Szilas A.P. Production and transport of oil and gas, Gathering and Transportation
Подождите немного. Документ загружается.

50
6.
GATHERING AND SEPARATION
OF
OIL
AND GAS
The mole number of gas discharged from the second separator is
nv2=zv2nLl=Zv2zLl"l.
The mole number
of
the hydrocarbon vapour discharged from the third separator
(or simply evaporating from it,
if
the tank is open) is
nv3
=Zv3nL2=Zv3ZLlZL2nl
'
In three-stage separation, the gas-oil ratio is generally the volume ratio of the gas
discharged from the first two stages to the liquid collecting in the third stage (stock-
tank oil), that is,
where
Vmo,
is the molar volume of the gas molecules in the standard state, in
m3/kmoles;
M,,
is the molar mass of the liquid collecting in the third stage, in
kg/kmoles; and
pL3
is the density of the liquid collecting in the third stage, in kg/m3.
Dividing both the numerator and denominator of the above formula by
n,
,
we get
and for two-stage separation,
6.4
-
9
The practical usefulness of equilibrium calculations depends first and foremost on
the accuracy
of
the equilibrium ratios
Ki.
The equilibrium ratio of the ith
component depends, in addition to separator temperature and pressure, also on the
composition
of
the well fluid, and accurate values may only be expected from the
laboratory testing of the fluid to
be
treated. The values
of
Ki
may, however, be
determined in a fair approximation also by various methods based on auxiliary
diagrams. The processes in current use relate the composition of the system to
convergence pressure. It is assumed that
if
two systems
of
hydrocarbons agree as to
convergence pressure, then the equilibrium ratios
of
their components will likewise
be equal at the given pressure and temperature. Hence, in order to find
Ki,
it
is
necessary to know the convergence pressure
of
the system and the equilibrium ratios
belonging to various convergence pressures. Determining in the laboratory the
equilibrium ratios of the components of a given hydrocarbon system at various
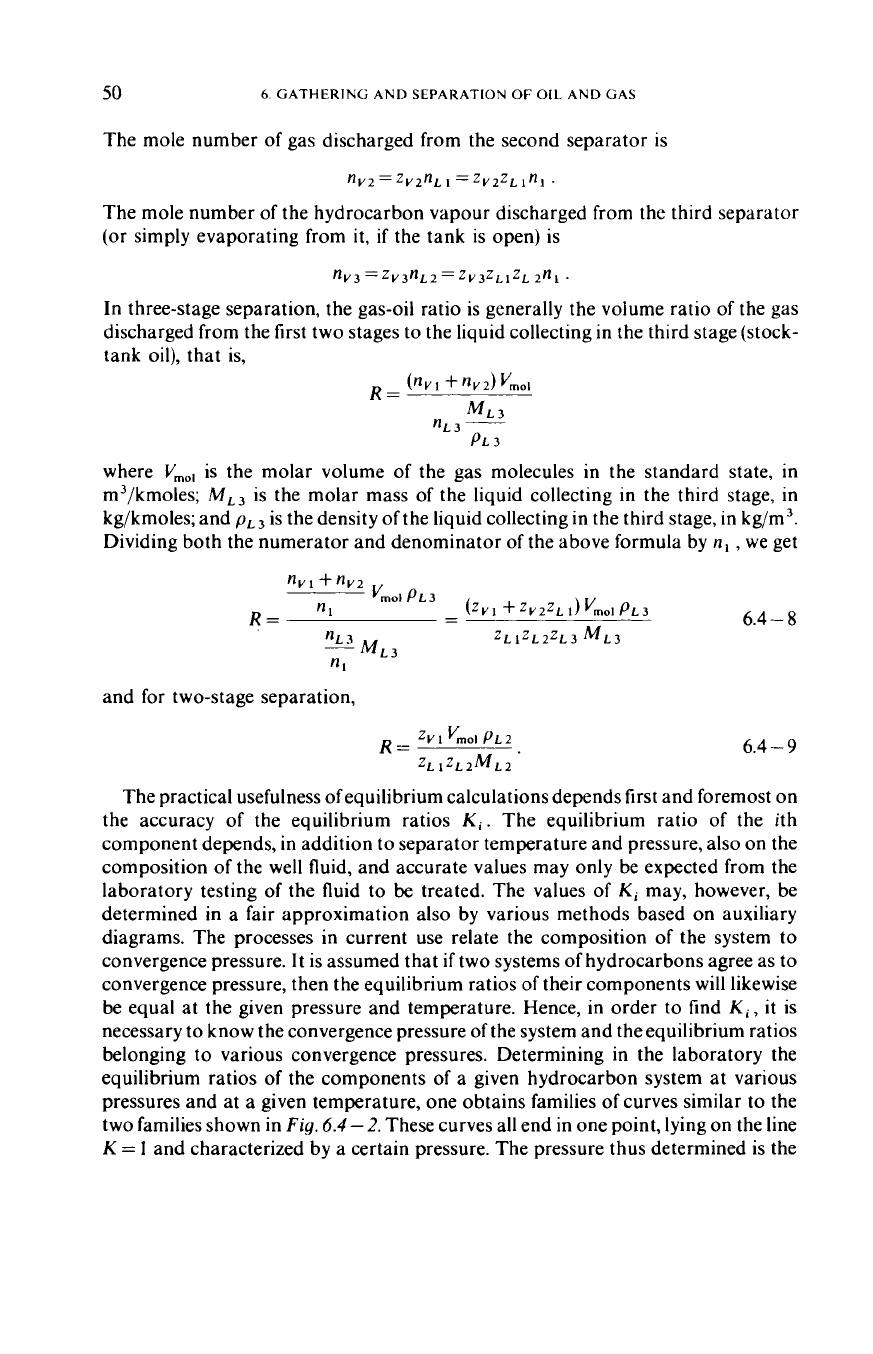
pressures and at a given temperature, one obtains families
of
curves similar to the
two families shown in Fig.
6.4
-
2.
These curves all end in one point, lying on the line
K
=
1
and characterized by a certain pressure. The pressure thus determined is the
6.4.
SEPARATION
OF
OIL
AND
GAS
51
apparent convergence pressure. It is
69
bars for the first family shown in the Figure,
and
345
bars for the second (Amyx
et
al.
1960).
If
the laboratory experiment is per-
formed at the critical temperature of the system, then the apparent convergence pres-
sure agrees with the critical pressure.
At
any other temperature, convergence is merely
apparent, because the system has its bubble point at a pressure lower than the
100
K
10
0.1
0.01
0
001
1
10
loo
p,bars
Fig.
6.4-2.
Equilibrium ratios
of
components
of
hydrocarbon systems
of
69
and
345
bars, apparent
convergence pressure; from Natural Gasoline Association
of
America’s
Equilibrium
Ratio
Data
Book
(1957;
used with permission
of
Natural Gas Processors Association)
apparent convergence pressure and there is just one phase instead of two at the
point of convergence. In the zone between the critical pressure and the apparent
convergence pressure, then, the equilibrium constant lacks a physical meaning: the
corresponding curve sections and the point
of
apparent convergence itself are
merely the results
of
extrapolation. The apparent convergence pressure of a given
system
of
hydrocarbons can
be
determined by various means. Its importance is
substantial especially at high pressures.
At
pressures below cca
7
bars, however, the
system’s composition, and hence, knowledge of its exact convergence pressure, lose
much of their importance. Experiment has shown that in establishing the
K
values
required for calculating equilibria in low-pressure separators, a satisfactory
accuracy will be achieved
if
convergence pressure
is
simply assumed to equal
345
bars
(5000
psia). K-isotherms may be found in the NGAA
Equilibrium Ratio
Data
Book
(1957).
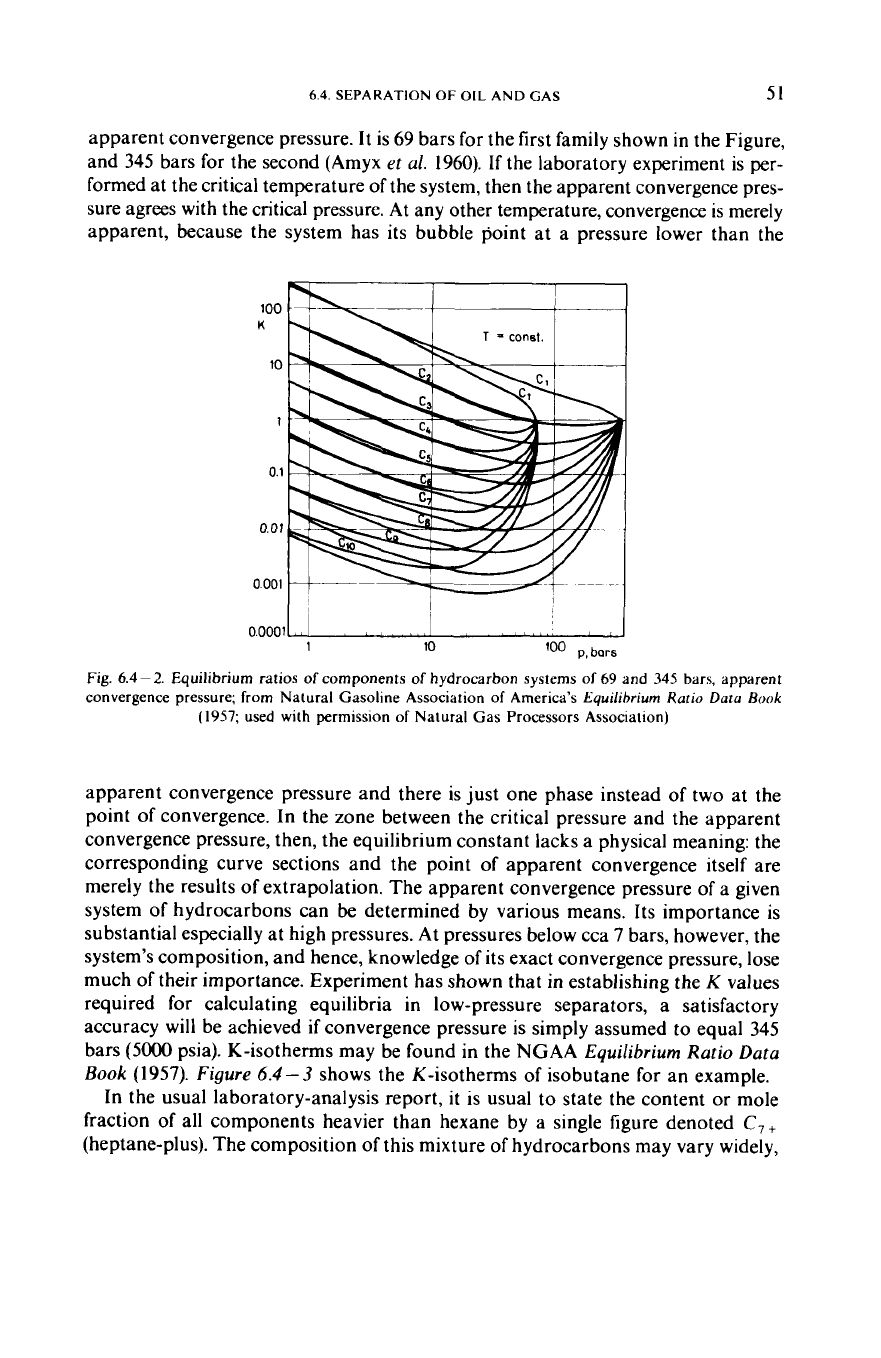
Figure
6.4-3
shows the K-isotherms
of
isobutane for an example.
In the usual laboratory-analysis report, it is usual
to
state the content
or
mole
fraction
of
all components heavier than hexane by a single figure denoted
C,,
(heptane-plus). The composition of this mixture of hydrocarbons may vary widely,
52
10
100
500
1000
3000
10000
P,
prio
b’ig.
6.4
3.
liquilihriuni ratios of iwhutane at
So00
psia
apparent convergence pressure; from Natural
(iasoline
Association of America’s
Eyuilihriirn~
Rtrlio
Dtrrci
Book
(1957;
used with permission of Natural
Gas Processors Association)
depending on the composition of the crude in hand. Since, however, the pressure
curves and critical parameters
of
components heavier than hexane differ very little,
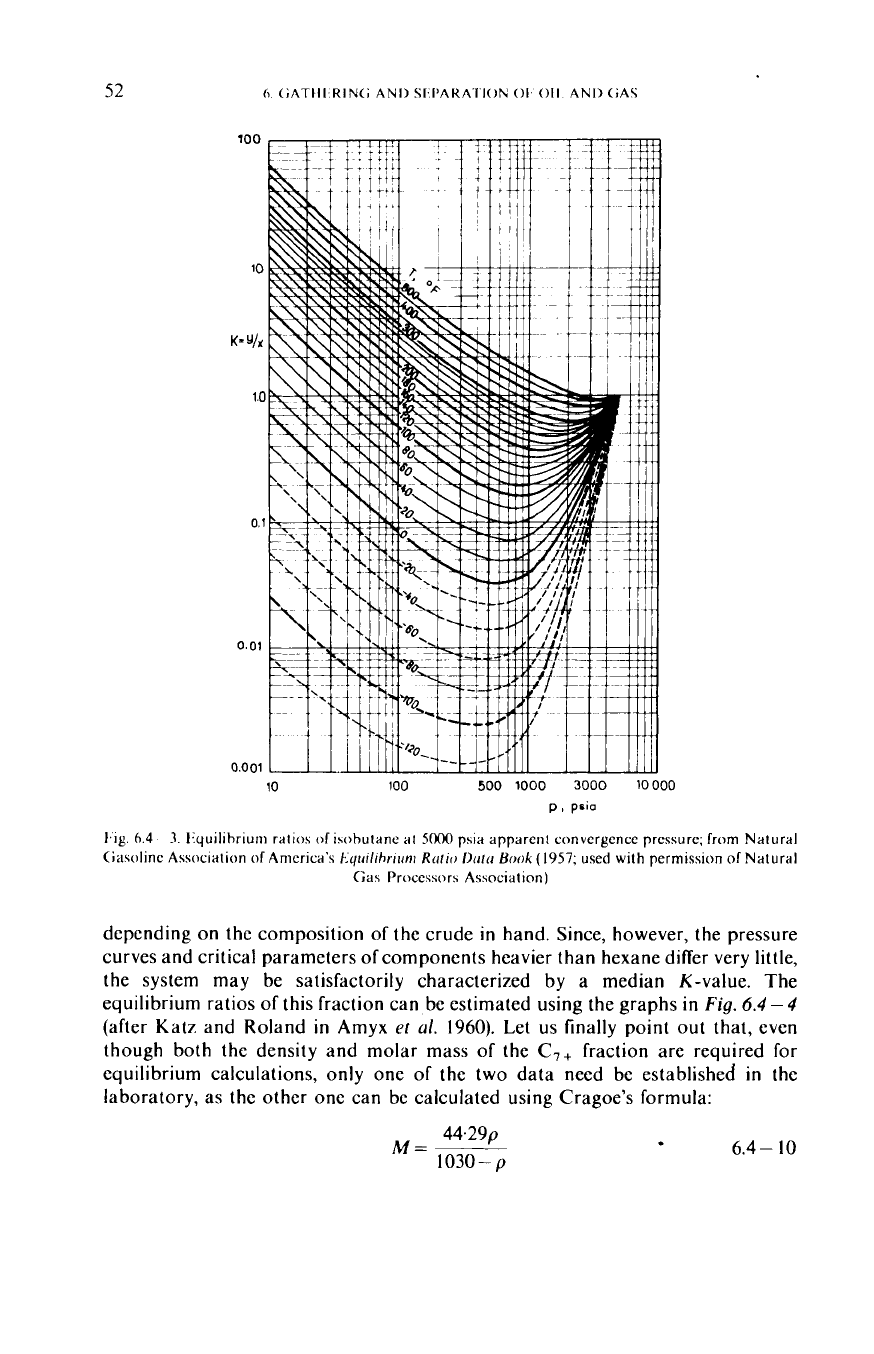
the system may be satisfactorily characterized by a median K-value. The
equilibrium ratios
of
this fraction can be estimated using the graphs in
Fig.
6.4-4
(after Katz and Roland in Amyx
et
al.
1960).
Let us finally point
out
that, even
though both the density and molar mass
of
the
C,,
fraction are required
for
equilibrium calculations, only one
of
the two data need be established in the
laboratory, as the other one can be calculated using Cragoe’s formula:
6.4-
10
6.4.
SEPARATION
OF
011
AND
(;AS
53
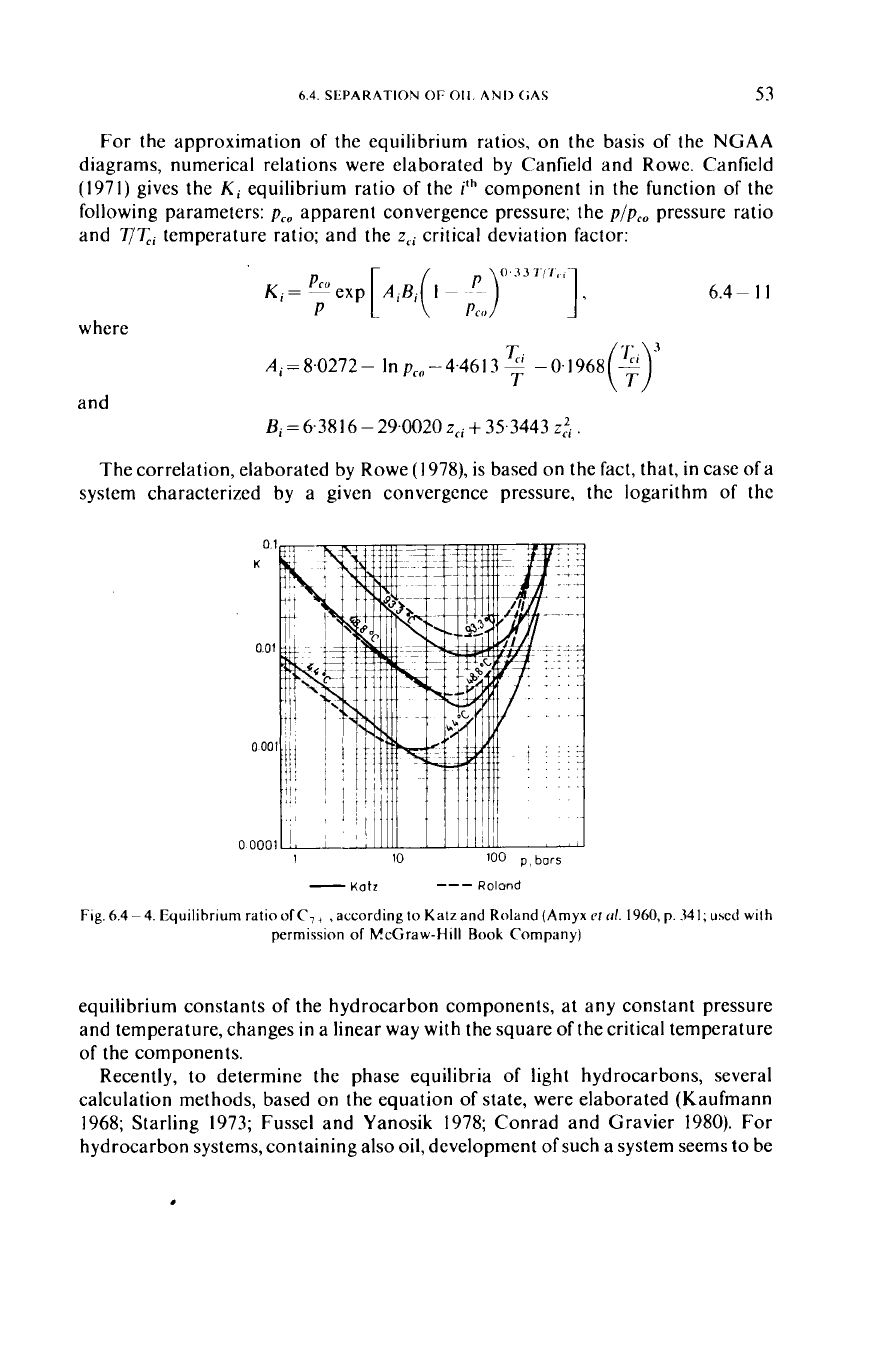
For the approximation
of
the equilibrium ratios, on the basis
of
the
NGAA
diagrams, numerical relations were elaborated by Canfield and Rowe. Canfield
(1971)
gives the
Ki
equilibrium ratio of the
ilh
component
in
the function
of
the
following parameters:
p,,
apparent convergence pressure; the
p/p,,
pressure ratio
and
TIT,,
temperature ratio; and the
zCi
critical deviation factor:
P
6.4-
11
where
Ai
=
8.0272
-
In
pE,,
-
4.46
1
3
T
and
Bi
=
6.3816
-
29.0020
zCi
+
35.3443
z:i
The correlation, elaborated by Rowe (1978), is based on the fact, that, in case of a
system characterized by a given convergence pressure, the logarithm of the
0
1
10
100
p,bors
Roland
---
Kotr
-
Fig.
6.4
-
4.
Equilibrium ratio
ofC,,
,according
lo
Katz
and Roland (Amyx
ci
ti/.
1960,
p.
341;
used with
permission
of
McGraw-Hill
Book
Company)
equilibrium constants
of
the hydrocarbon components, at any constant pressure
and temperature, changes in a linear way with the square
of
the critical temperature
of the components.
Recently,
to
determine the phase equilibria
of
light hydrocarbons, several
calculation methods, based on the equation
of
state, were elaborated (Kaufmann
1968; Starling 1973; Fussel and Yanosik 1978; Conrad and Gravier 1980). For
hydrocarbon systems, containing also oil, development of such a system seems
to
be
.
54
6.
<iATHIiKING AND SEPARATION
OF
OIL
AND GAS
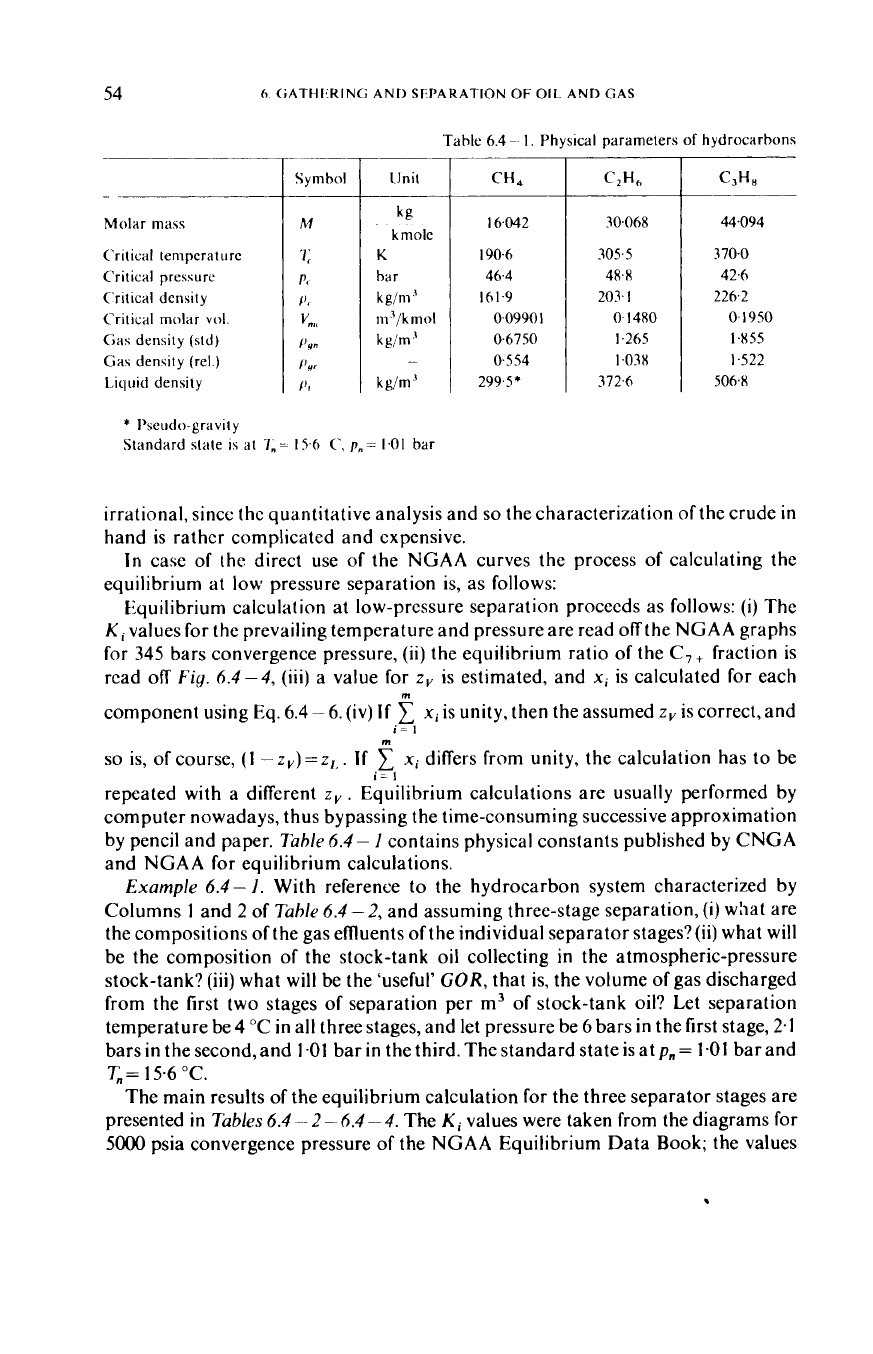
Molar
mass
Critical temperature
Critical pressure
Critical density
Critical molar
vol.
<;as density (std)
Gas density (rel.)
Liquid density
III
Symbol Unit
M
I
kg
kmole
Tahle
6.4
~
1,
Physical parameters
of
hydrocarbons
CH,
16.042
190.6
46.4
161.9
0.0990
I
0.6750
0.554
299.5*
30.068
305.5
4R.X
203.1
0.
I480
I
.265
1.038
312.6
44.094
370.0
42.6
226.2
0.
I950
1.855
1.522
506.X
*
I'seudo-gravity
Standard
state
is at
7,
=
15.6
C,
pn
=
1.01
bar
irrational, since the quantitative analysis and
so
the characterization of the crude
in
hand is rathcr complicated and expensive.
In
case
of
the
direct use
of
the
NGAA
curves the process
of
calculating the
equilibrium at
low
pressure separation is, as follows:
Equilibrium calculation at low-pressure separation proceeds as follows:
(i)
The
K,
values for the prevailing temperature and pressure are read
off
the
NGAA
graphs
for 345 bars convergence pressure,
(ii)
the equilibrium ratio of the
C,
+
fraction is
read off
Fig.
6.4-4,
(iii)
a value for
zy
is estimated, and
xi
is calculated
for
each
component using Eq. 6.4
-
6.
(iv)
If
1
xi
is unity, then the assumed
zy
is correct, and
so
is, of course,
(1
-z,)=z,,
.
If
xi
differs from unity, the calculation has to be
repeated with a different
zy
.
Equilibrium calculations are usually performed by
computer nowadays, thus bypassing the time-consuming successive approximation
by pencil and paper.
Tah/e
6.4
-
I
contains physical constants published by
CNGA
and
NGAA
for
equilibrium calculations.
Example
6.4
-
I.
With reference to the hydrocarbon system characterized
by
Columns
1
and
2
of
Table
6.4-2,
and assuming three-stage separation,
(1)
what are
the compositions of the gas etluents
of
the individual separator stages?
(ii)
what
will
be the composition of the stock-tank oil collecting in the atmospheric-pressure
stock-tank?
(iii)
what will be the 'useful'
GOR,
that is, the volume
of
gas discharged
from the first two stages
of
separation per m3
of
stock-tank oil? Let separation
temperature be
4
"C
in all three stages, and let pressure be
6
bars in the first stage,
2.1
bars in the second, and
1.01
bar
in
the third. The standard state is at
p.
=
1.01
bar and
T,=
15.6
"C.
The main results of the equilibrium calculation for the three separator stages are
presented
in
Tables
6.4
-
2
-
6.4
-
4.
The
K
values were taken from the diagrams
for
5000
psia convergence pressure
of
the
NGAA
Equilibrium Data Book; the values
m
i-
I
m
i=
I
6.4.
S1:l’AKATION
01-
011
ANI)
(;AS
55
i-C,H,, n-C4Hlc,
58-
120
58.
1
20
407.2 425-0
36.6
38-0
233.0
226.6
0.249
I
0.2578
2.446 2.446
2.006
24.N6
56
1.9
582-3
i-C’sH12
72. I46
461.1
33-2
234.2
0.3078
3.036
2.49
1
623.1
Component
I
CI
c2
C,
I-C,
n-C,
I-C,
n-C,
(-h
C,
+
Total
72.146
470.6
33.5
23
I
.9
0.3
109
3.036
2.491
629.0
114
‘(,ti
]
J
86171
507.8
29
9
234.6
0.367
I
3.626
2-975
662.5
Tahlc
6.4- 2.
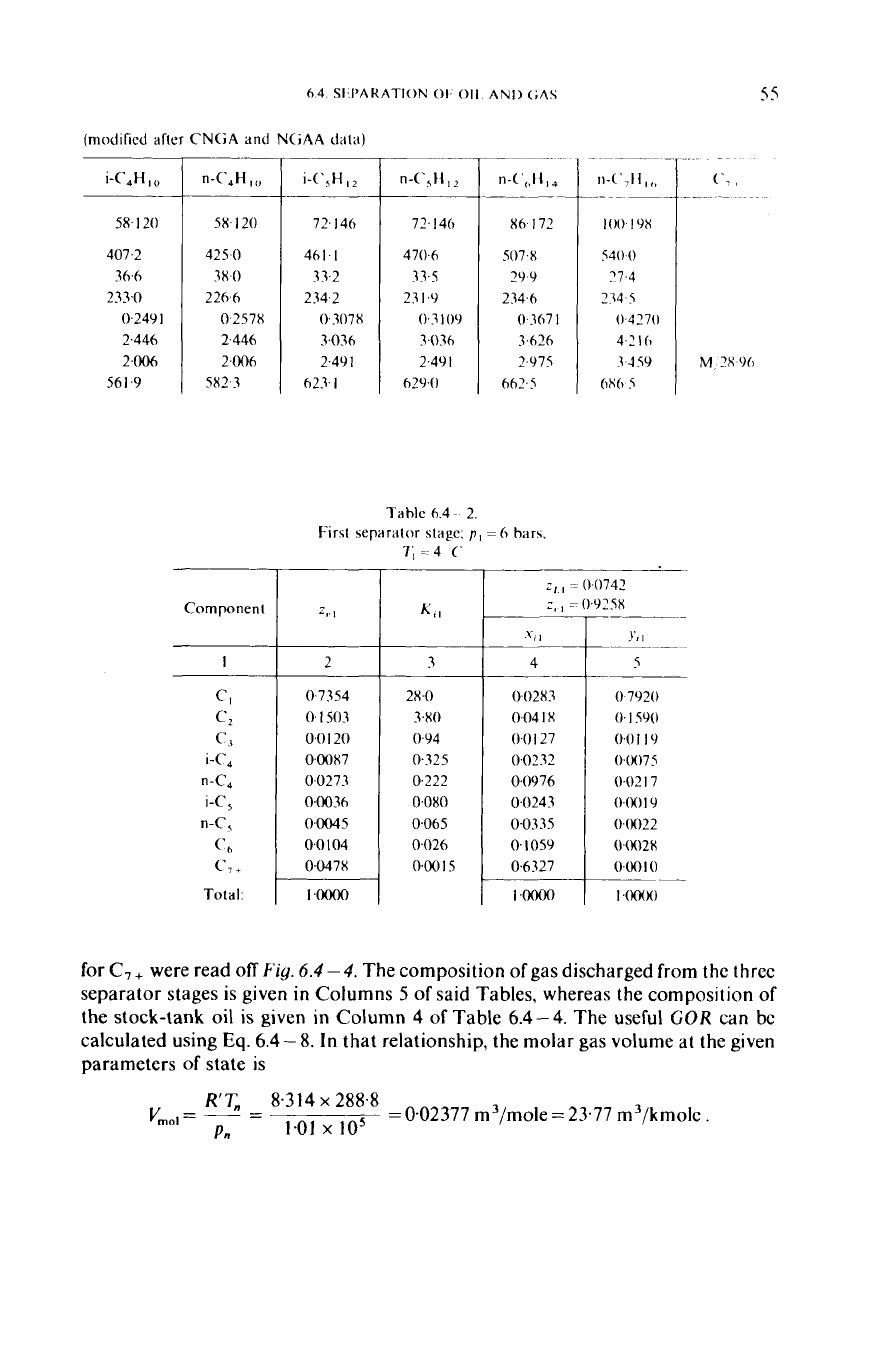
First separator stage;
pI
:
6 hars.
1;=~4
(
2
0.7354
0.1
503
0.0
1
20
0.00X7
0.0273
0.0036
0.0045
0.0
I04
09478
3
28.0
3.80
0.94
0.325
0.222
0.080
0.065
0.026
0~0015
I,
I
=
0
0742
z,
,
:
0
925x
____
.y,
I
4
~-
__-_
0.02~3
0.04
I
8
0.0
I
27
0.0232
0.0976
0.0243
0.0335
0.1059
0.6327
1
.oooo
5
for
C,
+
were read
off
Fig.
6.4
-
4.
The composition
of
gas discharged from the three
separator stages is given
in
Columns
5
of
said Tables, whereas the composition
of
the stock-tank
oil
is given in Column 4
of
Table 6.4-4. The useful
GOR
can be
calculated using
Eq.
6.4-8.
In
that relationship, the molar gas volume at the given
parameters
of
state is
R’T,
8.3 14
x
288.8
- -
=
0.02377
m3/mole
=
23.77
m3/kmole
1.01
x
105
Vmo,=
~
Pn
56
6.
GATHERING AND SEPARATION
OF
OIL
AND GAS
1
Let
us
point out that the exact value of the molar gas constant
in
the
S1
system is
R
=
8.3 I433
f
040044
J/mole
K.
We have used the value
8.3
14,
of sufficient accuracy
for
the task
in
hand. We have neglected
z,
,
the compressibility factor
in
the standard
state,
or
have put
it
equal to unity.
For
finding liquid density
pL3
we have used
Fig.
6.4-5,
which is Standing's
nomogram
(1952)
transposed into the
SI
system.
It
furnishes density of a liquid
2 3
Tahle
6.4
-
3.
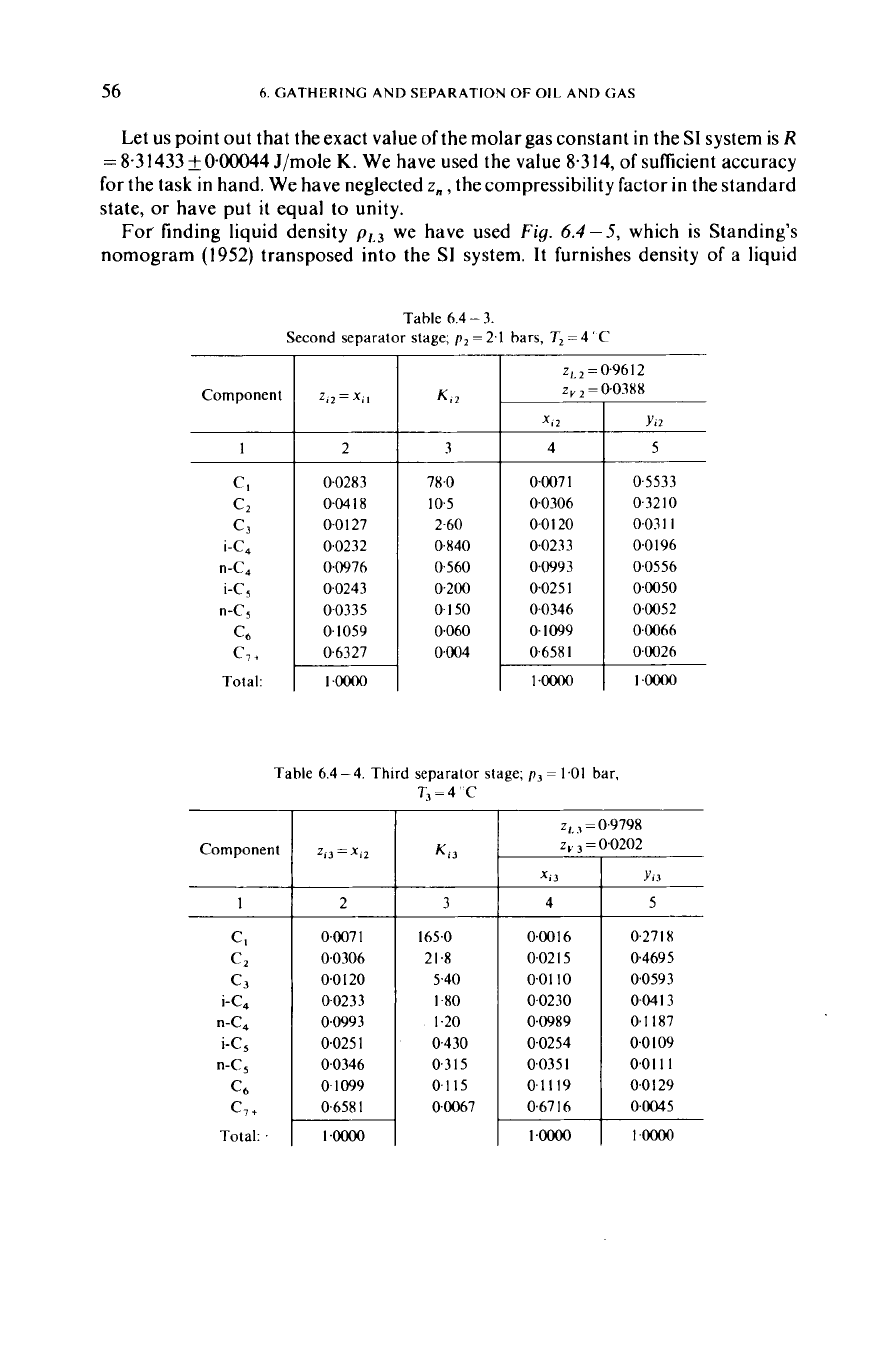
Second separator stage;
p2
=
2.1
bars,
T2
=
4
'
C
z~.~
=
0.961
2
zv,
=
o.o3xx
Component
zt2
=
x,
I
K,2
3
Y,,
1
2
4
5
05533
03210
0.031
1
0.0196
0.0556
00050
0.0052
0.0066
0.0026
I
~oooo
Cl
c2
c3
i-C,
n-C,
i-C,
n-C,
c6
c,
+
Total:
0.0283
0.04
18
0.01
27
0,0232
0.0976
0.0243
00335
0.1059
0.6327
7x.o
10.5
2.60
0x40
0.560
0200
0.
I
50
0.060
0,004
0.007
1
0.0306
0.0
1
20
0.0233
0,099 3
0.025
1
0.0346
0.1099
0.658
I
1
~oooo
Tahle
6.4-4.
Third separator stage;
p,
=
1.01
bar.
T3=4'C
zI,
,,
=
0.9798
zy
3
=
0.0202
YO3
5
4
0.0016
0.02
I
5
001
10
0.0230
0.0989
0.0254
0.035
I
0.1
I19
0.67 I6
02718
0.4695
0,0593
0.04
I
3
0.1
187
0.0
I09
0.01
I I
0.0
I29
0.0045
Cl
c2
c3
i-C,
n-C,
i-C,
n-C,
c6
c,
+
Total:
0007
1
0.0306
0.0
1
20
00233
0,0993
0.025
1
0.0346
0.
I099
0.693
I
165.0
21.8
5.40
1
.80
1.20
0430
0.3
I5
0.115
0.0067
64
SEPARATION
OF
OIL
AND
GAS
57
1000
,
900
L
?I
E
l
-01
800
1
1
>
7001
i:
0-
i
600
;
500
400
1
300
1
200
*
I
"00
'OOO
900
..I
PO9
-5
01
1
%C
FOC
500
kCC
300
c-
I
200
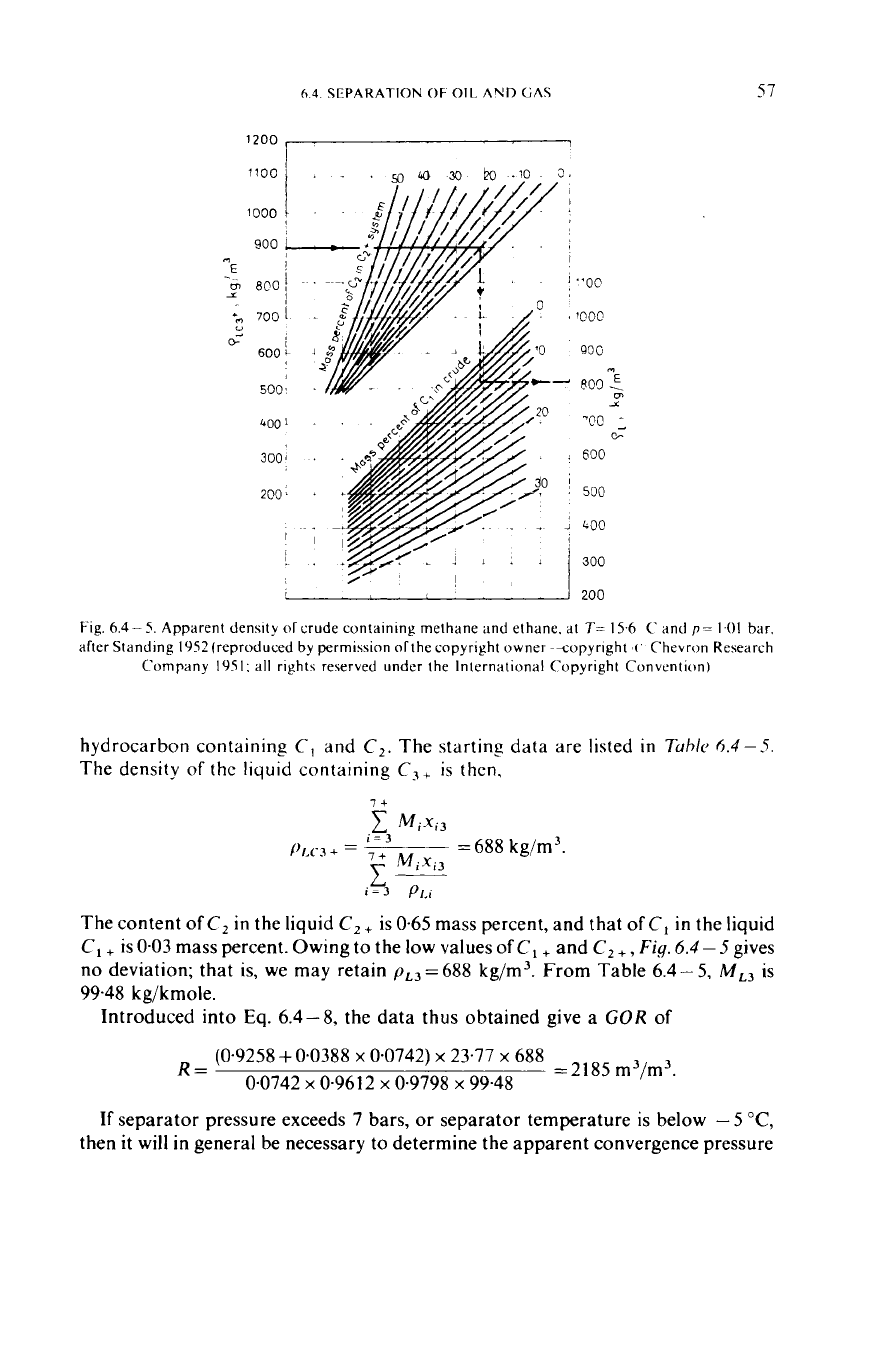
Fig.
6.4-
5.
Apparent density
of
crude containing methane and ethane, at
7-
15.6
C and
I)=
1.01
bar.
after Standing
1952
(reproduced by permission
of
the copyright owner -apyright
'('
Chevron Research
Company
1951:
all rights reserved under the International Copyright Convention)
hydrocarbon containing
C,
and
C,.
The starting data are listed
in
Tuhlc
6.4-5.
The density
of
thc liquid containing
C,,
is then,
Mixi3
'+
Mixi3
=
688 kg/m3.
i=3
PLC3
+
=
i
c--
=
3
The content
of
C,
in
the liquid
C,
+
is 0.65 mass percent, and that
of
C,
in
the liquid
C,
+
is 0.03 mass percent. Owing
to
the low values
of
C,
+
and
C,
+,
Fig.
6.4-
5
gives
no
deviation; that is, we may retain
pL3
=
688 kg/m3. From Table 6.4
-
5,
M,,
is
99.48 kg/kmole.
Introduced into
Eq.
6.4-8, the data thus obtained give a
GOR
of
R=
=2185 m3/m3.
(0.9258
+
0.0388
x
00742)
x
23.77
x
688
00742
x
0.96 12
x
0.9798
x
99.48
If
separator pressure exceeds 7 bars,
or
separator temperature is below
-
5
"C,
then it will in general be necessary to determine the apparent convergence pressure
58
6.
GATHERING AND SEPARATION
OF
OIL
AND GAS
Component
I
c,
cz
c,
I-c,
n-C,
I-c,
c,
C,
t
Total:
n-C,
Table 6.4 ~
5.
2
0.00
I6
0.02
1
5
001
10
0.0230
0.0989
0.0254
0.035
1
0.1
119
0.67 16
1
~oooo
30.07
58.12
sx-
12
72.1
5
72.
I5
86. I7
115.0
4
0.03
0.65
0.48
I
.34
5.75
1.83
253
9.64
17.23
99.48
5
506.8
561.9
623.
I
629.0
662.5
7
10.0
582.3
00010
00024
oQo99
0.0029
0.0040
00
146
010x8
01436
and to read the
Kis
off
the corresponding
NGAA
curve family. Since equilibrium
calculations are rather time-consuming,
it
is recommended to find out beforehand
whether two separate phases do exist at all at the temperature and pressure in
question.
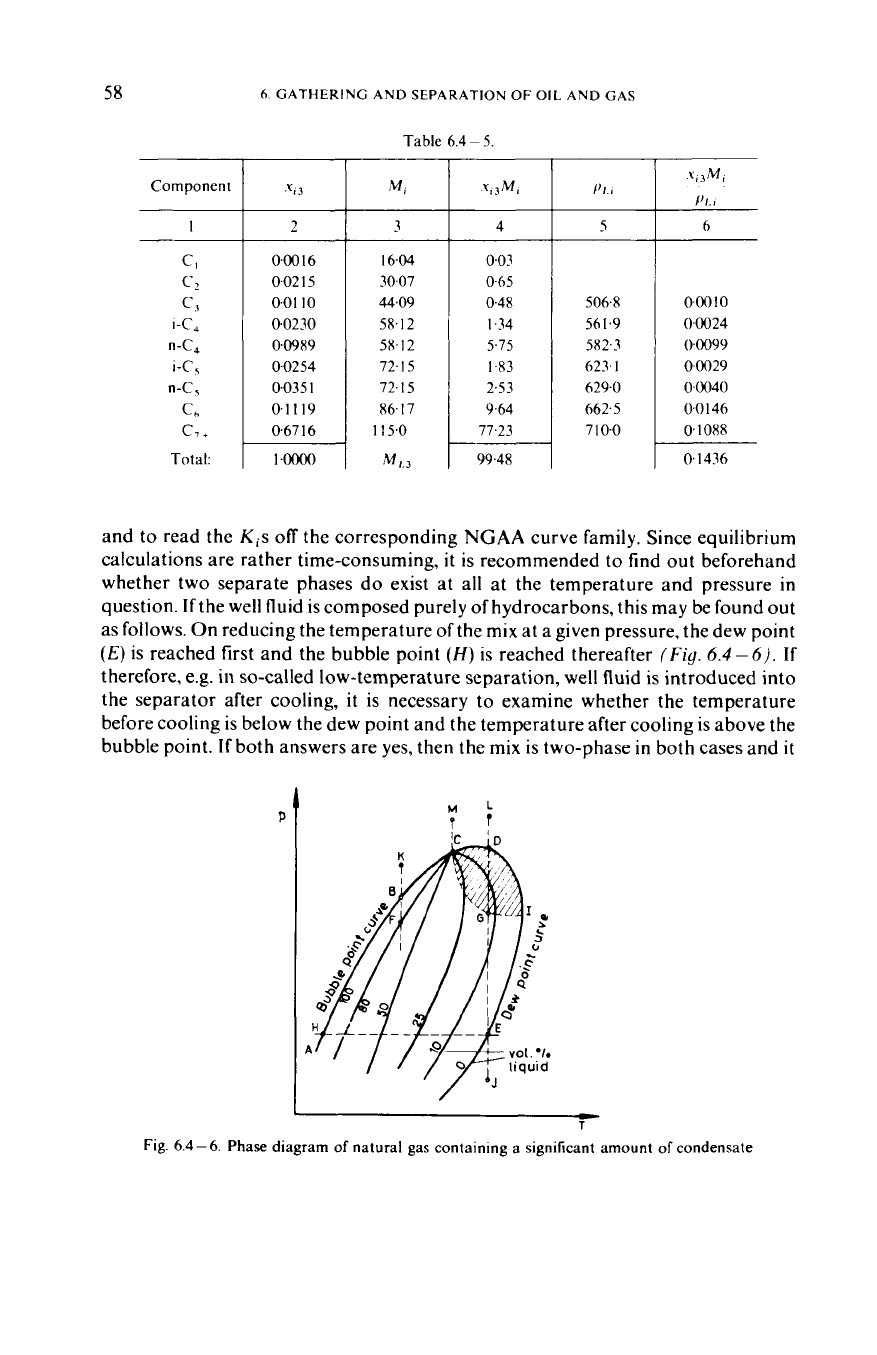
If
the well fluid is composed purely of hydrocarbons, this may be found out
as follows.
On
reducing the temperature
of
the mix at a given pressure, the dew point
(E)
is reached first and the bubble point
(H)
is reached thereafter
(Fig.
6.4-6).
If
therefore, e.g.
in
so-called low-temperature separation, well fluid is introduced into
the separator after cooling, it is necessary to examine whether the temperature
before cooling is below the dew point and the temperature after cooling is above the
bubble point.
If
both answers are yes, then the mix is two-phase
in
both cases and
it
P
t
ML
?t
A
Fig.
6.4-6.
Phase diagram
of
natural gas containing
a
significant amount
of
condensate
6.4.
SEPARATION
OF
OIL
AND GAS
59
is worth while to perform the equilibrium calculation.
At
the
dew
point,
one mole
(say)
of
vapour keeps equilibrium with a liquid drop
of
evanescent size. Hence, in a
very good approximation,
z,.
=
0
and
zy
=
1,
and by
Eqs
6.4
-
4 and 6.4
-
6,
m
5
xi=
c
zilKi=l.
6.4-
12
i-
1
i=
1
If
the value obtained is greater than
unity,
the mix is two-phase, because
in
order to
make said value approach unity
it
is necessary to increase the
Kis
and, by
Fig.
6.4
-3.
this requires raising the temperature
if
the pressure is fixed. Now by
Fig.
6.4-6,
this is possible only
if
the mix is
in
the two-phase domain.
At
the
bubble
point,
one
mole (say) of liquid keeps equilibrium with a gas bubble ofevanescent size. Hence,
in
a very good approximation,
zL
=
1
and
zy
=
0,
and Eqs 6.4
-
4 and 6.4
-
7
yield
m
m
1
yi=
c
ziKi=l.
i-
I
i:
I
6.4
-
13
If
the value of
the
equation is greater than unity, this means once more that the mix
is two-phase. because
in
order to make said value approach unity
it
is necessary to
reduce the
Kis:
now, by
Fig.
6.4-3,
this requires lowering the temperature
if
the
pressure is fixed, and by
Fig.
6.4
-6
this is possible only
if
the
mix
is in the two-phase
domain.
The well fluid often contains some water, too.
It
is necessary to keep
in
mind when
performing calculations concerning such
a
fluid that
the
common
vapour
pressure
qf
immiscible
liquids
is entirely independent of the proportion of the components
in
the
liquid phase, being equal
to
the sum of the vapour pressures of the components,
taken separately at the temperature considered.
Example
6.4-2.
Find the vapour pressure of a water-pentane mix at
50
"C.
H,O
1
~apou;.;;sure
bars
Component
CSH
I2
1.60
On heating this mix, the bubble point will occur at that temperature where the
sum
of
the two vapour pressures equals the external pressure. The bubble-point
temperature of the mix is, then, lower than that of any individual component (water-
vapour distillation). It is often necessary to determine the temperature at which
water starts to condense out
of
the hydrocarbon-water system (that is, to establish
the dew point
of
the mixture.) Since each component generates its partial vapour
pressure independently of the other components, the task is essentially
to
find the
temperature at which the sum
of
partial pressures equals the vapour pressure.
