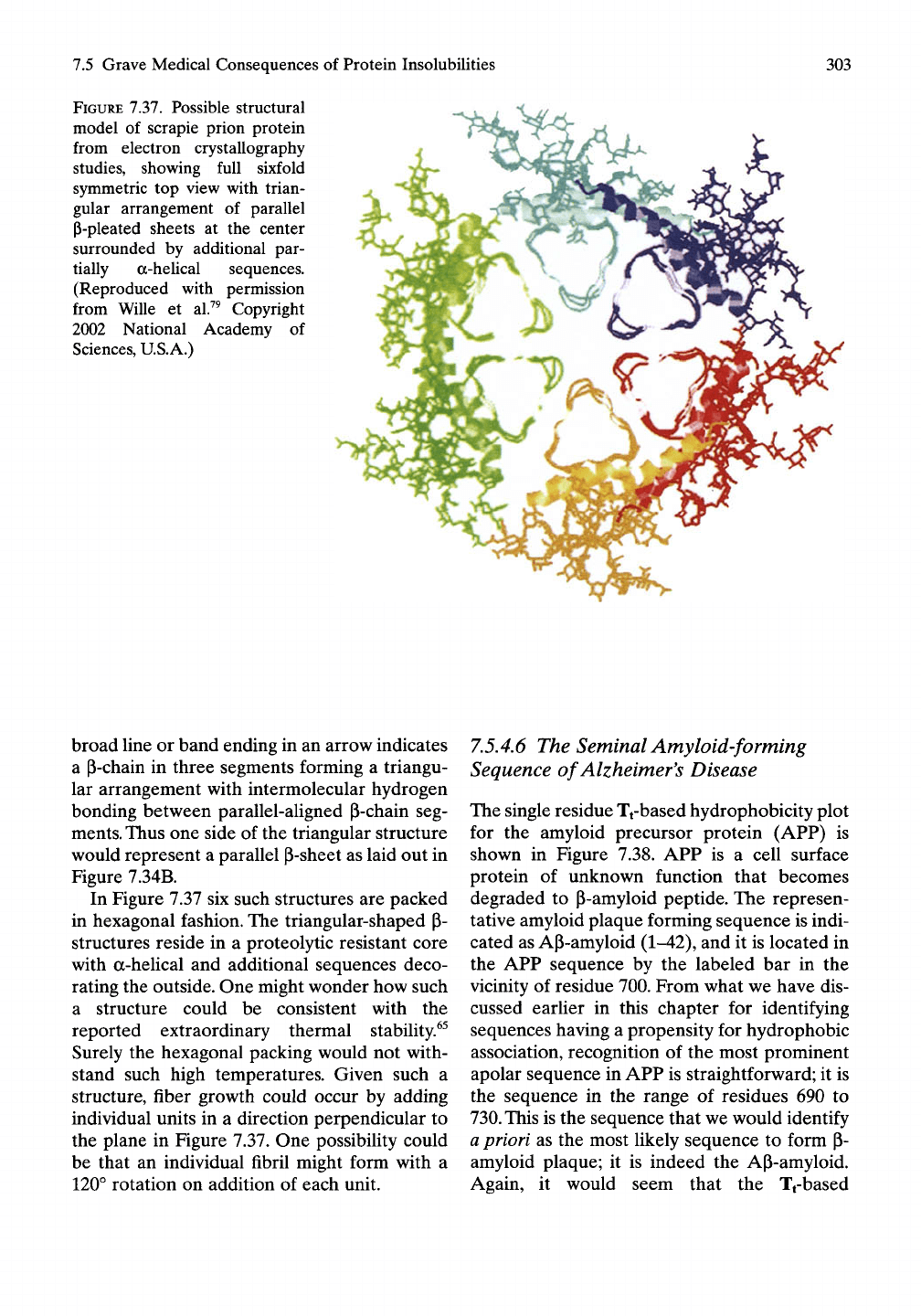
FIGURE 7.37. Possible structural
model of scrapie prion protein
from electron crystallography
studies, showing full sixfold
symmetric top view with trian-
gular arrangement of parallel
P-pleated sheets at the center
surrounded by additional par-
tially a-heUcal sequences.
(Reproduced with permission
from Wille et al.^^ Copyright
2002 National Academy of
Sciences, U.S.A.)
broad line or band ending in an arrow indicates
a P-chain in three segments forming a triangu-
lar arrangement with intermolecular hydrogen
bonding between parallel-aligned P-chain seg-
ments. Thus one side of the triangular structure
would represent a parallel P-sheet as laid out in
Figure 7.34B.
In Figure 7.37 six such structures are packed
in hexagonal fashion. The triangular-shaped p-
structures reside in a proteolytic resistant core
with a-helical and additional sequences deco-
rating the outside. One might wonder how such
a structure could be consistent with the
reported extraordinary thermal stability.^^
Surely the hexagonal packing would not with-
stand such high temperatures. Given such a
structure, fiber growth could occur by adding
individual units in a direction perpendicular to
the plane in Figure 7.37. One possibility could
be that an individual fibril might form with a
120° rotation on addition of each unit.
7.5.4.6
The Seminal Amyloid-forming
Sequence of Alzheimer's Disease
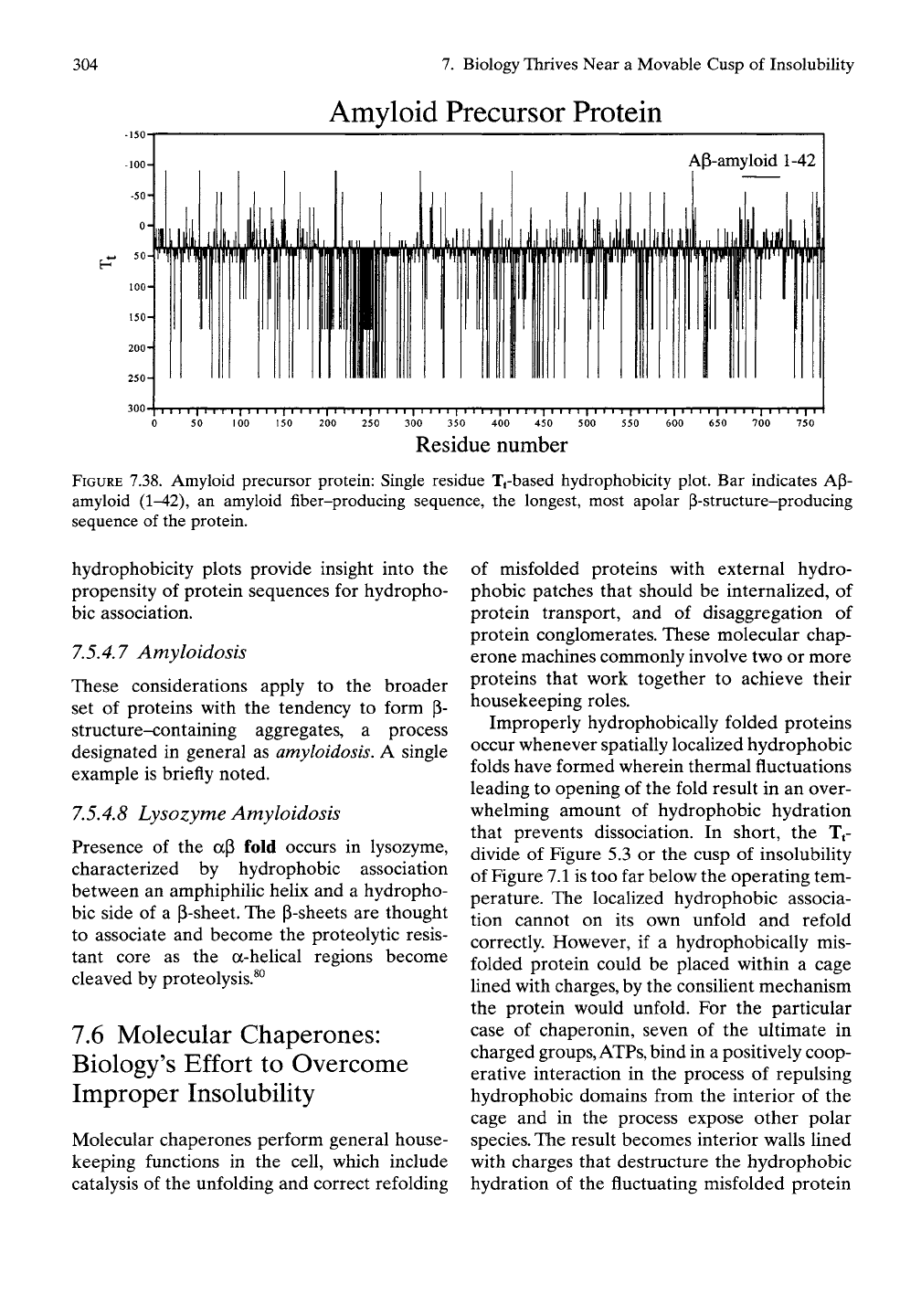
The single residue Tt-based hydrophobicity plot
for the amyloid precursor protein (AFP) is
shown in Figure 7.38. APP is a cell surface
protein of unknown function that becomes
degraded to P-amyloid peptide. The represen-
tative amyloid plaque forming sequence is indi-
cated as AP-amyloid (1-42), and it is located in
the APP sequence by the labeled bar in the
vicinity of residue 700. From what we have dis-
cussed eariier in this chapter for identifying
sequences having a propensity for hydrophobic
association, recognition of the most prominent
apolar sequence in APP is straightforward; it is
the sequence in the range of residues 690 to
730.
This is the sequence that we would identify
a priori as the most likely sequence to form P-
amyloid plaque; it is indeed the AP-amyloid.
Again, it would seem that the Tj-based
304
7.
Biology Thrives Near a Movable Cusp of Insolubility
Amyloid Precursor Protein
300
)
I I I I [ I i I I I I I I I I I I I I I I I I I I i I I I
;
I I I I I I I I I I I I I i
I
50 150 200 350
I I
' '
I i I
500
550
600
I
M
I
'
I I I
650 700
-r
750
Residue number
FIGURE 7.38. Amyloid precursor protein: Single residue T^based hydrophobicity plot. Bar indicates AP-
amyloid (1-42), an amyloid fiber-producing sequence, the longest, most apolar P-structure-producing
sequence of the protein.
hydrophobicity plots provide insight into the
propensity of protein sequences for hydropho-
bic association.
7.5 A J Amyloidosis
These considerations apply to the broader
set of proteins with the tendency to form P-
structure-containing aggregates, a process
designated in general as amyloidosis. A single
example is briefly noted.
7.5.4.8
Lysozyme Amyloidosis
Presence of the aP fold occurs in lysozyme,
characterized by hydrophobic association
between an amphiphilic helix and a hydropho-
bic side of a P-sheet. The P-sheets are thought
to associate and become the proteolytic resis-
tant core as the a-helical regions become
cleaved by proteolysis.^^
7.6 Molecular Chaperones:
Biology's Effort to Overcome
Improper Insolubility
Molecular chaperones perform general house-
keeping functions in the cell, which include
catalysis of the unfolding and correct refolding
of misfolded proteins with external hydro-
phobic patches that should be internalized, of
protein transport, and of disaggregation of
protein conglomerates. These molecular chap-
erone machines commonly involve two or more
proteins that work together to achieve their
housekeeping roles.
Improperly hydrophobically folded proteins
occur whenever spatially localized hydrophobic
folds have formed wherein thermal fluctuations
leading to opening of the fold result in an over-
whelming amount of hydrophobic hydration
that prevents dissociation. In short, the Tt-
divide of Figure 5.3 or the cusp of insolubility
of Figure 7.1 is too far below the operating tem-
perature. The localized hydrophobic associa-
tion cannot on its own unfold and refold
correctly. However, if a hydrophobically mis-
folded protein could be placed within a cage
lined with charges, by the consilient mechanism
the protein would unfold. For the particular
case of chaperonin, seven of the ultimate in
charged groups,
ATPs,
bind in a positively coop-
erative interaction in the process of repulsing
hydrophobic domains from the interior of the
cage and in the process expose other polar
species. The result becomes interior walls lined
with charges that destructure the hydrophobic
hydration of the fluctuating misfolded protein
