Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Synchrotron radiation X-ray diffraction 65
1450 °C. At elevated temperatures, above 1100 °C, the amounts of α and γ
are affected by the presence of the oxides and the oxygen in solid solution.
A number of scans at each temperature reveal the kinetics of the phase
transformations. Figure 3.20 shows the scans measured in 10 min. steps each
for two temperatures. The diffraction patterns of the several scans at each
Mole % phase
100
80
60
40
20
0
γ
α
2
B2
α
Liq
β
600 700 800 900 1000 1100 1200 1300 1400 1500 1600
T
(°C)
(a)
Mole % phase
100
80
60
40
20
0
γ
α/α
2
600 700 800 900 1000 1100 1200 1300 1400 1500 1600
T
(°C)
(b)
Ti
2
O
Al
2
O
3
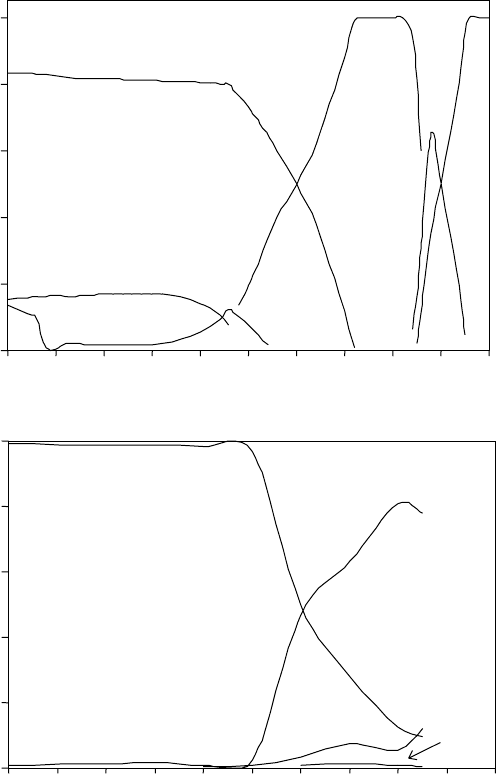
3.19
Mole percent versus temperature plot, representing the entire
set of the phases in the Ti-46Al-1.9Cr-3Nb alloy (a) calculated using
the Thermo-Calc software, and (b) derived after the fitting procedure
of the diffraction patterns.
Titanium alloys: modelling of microstructure66
Intensity (c.p.s.)
3000
2500
2000
1500
1000
500
0
29 30 31 32 33 34 35 36 37 38
2θ (°)
(a)
{111}γ
{011}B2
{021}α
2
{002}γ
{020}γ
3
2
1
0
1: 0 min
2: 10 min
3: 20 min
800 °C
Intensity (c.p.s.)
10000
8000
6000
4000
2000
0
29 30 31 32 33 34 35 36 37 38
2θ (°)
(b)
{111}γ
3
2
1
0
1: 0 min
2: 10 min
3: 20 min
1200 °C
{104}Al
2
O
3
{002}α
2
{100}Ti
2
O
{002}Ti
2
O
{011}B2
{101}Ti
2
O
{021}α
2
{012}α
2
+ {113}Al
2
O
3
{002}γ
{020} γ
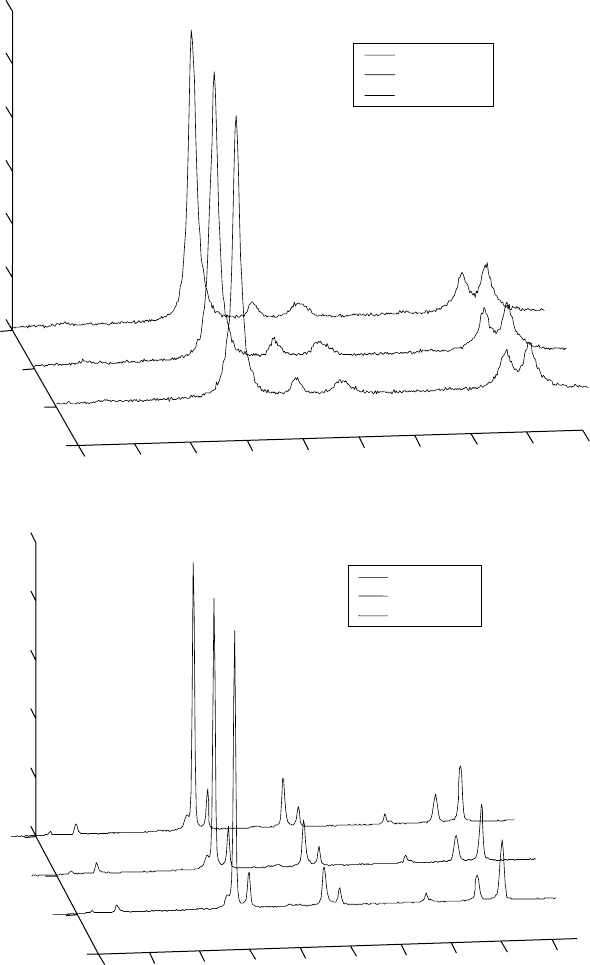
3.20
X-ray diffraction patterns for Ti-46Al-1.9Cr-3Nb alloy, showing
the kinetics of the transformation during isothermal exposure at (a)
800 and (b) 1200 °C.
Synchrotron radiation X-ray diffraction 67
temperature are similar. The phase transformations are very fast and
thermodynamic equilibria have been achieved in minutes or shorter. No
phase transformations are observed with prolonged time.
Metallography and scanning electron microscopy
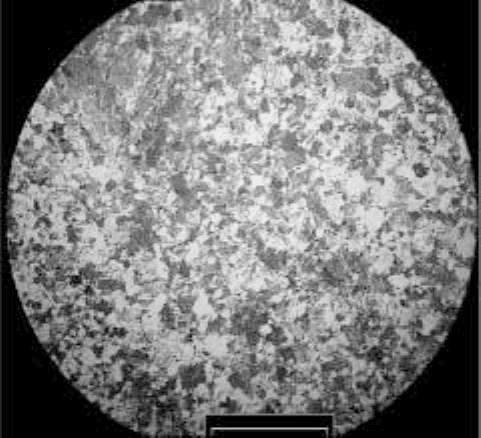
The microstructure after high-temperature exposure is of a duplex nature as
in the forged state (Chapter 2) (Fig. 3.21).
In Fig. 3.22, the oxidised layer is shown. The depth of the X-ray penetration
is 5–6.5 µm for scan in the range of 30–40° in 2
θ
, and 12 µm at 70° in 2
θ
.
3.4.3 Summary
The full-profile quantitative phase analysis of the set of X-ray diffraction
patterns collected at high temperatures allows measurement of the molar
fraction versus temperature for every phase in the Ti-46Al-1.9Cr-3Nb alloy.
A thermodynamically calculated phase diagram of the Ti-46Al-1.9Cr-3Nb
alloy corresponds well to the results of the molar phase fraction derived after
the fitting procedure of the X-ray diffraction patterns. The coefficients of
thermal expansion in the temperature range of 20–1450 °C are derived for
both γ and α /α
2
phases independently. X-ray diffraction and microscopy
100 µm
3.21
Optical micrograph, showing duplex microstructure of Ti-46Al-
1.9Cr-3Nb alloy after high-temperature measurements.

Titanium alloys: modelling of microstructure68
data prove the presence of a titanium and aluminium oxide layer at the alloy
surface. The Ti
2
O oxide forms in the surface of the gamma titanium aluminide
alloy at high temperature.
3.5 References
Berberich F, Matz W, Richter E, Schell N, Kreissig U and Moeller W (2000), ‘Structural
mechanisms of the mechanical degradation of Ti–Al–V alloys: In situ study during
annealing’, Surf Coat Technol, 128–129, 450–54.
Daróczi L, Beke D L, Lexcellent C and Mertinger V (2000), ‘Effect of hydrostatic
pressure on the martensitic transformation in CuZnAl(Mn) shape memory alloys’,
Scripta Mater, 43 (8), 691–97.
Ducher R, Viguier B and Lacaze J (2002), ‘Modification of the crystallographic structure
of γ-TiAl alloyed with iron’, Scripta Mater, 47 (5), 307–13.
Guo F A, Ji V, Zhang Y G and Chen C Q (2001), ‘A study of mechanical properties and
microscopic stress of a two-phase TiAl-based intermetallic alloy’, Mater Sci Eng A,
315 (1–2), 195–201.
Hao Y L, Yang R, Cui Y Y and Li D (2000), ‘The influence of alloying on the α
2
/(α
2
+γ)/
γ phase boundaries in TiAl based systems’, Acta Mater, 48 (6), 1313–24.
Li X Y, Taniguchi S, Matsunaga Y, Nakagawa K and Fujita K (2003), ‘Influence of
siliconizing on the oxidation behavior of a γ-TiAl based alloy’, Intermetallics, 11 (2),
143–50.
3.22
Scanning electron microscopy image, showing the surface
oxidised layer (arrow) of Ti-46Al-1.9Cr-3Nb alloy after high-
temperature measurements.
Synchrotron radiation X-ray diffraction 69
Li Z, Gao W, Yoshihara M and He Y (2003), ‘Improving oxidation resistance of Ti
3
Al and
TiAl intermetallic compounds with electro-spark deposit coatings’, Mater Sci Eng A,
347 (1–2), 243–52.
MacLean E J, Millington H F F, Neild A A and Tang C C (2000), ‘A versatile diffraction
instrument on Station 2.3 of the Daresbury Laboratory’, Mater Sci Forum, 321–324,
212–14.
Palm M, Zhang L C, Stein F and Sauthoff G (2002), ‘Phases and phase equilibria in the
Al-rich part of the Al–Ti system above 900 °C’, Intermetallics, 10 (6), 523–40.
Qin G W, Smith G D W, Inkson B J and Dunin-Borkowski R (2000), ‘Distribution
behaviour of alloying elements in α
2
(α)/γ lamellae of TiAl-based alloy’, Intermetallics,
8 (8), 945–51.
Tang Z, Niewolak L, Shemet V, Singheiser L, Quadakkers W J, Wang F, Wu W and Gil
A (2002), ‘Development of oxidation resistant coatings for γ-TiAl based alloys’,
Mater Sci Eng A, 328 (1–2), 297–301.
Zhang D, Dehm G and Clemens H (2000), ‘Effect of heat-treatments and hot-isostatic
pressing on phase transformation and microstructure in a β/B2 containing γ-TiAl
based alloy’, Scripta Mater, 42 (11), 1065–70.
Zhang W J, Reddy B V and Deevi S C (2001), ‘Physical properties of TiAl-base alloys’,
Scripta Mater, 45 (6), 645–51.
Zupan M and Hemker K J (2001), ‘High temperature microsample tensile testing of
γ-TiAl’, Mater Sci Eng A, 319–321, 810–14.

70
4
Differential scanning calorimetry and
property measurements
Abstract: Differential scanning calorimetry can reveal reproducibly the
thermal effects upon heating of titanium alloys and aluminides. The main
part of this chapter describes the kinetics of the γ + α to α phase
transformation in the Ti-46Al-1.9Cr-3Nb alloy, derived quantitatively from
the calorimetry data, giving phase compositions at any point during the
transformation upon continuous heating. This is followed by discussions of
mechanical property testing, and hydrogen penetration measurement. For the
latter purpose, alloys are cathodically charged with hydrogen in acid
solutions for various times at different current densities and temperature,
followed by X-ray diffraction to determine the nature of the microstructural
change.
Key words: phase transformation, microstructure, intermetallics, hydrogen
absorbing materials, high-temperature alloys.
4.1 Phase and structural transformations
This section explains the transformation behaviour in Ti-46Al-1.9Cr-3Nb
alloy, combining differential scanning calorimetry, microscopy, X-ray
diffraction, and thermodynamic calculations.
4.1.1 Forged alloy
The forged microstructure (Fig. 2.5) is as expected, considering the history
of heat and thermomechanical processing of the forged alloy (Chapter 2).
This duplex microstructure should result in good ductility.
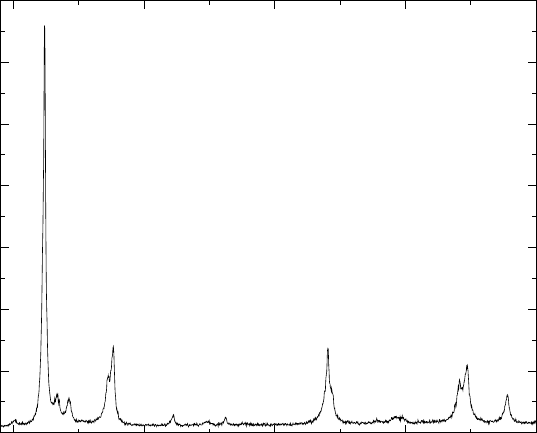
The X-ray diffraction analysis of the forged alloy shows the presence of
γ, α
2
and a small amount of B2 phases (Fig. 4.1). The B2 phase is clearly
detectable, especially with high-resolution synchrotron radiation diffraction.
More detailed interpretation of the X-ray results is given in Chapter 3.
4.1.2 Differential scanning calorimetry
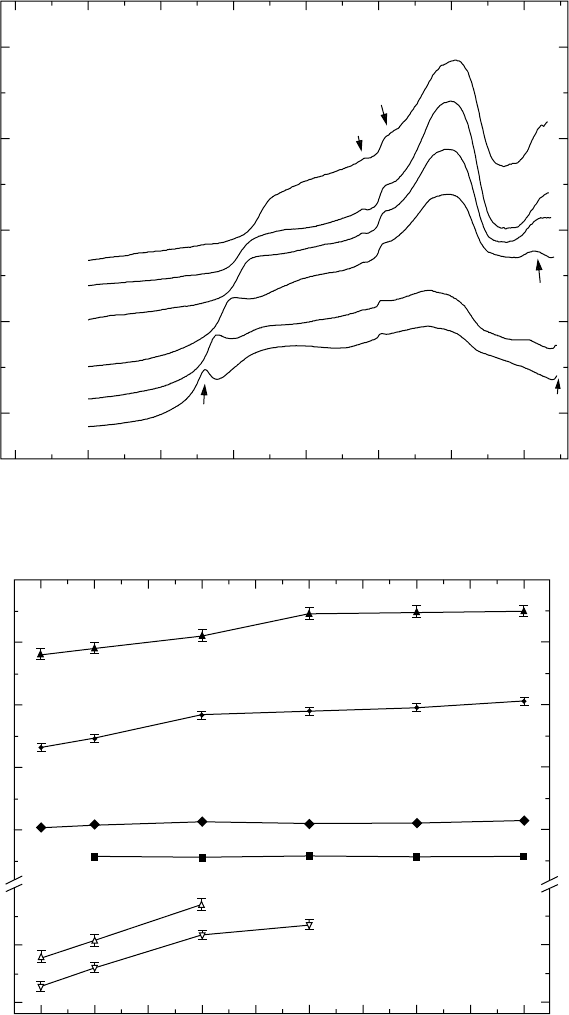
There are many peaks in the differential scanning calorimetry curves for
continuous heating (Fig. 4.2), with different character and appearance. We
now denote the different thermal effects present as TE1, TE2, TE3, TE4,
TE5, TE6 and TE7. All these thermal effects are reproducible during repeated
experiments.
Differential scanning calorimetry and property measurements 71
In the temperature range of 900–1150 °C, there are two thermal effects –
TE1 and TE2 (Fig. 4.2). These are quite well separated when heating with
slow heating rates (5, 10 and 20 °C/min). Thermal effect TE1 is sharp and
well defined. Its start temperature, however, is not clearly measurable. TE2
is very broad and ranges from 980 to 1160 °C at a heating rate of 5 °C/min.
TE1 is highly influenced by the heating rate and shifts significantly to higher
temperatures when faster heating rates are applied (Figs. 4.2 and 4.3). At
high heating rates, the two thermal effects, TE1 and TE2, are overlapped.
At a further increase of the temperature to about 1200 °C, there are two
small peaks, TE3 and TE4. TE4 is clearly observed at all heating rates. TE3
is clear at the higher heating rates (20 °C/min or higher) but is hardly visible
at the heating rates of 5 and 10 °C/min. There is a perfect reproducibility for
both peaks at different runs with error bar within a range of ±1.5 °C. Moreover,
both peaks appear at constant temperatures and are not influenced by the
heating rate (Figs. 4.2 and 4.3). The peak temperature of TE3 varies within
a narrow temperature interval of 1177–1179 °C and the peak temperature of
TE4 varies in the temperature interval of 1203–1207 °C, for different heating
rates (Fig. 4.3).
The major thermal effect, TE5, appears in the temperature interval from
1200 to 1350 °C. Its start temperature is not well defined, while the peak and
Intensity (c.p.s.)
3500
3000
2500
2000
1500
1000
500
0
30 35 40 45 50 55 60 65 70
2θ (°)
{111}γ
{020}α
2
{011}B2
{021}α
2
{002/020}γ
{021}γ
{022}α
2
{121}γ
{002}B2
{022}γ
{221}γ
{023}α
2
{112}B2
{222}γ
{113/131}γ
4.1
Synchrotron radiation X-ray diffraction pattern of forged Ti-46Al-
1.9Cr-3Nb alloy.
Titanium alloys: modelling of microstructure72
endo ⇒
50 °C/min
40 °C/min
30 °C/min
20 °C/min
10 °C/min
05 °C/min
TE1
TE2
TE7
TE6
TE3
TE4
TE5
Heat flow (W/g)
2.5
2.0
1.5
1.0
0.5
700 800 900 1000 1100 1200 1300 1400
T
(°C)
4.2
Differential scanning calorimetry curves for Ti-46Al-1.9Cr-3Nb
alloy employing different heating rates. For clarity, the calorimetry
curves are shifted along the vertical axis with respect to each other.
5 1015202530 35404550
Heating rate (°C/min)
T
(°C)
1400
1350
1300
1250
1200
1000
950
TE5 - End temperature
TE5 - Peak temperature
TE4 - Peak temperature
TE3 - Peak temperature
TE1 - End temperature
TE1 - Peak temperature
4.3
Influence of the heating rate on the different thermal effects
observed at continuous heating of Ti-46Al-1.9Cr-3Nb alloy.
Differential scanning calorimetry and property measurements 73
end temperatures are easily measurable and are influenced by the heating
rate. There is a small shift to higher temperatures when faster heating is
applied (Fig. 4.3).
TE6 is pronounced at heating rates of 10 and 20 °C/min. At higher heating
rates (30, 40 and 50 °C/min), this thermal effect is not completed. At a lower
heating rate (5 °C/min), TE6 is not clearly seen with only some signs, given
by the presence of an inflection point in the curve, at about 1340 °C. At
further increase of the temperature above 1440 °C, there is some indication
for the presence of another thermal effect, only at heating rates of 5 and
10 °C/min.
4.1.3 Interpretation of the calorimetry data
The variety of peaks and their appearances indicate several phase and structural
changes during heating. Such variety of peaks has not been observed during
differential thermal analysis (Ohnuma et al., 2000) of other gamma titanium
aluminides. Careful analysis is necessary in order to correlate the thermal
effects with the corresponding phase and structural changes. In order to
correctly interpret the calorimetry curves, additional theoretical and
experimental study is necessary involving: (i) thermodynamic calculations
of the phase equilibria in the Ti–Al–Cr–Nb system; (ii) additional experiments
involving microstructure investigations of alloy heated to different
temperatures; and (iii) calorimetry runs on repeat, or secondary, heating.
Thermodynamic calculations of the phase equilibria in the quaternary Ti–
Al–Cr–Nb system are made with Thermo-Calc using the TiAl-database. This
database can be used for the prediction of stable and metastable phase equilibria
in multi-component gamma titanium aluminides. The calculations are for the
actual alloy composition for both taking and not taking into account the
oxygen content of 700 ppm (0.07 wt.% or 0.17 at.%). The calculation results
are similar, with and without taking into account the oxygen content. The
extent of similarity can be assessed by comparing the calculated equilibrium
mole% of phases versus temperature plots with oxygen (this chapter) and
without oxygen (Chapter 3). Though there are five elements in the alloy
compositions, the term quaternary is used because the content of oxygen as
an impurity in these calculations is fixed and small.
Figure 4.4 shows the phase equilibrium diagram at different temperatures,
obtained by keeping the chromium concentration constant and varying the
aluminium and niobium contents. The isothermal diagrams are plotted for
ranges of aluminium and niobium that are of practical interest for gamma
titanium aluminides. The actual alloy composition is also depicted in the
diagrams. The thermodynamic calculations suggest the following conclusions:
• The B2 phase, which does not exist in the binary Ti–Al diagram, is an
equilibrium phase in the quaternary Ti–Al–Cr–Nb phase diagram at certain
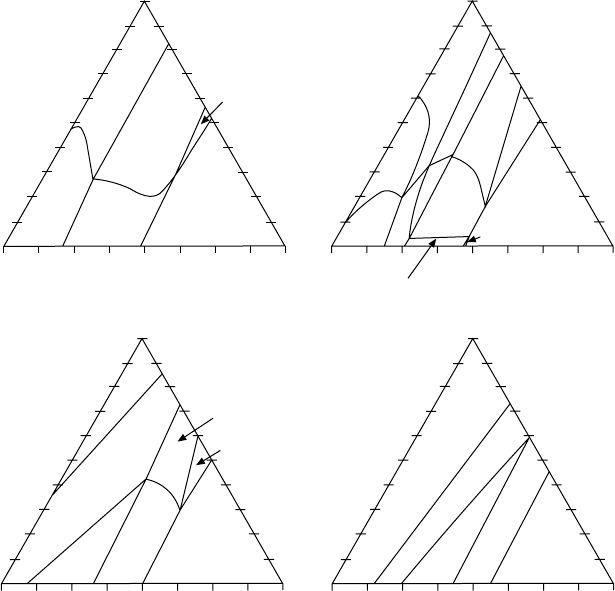
Titanium alloys: modelling of microstructure74
thermodynamic conditions. It exists in a wide concentration range,
including the composition of the alloy here, and forms a variety of two-
phase and three-phase equilibria involving the α, γ, and α
2
phases,
depending on the temperature.
• The alloy composition falls into different phase equilibrium fields,
depending on the temperature, that include α
2
+ γ + B2 at 1000 °C, α +
γ + B2 at 1100 °C, α + γ at 1200 °C and homogeneous α at 1350 °C (see
Fig. 4.4). This suggests that at heating/cooling, the alloy phase composition
would undergo a variety of phase transformations that are more complicated
as compared to those in the binary Ti–Al phase diagram. α + γ to α is the
major phase transformation and, on the calorimetry curve, should appear
as a prominent wide peak.
✽
✽
✽
✽
30 35 40 45 50 55 60 65 70
at.% Al
30 35 40 45 50 55 60 65 70
at.% Al
30 35 40 45 50 55 60 65 70
at.% Al
30 35 40 45 50 55 60 65 70
at.% Al
at.% Nb
10
9
8
7
6
5
4
3
2
1
(a) 1000 °C
(b) 1100 °C
(c) 1200 °C
(d) 1350 °C
at.% Nb
10
9
8
7
6
5
4
3
2
1
at.% Nb
10
9
8
7
6
5
4
3
2
1
at.% Nb
10
9
8
7
6
5
4
3
2
1
α
2
+ B2
γ + B2
α
2
+ γ + B2
α
2
+ γ
α
2
γ
α
2
+ B2
α + α
2
+ B2
α + B2
α + γ + B2
γ + B2
α
2
γ
α
2
+ γ
α + γ
α + α
2
α
B2
α + B2
α
2
+ γ + B2
α
α + γ
γ
γ
α + γ
α
β
α + β
γ + B2
4.4
Calculated isothermal sections of the quaternary Ti–Al–Cr–Nb
system with the composition of the alloy plotted on them. The
calculations are for Cr = 1.9 at.%. For clarity, only the range of
compositional interest for gamma titanium aluminides is plotted.
α + α
2
+ γ
