Tietenberg Tom, Lewis Lynne. Environmental & Natural Resource Economics
Подождите немного. Документ загружается.

511Introduction
Number of Substances. Of the tens of millions of known chemical compounds,
approximately 100,000 are actively used in commerce. Many exhibit little or no
toxicity, and even a very toxic substance represents little risk as long as it is isolated.
The trick is to identify problem substances and to design appropriate policies as
responses. The massive number of substances involved makes that a difficult
assignment.
Latency. The period of latency exhibited by many of these relationships
compounds the problem. Two kinds of toxicity are exhibited: acute and chronic.
Acute toxicity is present when a short-term exposure to the substance produces a
detrimental effect on the exposed organisms. Chronic toxicity is present when the
detrimental effect arises from exposure of a continued or prolonged nature.
The process of screening chemicals as potentially serious causes of chronic
illness is even more complicated than that of screening for acute illness. The
traditional technique for determining acute toxicity is the lethal-dose deter-
mination, a relatively quick test performed on animals that calculates the dose that
results in the death of 50 percent of the animal population. This test is less well
suited for screening substances that exhibit chronic toxicity.
The appropriate tests for discovering chronic toxicity typically have involved
subjecting animal populations to sustained low-level doses of the substance over an
extended period of time. These tests are very expensive and time-consuming. If the
EPA were to do the tests, given its limited resources, it could only test a few of
the estimated 700 new chemicals introduced each year. If the industries were to do
the tests, the expense could preclude the introduction of many potentially valuable
new chemicals that have limited, specialized markets.
Uncertainty. Another dilemma inhibiting policy makers is the uncertainty
surrounding the scientific evidence on which regulation is based. Effects uncovered
by laboratory studies on animals are not perfectly correlated with effects on
humans. Large doses administered over a three-year period may not produce
the same effects as an equivalent amount spread over a 20-year period. Some of
the effects are synergistic—that is, their effects are compounded by other variable
factors. They are either more serious or less serious in the presence of other
substances or conditions than they would be in the absence of those substances or
conditions. (Asbestos workers are 30 times more likely than their nonsmoking
fellow workers to get lung cancer if they smoke, for example). Once cancer is
detected, in most cases it does not bear the imprint of a particular source.
Policy makers have to act in the face of limited information (See Example 19.1).
From an economic point of view, how the policy process reacts to this dilemma
should depend on how well the market handles toxic substance problems. To
the extent that the market generates the correct information and provides the
appropriate incentives, policy may not be needed. On the other hand, when the
government can best generate information or create the appropriate incentive,
intervention may be called for. As the following sections demonstrate, the nature
and the form of the most appropriate policy response may depend crucially on how
the toxic source and the affected party or parties are related.

512 Chapter 19 Toxic Substances and Environmental Justice
The Arduous Path to Managing Risk: Bisphenol A
One example of a potentially toxic substance that is working its way through the
government regulatory bureaucracy is Bisphenol A (BPA). The food industry uses
more than six billion pounds of BPA every year to make the resins that line food
cans and the polycarbonate plastics used to make baby bottles and many
other products. The Centers for Disease Control and Prevention (CDC) says that
95 percent of us carry measurable amounts of BPA in our blood.
In April 2008, the National Toxicology Program (NTP) at the National Institutes
of Health (NIH) expressed some concern that exposure to BPA during pregnancy
and childhood could impact the developing breast and prostate, hasten puberty,
and affect behavior in American children. Not long after those concerns were
expressed, the Canadian government moved to ban polycarbonate infant bottles
containing BPA, the most popular type of bottle on the market.
Despite the absence of any such ruling from the U.S. government, after the
Canadian move the U.S. market reacted. Major BPA manufacturers, including Playtex
(which makes bottles and cups) and Nalgene, which makes portable water bottles,
announced a shift to BPA-free products. Major retailers, including Walmart and Toys
“R” Us, announced they would quickly phase out BPA-containing baby bottles.
In January 2010, the U.S. Food and Drug Administration (FDA), which had
previously found BPA to be safe, announced, “On the basis of results from recent
studies using novel approaches to test for subtle effects, both the National
Toxicology Program at the National Institutes of Health and FDA have some con-
cern about the potential effects of BPA on the brain, behavior, and prostate gland
in fetuses, infants, and young children. In cooperation with the National Toxicology
Program, FDA’s National Center for Toxicological Research is carrying out in-depth
studies to answer key questions and clarify uncertainties about the risks of BPA.”
Interestingly, while the federal government continued to study the problem,
some states moved ahead with regulation. In April 2010, Maryland became the
fifth state to ban the use of BPA in children’s products, including baby bottles and
sippy cups. New York followed suit in July passing a bill that had unanimous
support in the legislature.
How this risk was handled in the United States is especially noteworthy in that
both the market and the states reacted well before federal regulation was in place.
Sources
: The National Institutes of Health web site: http://www.niehs.nih.gov/news/media/questions/
sya-bpa.cfm (accessed November 21, 2010); Food and Drug Administration web site: http://www.fda.
gov/newsevents/publichealthfocus/ucm064437.htm (accessed November, 21, 2010); Environmental
Working Group web site: http://www.ewg.org/reports/infantformula (accessed November, 21, 2010);
American Chemical Society web site: http://pubs.acs.org/cen/news/88/i04/8804notw2.html (accessed
November 21, 2010)
EXAMPLE
19.1
Market Allocations and Toxic Substances
Toxic substance contamination can arise in a variety of settings. In order to define
the efficient policy response, we must examine what responses would be forthcom-
ing in the normal operation of the market. Let’s look at three possible relationships
513Market Allocations and Toxic Substances
between the source of the contamination and the victim: employer–employee,
producer–consumer, and producer–third party. The first two involve normal
contractual relations among the parties, while the latter involves noncontracting
parties, whose connection is defined solely by the contamination.
Occupational Hazards
Many occupations involve risk, including, for some people, exposure to toxic
substances. Do employers and employees have sufficient incentives to act in
concert toward achieving safety in the workplace?
The caricature of the market used by the most ardent proponents of regulation
suggests not. In this view, the employer’s desire to maximize profits precludes
spending enough money on safety. Sick workers can simply be replaced. Therefore,
the workers are powerless to do anything about it; if they complain, they are fired
and replaced with others who are less vocal.
The most ardent opponents of regulation respond that this caricature overlooks
or purposefully ignores significant market pressures, such as employee incentives
and the feedback effects of those incentives on employers. When the full story that
includes these pressures is considered, regulation may be unnecessary or even
counterproductive.
According to this market incentives worldview, employees will only accept work
in a potentially hazardous environment if appropriately compensated for taking
that risk. Riskier occupations should call forth higher wages. The increase in wages
should be sufficient to compensate them for the increased risk; otherwise they will
work elsewhere. These higher wages represent a real cost of the hazardous
situation to the employer. They also produce an incentive to create a safer work
environment, since greater safety would result in a lower risk premium and, hence,
lower wages. One cost could be balanced against the other. What was spent on
safety could be recovered in lower wages (see Figure 19.1).
The first type of cost, the marginal increase in wages, is drawn to reflect the fact
that the lower the level of precaution, the higher the wage bill. Two such curves are
drawn to reflect high-exposure and low-exposure situations. The high-exposure
case assumes larger numbers of workers are exposed than in the low-exposure case.
The low-exposure cost curve rises more slowly because the situation is less
dangerous at the margin.
The second type of curve, the marginal cost of providing precaution, reflects an
increasing marginal cost. The two different curves depict different production
situations. A firm with a few expensive precautionary options will face a steeply
sloped marginal cost curve, while a firm with many cheaper options will face a
lower marginal cost at every comparable degree of precaution chosen.
The graph depicts four possible outcomes—one for each possible combination
of these four marginal cost curves. Note that very different choices will be made,
depending on the circumstances. Also note that the level of risk chosen (as
indicated by the marginal damage, labeled MD) and the degree of precaution are
not perfectly correlated. The highest marginal risk is MD
2
, but the associated level
of precaution (Q
2
) is not the largest. The reason, of course, is that the cost of taking
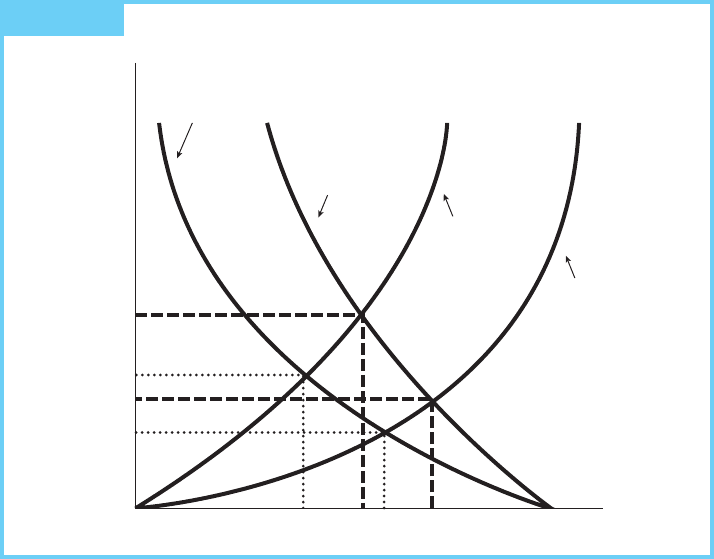
514 Chapter 19 Toxic Substances and Environmental Justice
Marginal Cost
(dollars
per unit)
Degree of
Precaution
(units)
Marginal Cost
of Precaution
(Many Options)
Marginal Cost
of Precaution
(Few Options)
Q
2
Q
1
MD
2
MD
1
MD
4
MD
3
Q
3
Q
4
Marginal Cost of
Increased Wages
(High Exposure)
Marginal Cost of
Increased Wages
(Low Exposure)
FIGURE 19.1 Market Provision of Occupational Safety
precautions matters, and sometimes it is cheaper to accept the risk and compensate
for it than it is to prevent it.
Because the marginal increased wages curve accurately reflects marginal
damages (since the higher wages are demanded by workers to compensate them
for damages), these market equilibria are also efficient. Thus, the efficient
resolution of the occupational hazards problem varies not only from substance
to substance, but also from plant to plant. As long as this stylized view of the
world is correct, the market will tailor the appropriate degree of precaution to
the situation.
Proponents of this view point out that this allocation would also allow more
choices for workers than would, for example, a system requiring all workplaces to
be equally safe. With varying occupational risk, those occupations with more risk
(such as working to clean up toxic spills) would attract people who were less
averse to risk. These workers would receive higher-than-average wages (to
compensate them for the increased risk), but paying these higher wages would be
cheaper to the firm (and hence, consumers) than requiring every workplace to
meet the same risk standard. The risk-averse workers would be free to choose less
risky occupations.
Do wages actually reflect risk? Existing empirical studies make clear that wages in
risky occupations do contain a risk premium (Viscusi and Aldy, 2003). Two conclu-
sions about these risk premiums seem clear from these studies: (1) the willingness

515Market Allocations and Toxic Substances
to pay for apparently similar risk reductions varies significantly across individuals;
and (2) the revealed willingness to pay for risk reduction is substantial.
In those cases where wages accurately reflect risk is there any appropriate role
for the government in controlling contamination in the workplace? Perhaps. The
efficient solution may not always be considered the most ethical solution, a point
that has been addressed in the courts. For example, if the employee is a pregnant
woman and the occupational hazard involves potential damage to the fetus, does
the expectant mother have the right to risk the unborn child, or is some added
protection for the fetus needed? Furthermore, if the lowest-cost solution is to ban
pregnant, or even fertile, women from a workplace that poses a risk to a fetus, is
that an acceptable solution, or is it unfair discrimination against women? As
Example 19.2 suggests, these are not idle concerns.
Ethical concerns are not the only challenges for market solutions. Wages may
not reflect the actual risk. The ability of the worker to respond to a hazardous
situation depends on his or her knowledge of the seriousness of the danger. With
EXAMPLE
19.2
Susceptible Populations in the Hazardous
Workplace
Some employees are especially susceptible to occupational hazards. Pregnant
women and women in the childbearing years are particularly vulnerable. When an
employer attempts to manage a work situation that poses a hazardous threat,
either the susceptible population can be separated from the hazard or the hazard
can be controlled to a sufficient level that its risk is acceptable to even the most
susceptible employees.
The economic aspects of this choice are easily deduced from Figure 19.1.
Suppose that the firm has few control options and is on the uppermost of the two
marginal cost of precaution curves. By removing the susceptible population, it
could face the low-exposure curve. Removal of the susceptible population results
in lower marginal risk to the workers, lower costs to the firm, and less precaution
taken. But is it fair to those who are removed from their jobs?
This issue came to a head in 1978 when American Cyanamid decided to
respond to an occupational risk by banning all fertile women from jobs in the
section manufacturing lead chromate pigment at Willow Island, West Virginia.
After reviewing the decision, the Occupational Safety and Health Administration
(OSHA) cited the company under the general duty clause of the Occupational
Safety and Health Act, which requires an employer to provide a workplace free of
hazards, and fined it $10,000. That was not the last of it. In early 1980, the Oil,
Chemical, and Atomic Workers Union sued the company under the 1964 Civil
Rights Act on the grounds that the company had discriminated unfairly against
women. In March 1991, the Supreme Court ruled that banning fertile women from
any workplace posing a risk to a fetus was not an acceptable way to control risk.
The hazards must be reduced.
Source
:
International Union v. Johnson Controls,
499 U.S. 187 (1991).

516 Chapter 19 Toxic Substances and Environmental Justice
toxic substances, that knowledge is likely to be incomplete. Consequently, the
marginal increased wages function may be artificially rotated toward the origin. In
this case the employer would choose too little precaution. By having access to the
health records of all employees, the employer may be in the best position to assess
the degree of risk posed, but the employer also has an incentive to suppress that
information since publicizing the risk would mean demands for higher compen-
satory wages and possible lawsuits.
Information on the dangers posed by exposure to a particular toxic substance is
a public good to employees; each employee has an incentive to be a free rider on
the discoveries of others. Individual employees do not have an incentive to bear the
cost of doing the necessary research to uncover the degree of risk. Thus, it seems
neither employers nor employees can be expected to produce the efficient amount
of information on the magnitude of risk.
1
As a result, the government may play a substantial role in setting the bound-
aries on ethical responses, in stimulating research on the nature of hazards, and
in providing for the dissemination of information to affected parties. It does
not necessarily follow, however, that the government should be responsible for
determining the level of safety in the workplace once this information is
available and the ethical boundaries are determined. For situations that are
sufficiently dangerous that no rational worker would voluntarily choose to
work there, the role of the government would be to set and enforce a safety
threshold.
Our analysis suggesting that the market will not provide an efficient level of in-
formation on occupational risk is consistent with the enactment of “right-to-know”
laws in several states. These laws require businesses to disclose to their employees
and to the public any potential health hazards associated with toxic substances
used on the job. Generally employers are required to (1) label toxic substance
containers, (2) inventory all toxic substances used in the workplace, and (3) provide
adequate training on the handling of these substances to all affected employees.
Significantly, proponents of these laws suggest that the targets are not the large
chemical companies, which generally have excellent disclosure programs, but the
smaller, largely nonunion plants.
Product Safety
Exposure to a hazardous or potentially hazardous substance can also occur as a
result of using a product, as when eating food containing chemical additives. Does
the market efficiently supply safe products?
One view holds that the market pressures on both parties (consumers and
producers) are sufficient to yield an efficient level of safety. Safer products are
generally more expensive to produce and carry a higher price tag. If consumers feel
1
Unions would be expected to produce more efficient information flows since they represent many
workers and can take advantage of economies of scale in the collection, interpretation, and dissemi-
nation of risk information. Available evidence suggests that the preponderance of wage premiums for
risk has been derived from unionized workers.

517Market Allocations and Toxic Substances
2
A classic example is provided by the manner in which Americans choose their automobiles. It is quite
clear that some larger cars are safer and more expensive than smaller, cheaper ones, at least to their
owners. Some consumers are willing to pay for this additional level of safety, and others are not.
that the additional safety justifies the cost, they will purchase the safer product.
Otherwise they won’t. Producers supplying excessively risky products will find
their market drying up, because consumers will switch to competing brands that
are safer, despite their higher price. Similarly, producers selling excessively safe
products (meaning they eliminate, at great cost, risks consumers are perfectly
willing to take in return for a lower purchase price) find their markets drying up as
well. Consumers will choose the cheaper, riskier product.
This theory also suggests that the market will not (and should not) yield a
uniform level of safety for all products. Different consumers will have different
degrees of risk aversion. While some consumers might purchase riskier, but
cheaper, products, others might prefer safer, but more expensive, products.
2
Thus, it would be common to find products with various safety levels supplied
simultaneously, reflecting and satisfying different consumer preferences for risk.
Forcing all similar products to conform to a single level of risk would not be efficient.
Uniform product safety is no more efficient than uniform occupational safety.
If this view of the market were completely accurate, government intervention to
protect consumers would not be necessary to ensure the efficient level of risk. By
the force of their collective buying habits, consumers would protect themselves.
The problem with the market’s ability to provide such self-regulation is the
availability of information on product safety. The consumer acquires his or her
information about a product generally from personal experience, but also from
labels and warnings. With toxic substances the latency period may be so long as to
preclude any effective market reaction. Even when some damage results, it is
difficult for the consumer to associate it with a particular source. While an
examination of the relationships between purchasing patterns of a large number of
consumers and their subsequent health might well reveal some suggestive
correlations, it would be difficult for any individual consumer to deduce this
correlation. Furthermore, it may be that the risk is so large that no knowledgeable
consumer would accept that risk so that banning the product is the appropriate
remedy. (Note that banning was the choice of several states in managing the risk
from BPA, as described in Example 19.1.)
In situations where adequate information is available on the risks, consumers
should have a substantial role in choosing the acceptable level of risk through their
purchases, but varying levels of access to information can make this problematic.
(Recall Debate 18.1 on fish consumption advisories.)
Third Parties
The final case involves third parties, victims who have no contractual relationship to
the source. Oil spills are one example. Another occurs when groundwater is
contaminated by a neighboring wastewater treatment facility, by surreptitious

518 Chapter 19 Toxic Substances and Environmental Justice
dumping of toxic wastes, or by the improper applications of a pesticide. In both of
these examples the victims are third parties. In any of these situations, the affected
party cannot bring any direct market pressure to bear on the source so the case for
additional government intervention is strongest for third-party situations.
This does not necessarily imply, however, that executive or legislative remedies
are appropriate. The most appropriate response may come from simply requiring
better information on the risk or from using the judicial system to impose liability.
Liability law provides one judicial avenue for internalizing the external costs in
third-party situations. If the court finds (1) that damage occurred; (2) that it was
caused by a toxic substance; and (3) that a particular source was responsible for the
presence of the substance, the source can be forced to compensate the victim
for the damages caused. Unlike regulations that are uniformly (and, hence,
inefficiently) applied, a court decision can be tailored to the exact circumstances
involved in the suit. Furthermore, the impact of any particular liability assignment
can go well beyond the parties to that case. A decision for one plaintiff can remind
other sources that they should take the efficient level of precaution now to avoid
paying damages later.
In principle, liability law can force potential sources of toxic discharges, including
nonpoint sources, to choose efficient levels of precaution. Unlike regulation, liabi-
lity law can provide compensation to the victims. How well it functions in practice
will be clarified in later sections of this chapter.
The Incidence of Hazardous Waste
Siting Decisions
Another element of managing risks involves dealing with the fairness of third-party
situations that arise when hazardous waste facilities are being sited. Are market
siting decisions likely to be both efficient and fair?
History
In 1979, Robert Bullard, then a sociologist at Texas Southern University,
completed a report describing a futile attempt by an affluent African American
neighborhood in Houston, Texas, to block the location of a hazardous waste site
within their community. His analysis suggested that race, not just income status,
was a probable factor in this local land use decision.
Environmental justice, as revealed though the siting of hazardous waste plants,
became a national issue in 1982 when some 500 demonstrators protested against
the location of a proposed PCB landfill in a predominantly low-income community
in North Carolina. On returning from the protests, Walter Fauntroy, the District
of Columbia congressional delegate, asked the General Accounting Office (GAO)
to study the characteristics of hazardous waste sites in the EPA’s Region 4 (Georgia,
Florida, Mississippi, Alabama, Kentucky, Tennessee, North Carolina, and South
Carolina). The 1983 study found that three out of four commercial hazardous
519The Incidence of Hazardous Waste Siting Decisions
waste facilities were in predominantly African American communities, and the
fourth was in a low-income community.
In 1987, the United Church of Christ Commission for Racial Justice examined
the issue of hazardous waste siting for the nation as a whole. According to their
statistical analysis of communities with commercial hazardous waste facilities, they
determined the following:
●
“Race proved to be the most significant among the variables tested . . . ”
●
“Communities with the greatest number of commercial hazardous waste
facilities had the highest composition of racial and ethnic residents. In
communities with two or more facilities or one of the nation’s five largest
landfills, the minority population was more than three times that of
communities without such facilities (38 percent vs. 12 percent).”
●
In communities with uncontrolled toxic waste sites, they found the following:
●
“Three out of every five Black and Hispanic Americans lived in communities
with uncontrolled toxic waste sites.
●
Approximately half of all Asian/Pacific Islanders and American Indians lived
in communities with uncontrolled toxic waste sites.”
In 1994, the Center for Policy Alternatives issued “Toxic Wastes and Race
Revisited: An Update of the 1987 Report.” That study found that commercial toxic
waste facilities were even more likely to be located in minority communities at that
time than in 1980, despite growing national attention to the issue.
Not all studies have reached this conclusion, but in a detailed review of the
literature, Hamilton (2003) finds that for most U.S. studies, low-income and
minority residents do indeed face higher risks from hazardous waste facilities. Less
detailed information exists on the exposure of these populations to hazardous waste
risks in other industrialized countries.
Recent Environmental Justice Research
and the Emerging Role of Analysis Using GIS
The application of geographic information systems (GIS) technology has allowed
studies of the distributional inequities with respect to either pollution or hazardous
waste site location to become more sophisticated. GIS technology also allows
analyses to be conducted at the facility level, the city level, or another geographical
area. Most regional offices of the EPA, for example, now use demographic data from
the U.S. Census Bureau, combined with GIS mapping. This technique allows for
the overlay of census data onto concentric rings around a hazardous waste facility or
a Superfund site, for example, in order to discover who lives in close proximity to
the site. The distribution of risks can also be mapped with assumptions about the
radius of the externalities around a facility and data from epidemiological studies
(Hamilton, 2006). What have these most recent studies found?
The results from these studies are quite varied. Using only one measure of
equity, such as low income, could prove misleading. Hamilton and Viscusi (1999),
520 Chapter 19 Toxic Substances and Environmental Justice
for example, consider multiple measures of equity, including racial distribution,
mean household income, and potential cancer risks, and their work demonstrates
how sensitive the results are to the specific measure that is used.
Other studies have utilized the EPA’s Toxic Release Inventory (TRI) data. This data
set contains self-reported information on toxic releases from all reporting plants. Using
an air pollution index by zip code, Brooks and Sethi (1997) find that demographic
groups most likely to face the threat of exposure to toxic air emissions include mino-
rities, renters, people with incomes below the poverty line, and individuals with fewer
years of schooling. Similar results were found by Sadd et al. (1999) for metropolitan
Los Angeles. Using both GIS and Census Tract data, they find that census tracts with
an emitting facility in the data set had higher percentages of minorities, including
Latino residents, lower incomes (both per capita and household), higher percentages of
industrial land, lower property values, and higher percentages of persons employed in
manufacturing. Similar results were found for Hillsborough County in Florida
(Chakraborty, 2001). Studies have also found significant negative effects of pollution on
house values and incomes for New England states (Example 19.3).
What explains these findings? What do these findings imply for policy?
The Economics of Site Location
One point of departure is to attempt to understand the dynamics of site location and
how both income and race might play a role. Our analysis begins by recognizing
that hazardous waste facilities are generally unpopular neighbors. Even if the
Do New Polluting Facilities Affect Housing Values
and Incomes? Evidence in New England
Using census data for New England for 1980 and 1990 and adding Toxics Release
Inventory (TRI) data for manufacturing firms that began operations during that
period, Hanna (2007) explores the effect of polluting facilities on the surrounding
neighborhoods. The study looks specifically at how prices, wages, pollution, and
incomes vary among census tracts in the New England states.
TRI data have only been collected since 1987, so Hanna uses data on new
plants in order to measure how pollution changed over the 1980s. Hanna created
an index of pollution exposure that is a weighted sum of the distance between the
census tract and the pollution source times the TRI-reported releases for that
pollution source. Some 167 sites were in the TRI data for this time period in
New England. Ten percent of the new plant emissions were of dichloromethane,
an airborne contaminant classified as a probable human carcinogen. Significant
negative effects of pollution on house values and incomes were found. Their
estimates suggest that a house located one mile closer to a polluting manu-
facturing plant reduces its value by 1.9 percent.
Source
: Brid Gleeson Hanna. “House Values, Incomes, and Industrial Pollution,”
Journal of Environmental
Economics and Management
Vol. 54 (2007): 100–112.
EXAMPLE
19.3
