Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


7.3 Hemoglobin Structures Demonstrate
the
Consilient Mechanism
265
hydrophobic dominance, separate
or
reorient
in
a way to
minimize AGap.
The
separated
and
reoriented charged groups
now
reach
out for
more hydration, such that
the
oxygen-binding
event
at one
heme decreases
the
hydrophobic-
ity
(the
amount
of
hydrophobic hydration)
of
the second heme
at the
other
end of the
crevasse
and
facilitates
the
binding oxygen
to
the second heme.
The
decrease
in
hydropho-
bicity
of the
second heme
due to
oxygen
binding
to the
first heme uniquely becomes
the consilient mechanism's explanation
for the
sigmoid binding curve, that is,
for the
effective
oxygen transport function
of
hemoglobin.
In short,
we
propose that first polar oxygen,
in
its
approach
to the
heme binding site
of
hemoglobin, experiences repulsion
due to the
hydrophobicity
of the
heme
and its
environ-
ment. The barrier results from
the
loss
of
hydra-
tion available
to the
oxygen molecule
as it
approaches
the
hydrophobic hydration
of the
exposed heme
and its
surrounding hydrophobic
groups. Once
the
first polar oxygen molecule
binds,
however,
the
resulting decrease
in
hydrophobicity transmits through
the
water-
filled crevasse,
by
reoriented, more competitive
charged groups,
to the
second heme
and
facili-
tates binding
of the
second oxygen. Structural
justifications
for
this view follow.
7.3.3 Examination
of
the Structural
Changes Resulting from
Oxygen Binding
7.3.3.1
General Appearance
of the
Crystal
Structures
of
Deoxyhemoglobin
and Oxyhemoglobin
7.3.3.1.1
The
Size
of the
Aqueous Core
of the
T State
of
Hemoglobin
A space-filling representation. Figure 7.11A,B
presents stereo views
of
deoxyHb
and
oxyHb(T) from
the
perspective
of the
twofold
(diad) axis.
A
first point
to
note with this per-
spective
is
that
it
gives
the
most minimal size
of
the aqueous core
of the
hemoglobin molecule.
Looking
by
stereo view into
the
aqueous core
at several slight angles from
the
diad axis,
one
sees
a
much larger water-filled
core.
Thus,
water
is
not
only accessible from
the
outside
of the
molecule
to the
areas
of
association between
subunits,
but
water
is
also available
to the
qua-
ternary structure from
the
inside.
In
fact,
as
measured from slabs taken perpendicular
to
the twofold axis,
the
water-filled core, which
waters would constitute part
of the
"waters
of
Thales," approaches 40%
of the
total diameter.
The thickness
of
the wall
on one
side
can be
less
than
the
width
of the
aqueous channel.
7.3.3.1.2
The
Limited External Differences
Between deoxyHb
and
oxyHb(T)
From
the
perspective
of
Figure 7.11, small
dif-
ferences
are
seen between
the
P-chains
and
between
the
a-chains within
the
same tetramer.
Surprisingly, these small intramolecular
dif-
ferences appear
to be as
great
as the
inter-
molecular differences between deoxyHb
and
oxyHb(T). Attempts
to
oxygenate crystalline
deoxyHb
at
room temperature destroy crys-
talline order. However, crystals
of
deoxyHb
formed
at
4° C, carefully changed
to a
40% poly-
ethylene glycol-water solution,
and
oxygenated
at
4°C
survive with oxygen bound
at all
four
hemes.^^
The
result
is
T-state oxyhemoglobin,
that
is,
oxyHb(T).
The
protocol
for
forming
oxyHb(T)
is a
prescription
for
reigning
in the
consilient mechanism certainly
at the
level
of
changing quaternary structure. Nonetheless,
we
choose
to
utilize oxyHb(T) because,
if
oxygen
binding does decrease
the
hydrophobicity
of
the
heme group, evidence
of
this should
be in the
heme environment
as
long
as
there does remain
adequate water
in the
crystal
to
evidence
the
competition
for
hydration.
7.3.3.1.3 Looking
to a
Molecular Basis
for the
Coherence
of
Phenomena
The phenomenological observations detailed
above
in
section
7.2.4
clearly exhibit
a
coher-
ence
of
phenomena with
the
consiUent mecha-
nism.
Our
objective, therefore,
is to
determine
the particular expressions
of the
consilient
mechanism
at the
molecular level.
As a
starting
point, we look from
the
aqueous medium
to the
heme group
in its
protein setting
and
make
gross comparison between
the
heme environ-
ment
in
myoglobin
and the
environments
of the
hemes
of
hemoglobin.
In our
view,
the
first clue
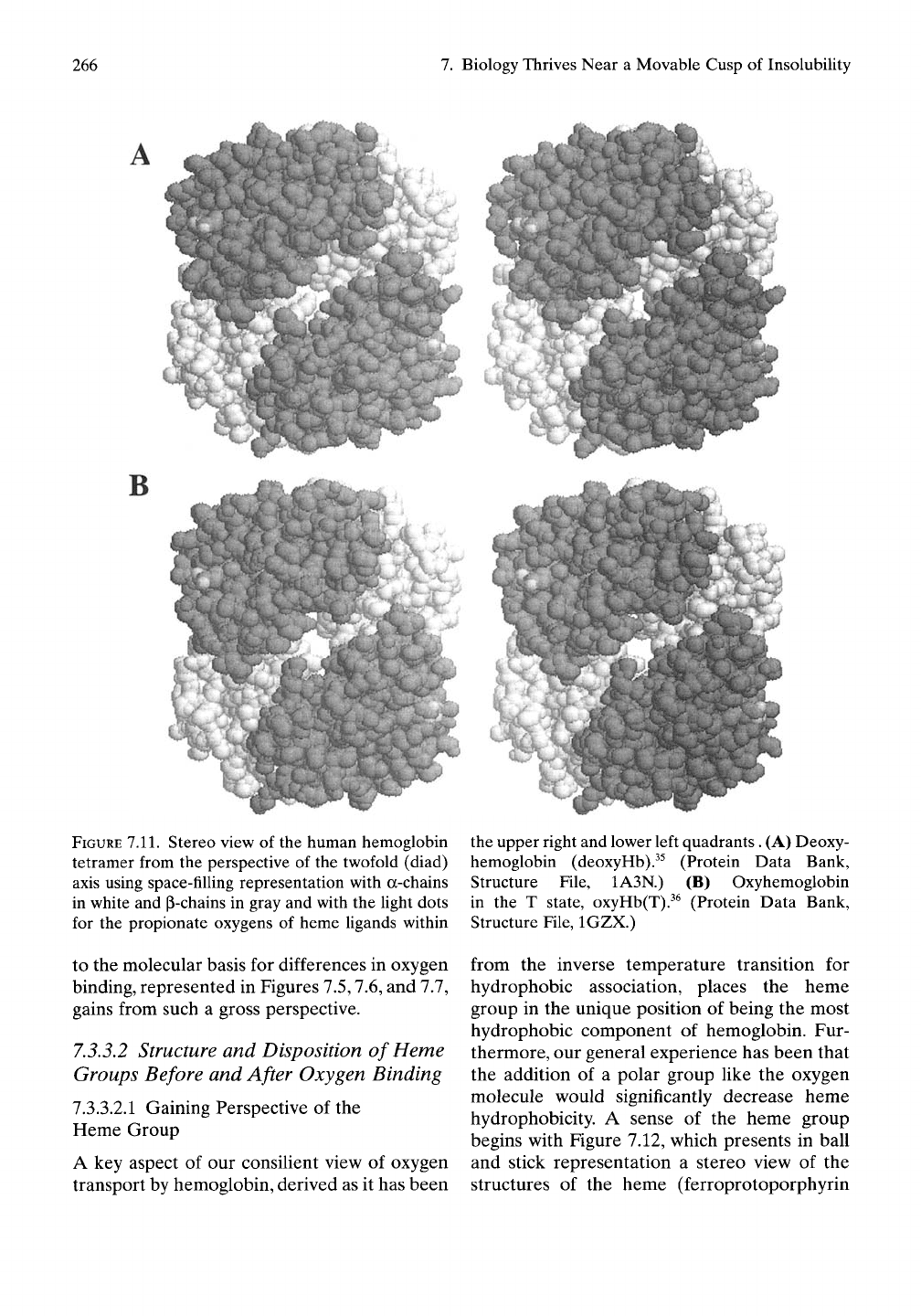
266
7.
Biology Thrives Near a Movable Cusp of Insolubility
B
FIGURE
7.11.
Stereo view of the human hemoglobin
tetramer from the perspective of the twofold (diad)
axis using space-filling representation with a-chains
in white and ^-chains in gray and with the light dots
for the propionate oxygens of heme ligands within
to the molecular basis for differences in oxygen
binding, represented in Figures 7.5,7.6, and 7.7,
gains from such a gross perspective.
7.3.3,2
Structure and Disposition of Heme
Groups Before and After Oxygen Binding
7.3.3.2.1 Gaining Perspective of the
Heme Group
A key aspect of our consilient view^ of oxygen
transport by hemoglobin, derived as it has been
the upper right and lower left quadrants
.
(A) Deoxy-
hemoglobin (deoxyHb).^^ (Protein Data Bank,
Structure File, 1A3N.) (B) Oxyhemoglobin
in the T state, oxyHb(T).^^ (Protein Data Bank,
Structure File, IGZX.)
from the inverse temperature transition for
hydrophobic association, places the heme
group in the unique position of being the most
hydrophobic component of hemoglobin. Fur-
thermore, our general experience has been that
the addition of a polar group like the oxygen
molecule would significantly decrease heme
hydrophobicity. A sense of the heme group
begins with Figure 7.12, which presents in ball
and stick representation a stereo view of the
structures of the heme (ferroprotoporphyrin
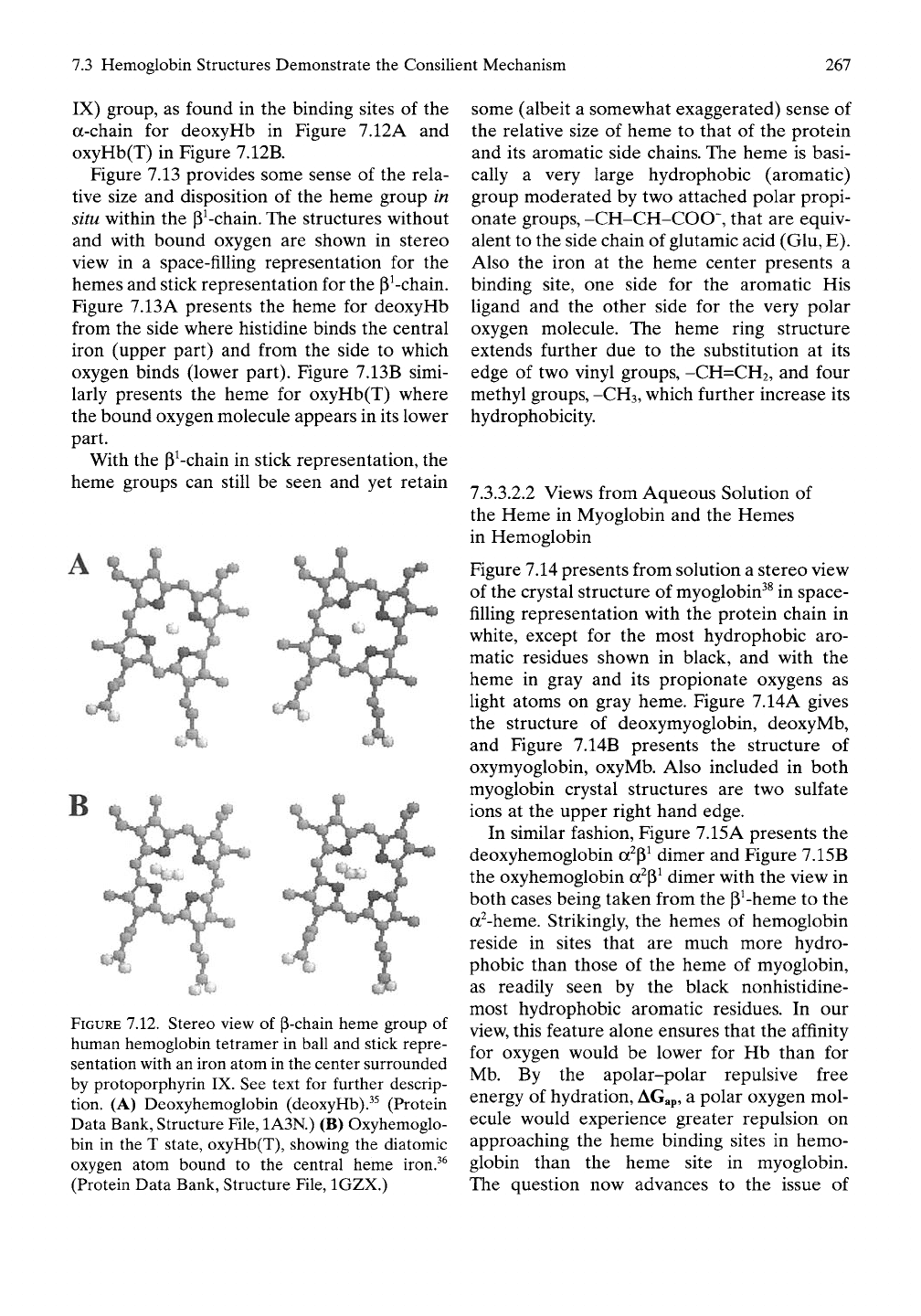
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
267
IX) group, as found in the binding sites of the
a-chain for deoxyHb in Figure 7.12A and
oxyHb(T) in Figure 7.12B.
Figure 7.13 provides some sense of the rela-
tive size and disposition of the heme group in
situ within the p^-chain. The structures without
and with bound oxygen are shown in stereo
view in a space-filling representation for the
hemes and stick representation for the p^-chain.
Figure 7.13A presents the heme for deoxyHb
from the side where histidine binds the central
iron (upper part) and from the side to which
oxygen binds (lower part). Figure 7.13B simi-
larly presents the heme for oxyHb(T) where
the bound oxygen molecule appears in its lower
part.
With the P^-chain in stick representation, the
heme groups can still be seen and yet retain
B
FIGURE 7.12. Stereo view of P-chain heme group of
human hemoglobin tetramer in ball and stick repre-
sentation with an iron atom in the center surrounded
by protoporphyrin IX. See text for further descrip-
tion. (A) Deoxyhemoglobin (deoxyHb).^^ (Protein
Data Bank, Structure File, 1A3N.) (B) Oxyhemoglo-
bin in the T state, oxyHb(T), showing the diatomic
oxygen atom bound to the central heme iron.^^
(Protein Data Bank, Structure File, IGZX.)
some (albeit a somewhat exaggerated) sense of
the relative size of heme to that of the protein
and its aromatic side chains. The heme is basi-
cally a very large hydrophobic (aromatic)
group moderated by two attached polar propi-
onate groups, -CH-CH-COO~, that are equiv-
alent to the side chain of glutamic acid (Glu, E).
Also the iron at the heme center presents a
binding site, one side for the aromatic His
Ugand and the other side for the very polar
oxygen molecule. The heme ring structure
extends further due to the substitution at its
edge of two vinyl groups, -CH=CH2, and four
methyl groups, -CH3, which further increase its
hydrophobicity.
7.3.3.2.2 Views from Aqueous Solution of
the Heme in Myoglobin and the Hemes
in Hemoglobin
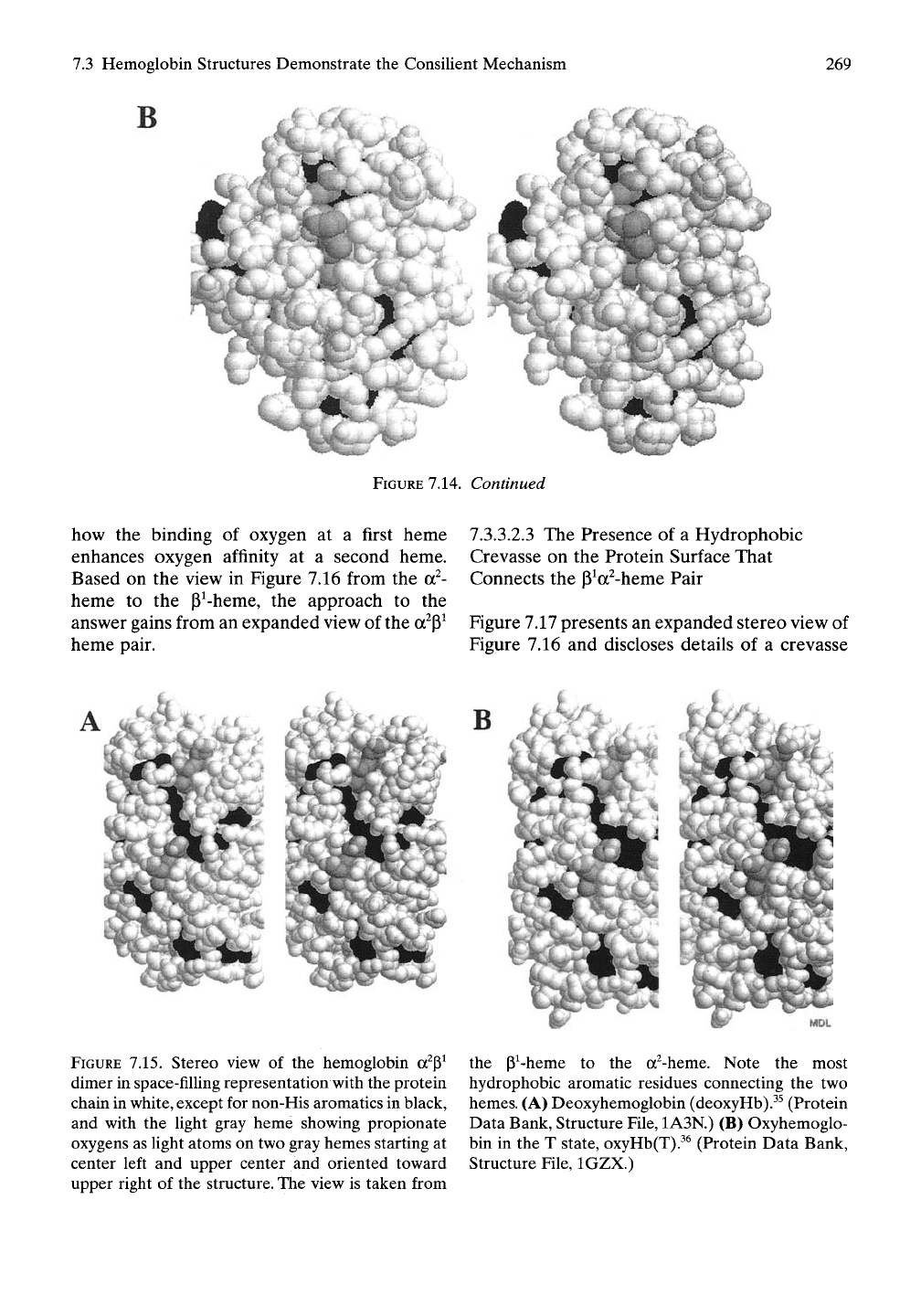
Figure 7.14 presents from solution a stereo view
of the crystal structure of myoglobin^^ in space-
fiUing representation with the protein chain in
white, except for the most hydrophobic aro-
matic residues shown in black, and with the
heme in gray and its propionate oxygens as
light atoms on gray heme. Figure 7.14A gives
the structure of deoxymyoglobin, deoxyMb,
and Figure 7.14B presents the structure of
oxymyoglobin, oxyMb. Also included in both
myoglobin crystal structures are two sulfate
ions at the upper right hand edge.
In similar fashion. Figure 7.15A presents the
deoxyhemoglobin a^p^ dimer and Figure 7.15B
the oxyhemoglobin a^p^ dimer with the view in
both cases being taken from the p^-heme to the
a^-heme. Strikingly, the hemes of hemoglobin
reside in sites that are much more hydro-
phobic than those of the heme of myoglobin,
as readily seen by the black nonhistidine-
most hydrophobic aromatic residues. In our
view, this feature alone ensures that the affinity
for oxygen would be lower for Hb than for
Mb.
By the apolar-polar repulsive free
energy of hydration, AGap, a polar oxygen mol-
ecule would experience greater repulsion on
approaching the heme binding sites in hemo-
globin than the heme site in myoglobin.
The question now advances to the issue of
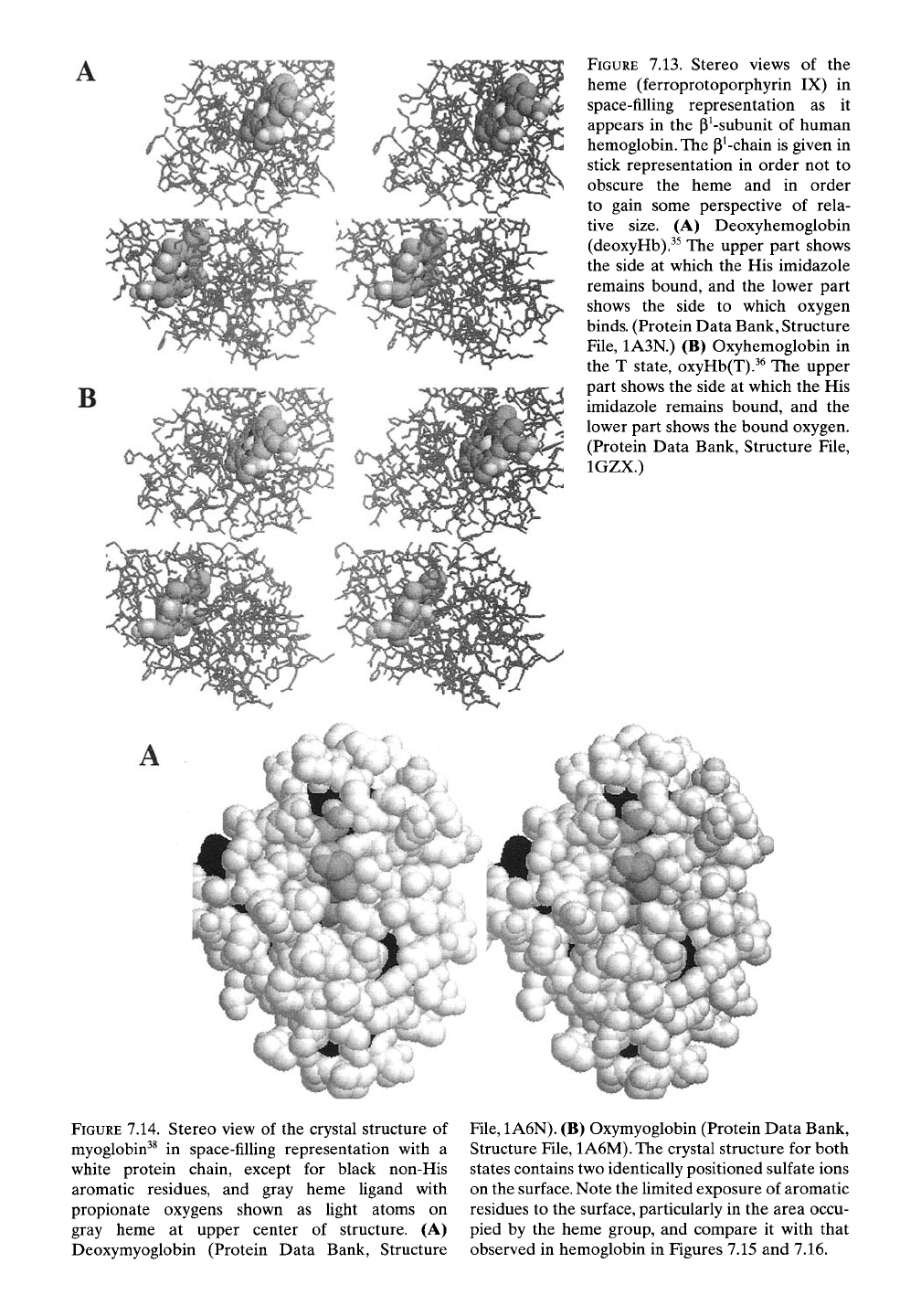
FIGURE
7.13. Stereo views of the
heme (ferroprotoporphyrin IX) in
space-filhng representation as it
appears in the p^-subunit of human
hemoglobin. The P^-chain is given in
stick representation in order not to
obscure the heme and in order
to gain some perspective of rela-
tive size. (A) Deoxyhemoglobin
(deoxyHb).^^ The upper part shows
the side at which the His imidazole
remains bound, and the lower part
shows the side to which oxygen
binds.
(Protein Data Bank, Structure
File,
1A3N.) (B) Oxyhemoglobin in
the T state, oxyHb(T).^^ The upper
part shows the side at which the His
imidazole remains bound, and the
lower part shows the bound oxygen.
(Protein Data Bank, Structure File,
IGZX.)
FIGURE
7.14. Stereo view of the crystal structure of
myoglobin^^ in space-filling representation with a
white protein chain, except for black non-His
aromatic residues, and gray heme ligand with
propionate oxygens shown as Ught atoms on
gray heme at upper center of structure. (A)
Deoxymyoglobin (Protein Data Bank, Structure
File,
1A6N). (B) Oxymyoglobin (Protein Data Bank,
Structure File, lA6M).The crystal structure for both
states contains two identically positioned sulfate ions
on the surface. Note the limited exposure of aromatic
residues to the surface, particularly in the area occu-
pied by the heme group, and compare it with that
observed in hemoglobin in Figures 7.15 and 7.16.
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
269
B
FIGURE
7.14.
Continued
how the binding of oxygen at a first heme
enhances oxygen affinity at a second heme.
Based on the view in Figure 7.16 from the a^-
heme to the p^-heme, the approach to the
answer gains from an expanded view of the a^P^
heme pair.
7.3.3.2.3 The Presence of a Hydrophobic
Crevasse on the Protein Surface That
Connects the P^a^-heme Pair
Figure 7.17 presents an expanded stereo view of
Figure 7.16 and discloses details of a crevasse
B
FIGURE 7.15. Stereo view of the hemoglobin a^p^
dimer in space-filling representation with the protein
chain in
white,
except for non-His aromatics in black,
and with the light gray heme showing propionate
oxygens as light atoms on two gray hemes starting at
center left and upper center and oriented toward
upper right of the structure. The view is taken from
the P^-heme to the a^-heme. Note the most
hydrophobic aromatic residues connecting the two
hemes.
(A) Deoxyhemoglobin (deoxyHb).^^ (Protein
Data Bank, Structure File, 1A3N.) (B) Oxyhemoglo-
bin in the T state, oxyHb(T).^^ (Protein Data Bank,
Structure File, IGZX.)
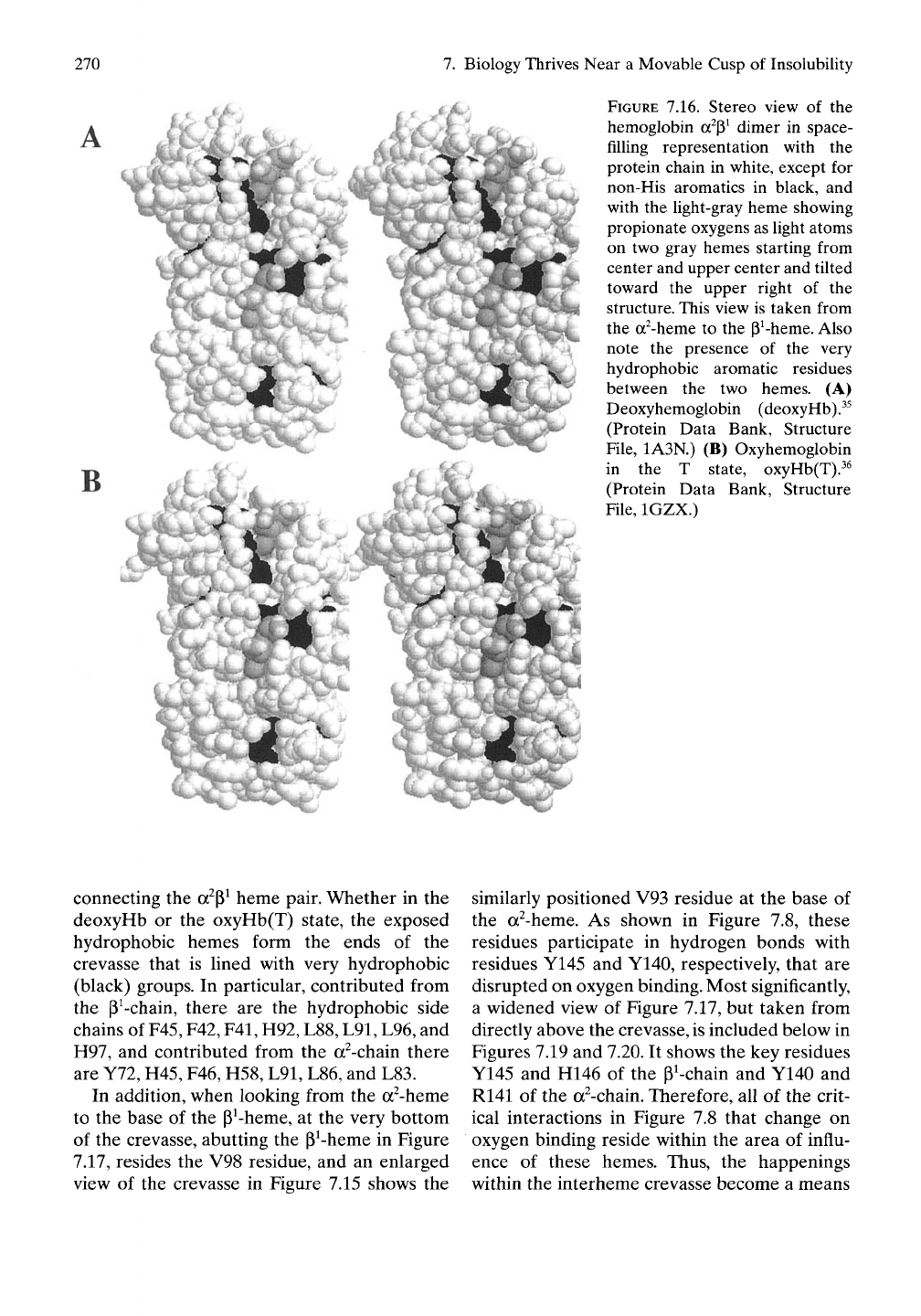
270
7.
Biology Thrives Near a Movable Cusp of Insolubility
B
FIGURE 7.16. Stereo view of the
hemoglobin a^P^ dimer in space-
filling representation with the
protein chain in white, except for
non-His aromatics in black, and
with the hght-gray heme showing
propionate oxygens as light atoms
on two gray hemes starting from
center and upper center and tilted
toward the upper right of the
structure. This view is taken from
the a^-heme to the P^-heme. Also
note the presence of the very
hydrophobic aromatic residues
between the two hemes. (A)
Deoxyhemoglobin (deoxyHb).^^
(Protein Data Bank, Structure
File,
1A3N.) (B) Oxyhemoglobin
in the T state, oxyHb(T).^^
(Protein Data Bank, Structure
File,
IGZX.)
connecting the a^P^ heme pair. Whether in the
deoxyHb or the oxyHb(T) state, the exposed
hydrophobic hemes form the ends of the
crevasse that is lined with very hydrophobic
(black) groups. In particular, contributed from
the P^-chain, there are the hydrophobic side
chains of
F45,
F42,
F41,
H92, L88,
L91,
L96, and
H97,
and contributed from the a^-chain there
are Y72, H45, F46, H58,
L91,
L86, and L83.
In addition, when looking from the a^-heme
to the base of the P^-heme, at the very bottom
of the crevasse, abutting the P^-heme in Figure
7.17, resides the V98 residue, and an enlarged
view of the crevasse in Figure 7.15 shows the
similarly positioned V93 residue at the base of
the a^-heme. As shown in Figure 7.8, these
residues participate in hydrogen bonds with
residues Y145 and Y140, respectively, that are
disrupted on oxygen binding. Most significantly,
a widened view of Figure 7.17, but taken from
directly above the crevasse, is included below in
Figures 7.19 and 7.20. It shows the key residues
Y145 and H146 of the p^-chain and Y140 and
R141 of the a^-chain. Therefore, all of the crit-
ical interactions in Figure 7.8 that change on
oxygen binding reside within the area of influ-
ence of these hemes. Thus, the happenings
within the interheme crevasse become a means

7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
271
of communication not only between the hemes
of the particular crevasse but also provide com-
munication between the a^p^-heme pair and the
a^p^-heme pair.
Returning to the interheme crevasse, the
unique grouping of hydrophobic residues
associated with the a^p^-heme pair and with
the equivalent a^p^-heme pair constitutes two
remarkably hydrophobic domains for exposure
at an aqueous surface. It is true, however, that
much of the hydrophobicity Ues in the depths
of the water-filled crevasse. These are circum-
stances under which the consilient mechanism
dictates an acute competition for hydration
between the hydrophobic residues of each
crevasse and its associated surface charged
groups. Such a competition gives rise to a repul-
sion between hydrophobic and charged groups
as each requires water unperturbed by the
other to lower their free energy. Based on the
studies reviewed in Chapter 5, this, of course, is
what we have called an apolar-polar repulsive
free energy of hydration and have indicated by
AGap. As the hydrophobic groups are held as
a more rigid part of the protein structure, it
would seem to be left to the charged side chains
at the ends of their aUphatic chains with their
greater range of motion to reflect the repulsion.
7.3.3.2.4 The Disposition of Charged Side
Chains Associated with the Crevasse
Connecting the P^- and a^-Heme Groups
for deoxyHb
There are basically two ways in which charged
side chains of amino acid residues can lower
their free energy when confronted with a
hydrophobic domain. One way is to form ion
pairs.
The second way, when possible, is to move
toward locations more remote from the most
hydrophobic surface. Both examples are shown
in the crevasse that connects the
P^
and a^ heme
groups as well as in the equivalent crevasse con-
necting the a^p^-heme groups.
As shown in Figure 7.17A, there are seven
charged residues in the immediate vicinity of
the P^a^ crevasse. Starting at six o'clock and
reading clockwise, they are
K61,
K90, R92, R40,
E43,
K66, and K95. Interestingly, this means six
positively charged and one negatively charged
side chains. However, the two heme groups add
four more negatively charged groups associated
with each crevasse. Those residues in position
for ion pairing are K61, R92, E43, and K66.
Despite literally being bathed in water, ion
pairs form. The positively charged side chains
of K90, R40, and K95 are expected to have
moved to their positions of lowest accessible
free energy, that is, to minimize repulsion
coming from the apolar crevasse. Thus these
charges are expected to be pointing in the
direction of lower hydrophobicity. This finding
indicates that the region of the a-heme is
less hydrophobic than that of the p-heme.
This observation is consistent with a number of
reports that the T state exhibits a fivefold
greater binding at the a-heme than at the
P-heme.^^^2
7.3.3.2.5 The Effect of Oxygen Binding on the
Disposition of Charged Side Chains Proximal
to the Crevasse Connecting the p^- and
a^-Heme Groups
As shown in Figure 7.17B, oxygen binding
effects a remarkable change in the disposition
of the seven charged side chains. The four side
chains positioned for ion paring separate from
their ion paired positions, and the charges of
the three positively charged, non-ion-paired
residues, K90, R40, and K95, reorient their
charges. Yet the forces that effect these changes
cannot have come through the protein struc-
ture.
In our view, the driving force for these
oxygenation-effected side chain reorientations
came through the "waters of Thales" of the
hydrophobic crevasse. We beUeve this demon-
strates that oxygen binding, even though it
occurs on the other side of the hemes, decreases
the hydrophobicity of the hydrophobic crevasse
sufficiently to allow improved hydration of
these charged species. Thus, we arrive at a spe-
cific set of structural changes on oxygenation
consistent with the consilient mechanism.
7.3.4 Consideration of
Communication Between the a^p^-
and a^p^-Heme Pairs
The consilient mechanism provides a means of
communication between the pair of hemes of
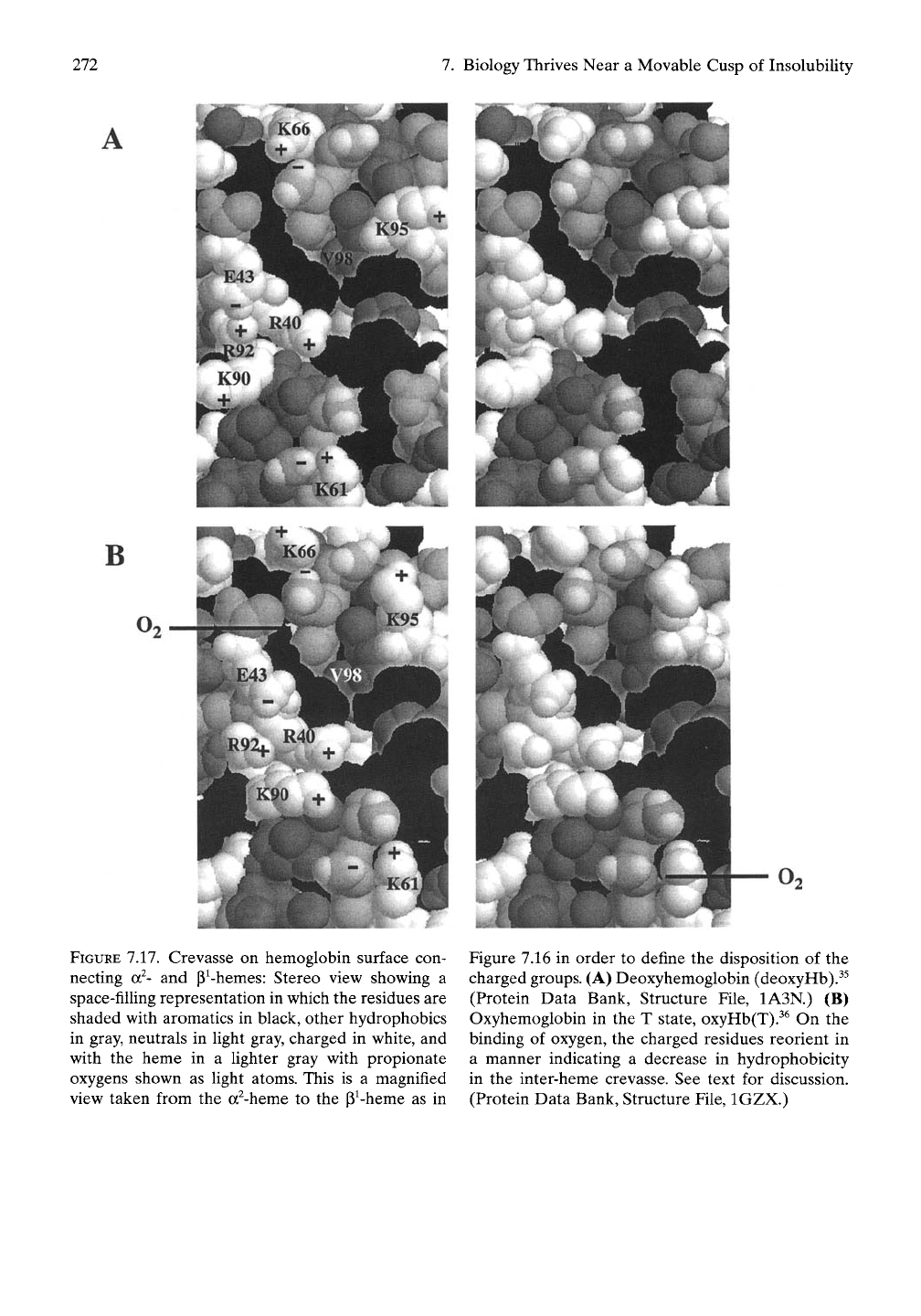
272
7.
Biology Thrives Near a Movable Cusp of Insolubility
B
o.
FIGURE 7.17. Crevasse on hemoglobin surface con-
necting a^- and p^-hemes: Stereo view showing a
space-filling representation in which the residues are
shaded with aromatics in black, other hydrophobics
in gray, neutrals in light gray, charged in white, and
with the heme in a lighter gray with propionate
oxygens shown as Hght atoms. This is a magnified
view taken from the a^-heme to the P^-heme as in
Figure 7.16 in order to define the disposition of the
charged groups. (A) Deoxyhemoglobin (deoxyHb).^^
(Protein Data Bank, Structure File, 1A3N.) (B)
Oxyhemoglobin in the T state, oxyHb(T).^^ On the
binding of oxygen, the charged residues reorient in
a manner indicating a decrease in hydrophobicity
in the inter-heme crevasse. See text for discussion.
(Protein Data Bank, Structure File, IGZX.)
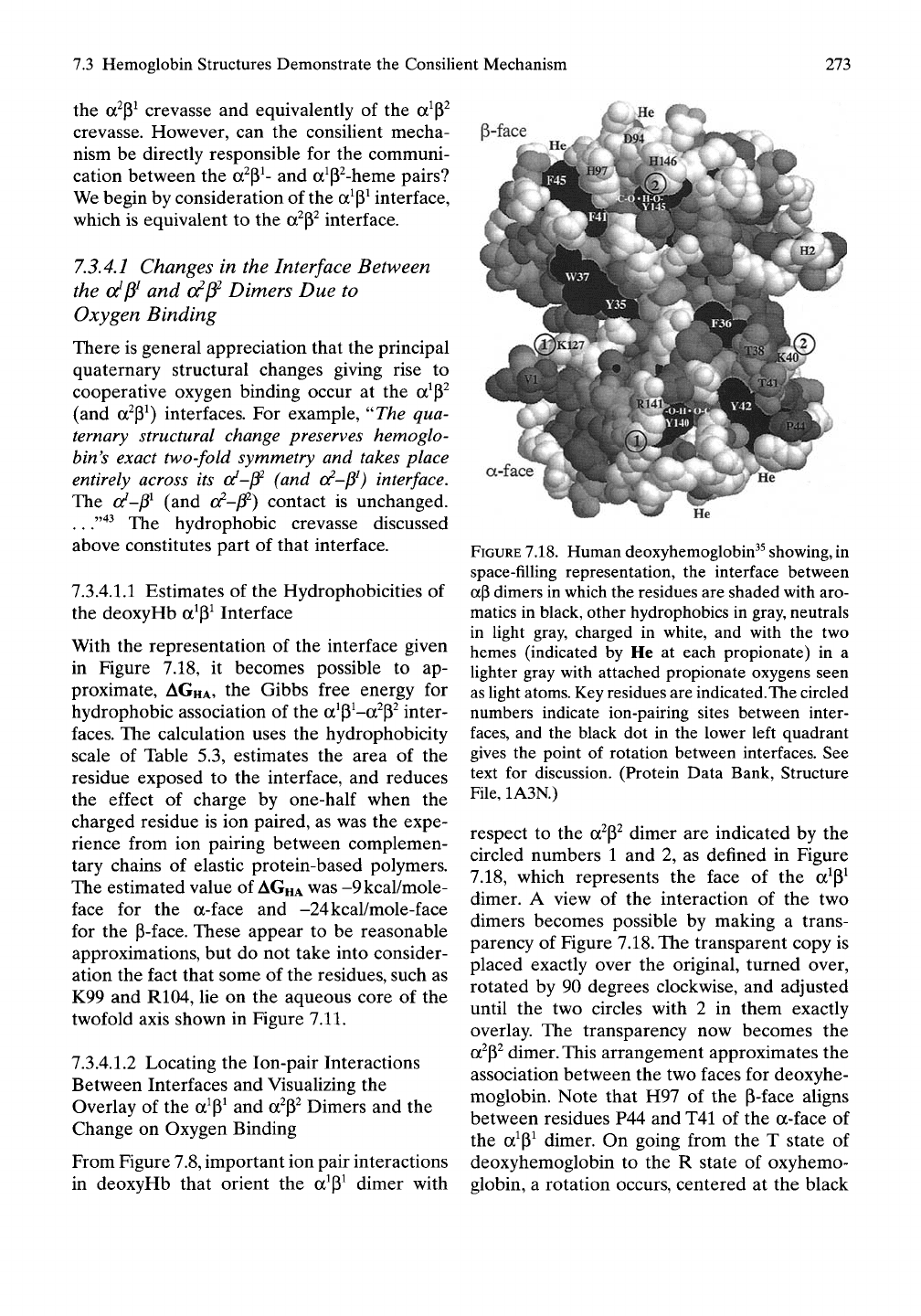
7.3 Hemoglobin Structures Demonstrate the Consilient Mechanism
273
the a^p^ crevasse and equivalently of the a^p^
crevasse. However, can the consilient mecha-
nism be directly responsible for the communi-
cation between the a^p^- and a^p^-heme pairs?
We begin by consideration of the a^P^ interface,
which is equivalent to the a^p^ interface.
7.3.4.1
Changes in the Interface Between
the o^fi^ and df^ Dimers Due to
Oxygen Binding
There is general appreciation that the principal
quaternary structural changes giving rise to
cooperative oxygen binding occur at the a^p^
(and a^p^) interfaces. For example, "The qua-
ternary structural change preserves hemoglo-
bin's exact two-fold symmetry and takes place
entirely across its d-f^ (and o^-pf) interface.
The d-P^ (and o?-/?) contact is unchanged.
.. .'"^^ The hydrophobic crevasse discussed
above constitutes part of that interface.
7.3.4.1.1 Estimates of the Hydrophobicities of
the deoxyHb a^P^ Interface
With the representation of the interface given
in Figure 7.18, it becomes possible to ap-
proximate, AGHA, the Gibbs free energy for
hydrophobic association of the a^p^-a^p^ inter-
faces.
The calculation uses the hydrophobicity
scale of Table 5.3, estimates the area of the
residue exposed to the interface, and reduces
the effect of charge by one-half when the
charged residue is ion paired, as was the expe-
rience from ion pairing between complemen-
tary chains of elastic protein-based polymers.
The estimated value of
AGHA
was -9kcal/mole-
face for the a-face and -24kcal/mole-face
for the P-face. These appear to be reasonable
approximations, but do not take into consider-
ation the fact that some of the residues, such as
K99 and R104, lie on the aqueous core of the
twofold axis shown in Figure 7.11.
7.3.4.1.2 Locating the Ion-pair Interactions
Between Interfaces and Visualizing the
Overlay of the a^p^ and a^p^ Dimers and the
Change on Oxygen Binding
From Figure
7.8,
important ion pair interactions
in deoxyHb that orient the a^p^ dimer with
a-face
FIGURE
7.18.
Human deoxyhemoglobin^^
showing,
in
space-filling representation, the interface between
ap dimers in which the residues are shaded with aro-
matics in black, other hydrophobics in gray, neutrals
in light gray, charged in white, and with the two
hemes (indicated by He at each propionate) in a
lighter gray with attached propionate oxygens seen
as light atoms. Key residues are indicated. The circled
numbers indicate ion-pairing sites between inter-
faces,
and the black dot in the lower left quadrant
gives the point of rotation between interfaces. See
text for discussion. (Protein Data Bank, Structure
File,
1A3N.)
respect to the a^p^ dimer are indicated by the
circled numbers 1 and 2, as defined in Figure
7.18, which represents the face of the a^P^
dimer. A view of the interaction of the two
dimers becomes possible by making a trans-
parency of Figure 7.18. The transparent copy is
placed exactly over the original, turned over,
rotated by 90 degrees clockwise, and adjusted
until the two circles with 2 in them exactly
overlay. The transparency now becomes the
a^P^ dimer. This arrangement approximates the
association between the two faces for deoxyhe-
moglobin. Note that H97 of the p-face aligns
between residues P44 and T41 of the a-face of
the a^p^ dimer. On going from the T state of
deoxyhemoglobin to the R state of oxyhemo-
globin, a rotation occurs, centered at the black

274
7.
Biology Thrives Near a Movable Cusp of Insolubility
dot, such that H97 now aligns between residues
T41 and T38. This rotation, otherwise indicated
in Figure 7.10, represents the quaternary struc-
tural change between the a^p^ and a^(3^ dimers
that results from oxygen binding.
7.3.4.1.3 Visualizing the Relationship
Between the a^p^- and a^P^-heme Pairs
Before and After Oxygen Binding
The He labels on Figure 7.18, located at heme
propionates, indicate the widths of the a^- and
P^-hemes. With the transparency, representing
the a^P^ dimer properly placed over the a^p^
dimer as indicated above, the relationship
between the o?- and P^-hemes is shown at the
upperleft and the relationship between the a^-
and P^-hemes is shown at the lower right. Per-
forming the above indicated rotation increases
the distance between the a^p^-hemes and the
nearly equivalent a^P^-hemes. The relationship
between the a^P^ hemes is shown in Figures
7.15A and 7.16A.
7.3.4.2
Looking Directly into the o^pf
(of^) Interheme Crevasse also to See the
Classic Intersubunit Interactions That
Change on Oxygen Binding
Having gained a sense of the three-dimensional
relationship between the hemes and realizing
that the quaternary structural changes are
principally due to disruption of the ion pairs
involving the H146 and R141 C-terminal
carboxylates, we now look for the relationship
of these functional groups to the a^P^ (oc^P^)
interheme hydrophobic crevasse. For that
purpose. Figure 7.19 provides a look directly
into the a^p^ crevasse for the full width of that
interfacial interaction with a few of the critical
residues labeled.
7.3.4.2.1 Are the Critical Ion Pairs for
Quaternary Structural Change Within Reach
of the Heme-based Consilient Mechanism?
Importantly, the H146-K40 ion pair that oc-
curs between the a^p^ interface, located by the
circled '2's in Figure 7.18, is readily seen in the
expanded view of the a^P^ interheme crevasse
of Figure 7.19A. Also, the C-terminal carboxy-
late of the o? R141 is visible that contributes its
negative charge to the ion pair with a^ K127
(the circled 'I's interactions in Figure 7.18). The
question now becomes, can oxygenation of
a heme in the a^P^ (or the a^P^) interheme
hydrophobic crevasse weaken these ion pairs
that are thought to trigger the quaternary
structural changes considered responsible for
the interaction between a^P^- and a^P^-heme
pairs? To do so by the consilient mechanism
would seem to require intervening "waters of
Thales."
7.3.4.2.2 Demonstrating the Presence of the
"Waters of Thales"
Figure 7.19B shows the observed "waters of
Thales" when the chains are given in space-
filling representation. The observed water mol-
ecules in a crystallographic structure represent
a minimal number of water molecules actually
present, as many more waters are too mobile to
be positioned by X-ray diffraction. Conversion
of the chains in Figure 7.19 to the stick repre-
sentation in Figure 7.20 allows delineation of
the unseen residues in Figure 7.19. Further-
more, the stick representation allows for
three-dimensional visualization of all of the
dif-
fraction-detected waters in this region. From
Figure 7.20B with the detected water mole-
cules,
it is not unthinkable that the consilient
mechanism might play a role in the relaxation
of these critical ion pairs and thereby con-
tribute to the communication between a^P^-
and a^P^-heme pairs that facilitates oxygen
binding. After all, formation of oxyhemoglobin
occurs with the uptake of water, in our view, as
ion pairs and hydrophobic regions dissociate.
Figure 7.21 gives an expanded view of the part
of the p^-chain that undergoes disruption of
ion pairs and hydrogen bond. Figure 7.21 can
also facilitate identification of residues in
Figure 7.20 without the distraction of so many
labels.
