Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Synchrotron radiation X-ray diffraction 35
3.2.1 Ti-6Al-4V
Ti 6-4 alloy in five different heat treatment conditions is examined:
(i) annealed (this is the starting state of the alloy, which is rolled with a
reduction degree not less than 60% at temperatures in the α + β field,
followed by recrystallisation annealing at 800 °C for two hours);
(ii) furnace cooling, with a cooling rate of 0.5 °C/s, after β-homogenisation
at 1100 °C;
(iii) water quenching after β-homogenisation at 1100 °C;
(iv) water quenching after homogenisation in α + β region at 850 °C; and
(v) water quenching after homogenisation in α + β region at 850 °C,
followed by ageing at 600 °C for 20 hours.
Ti-6Al-2Sn-4Zr-2Mo-0.08Si Room temperature
30 32 34 36 38 40
2θ (°)
Relative intensity
3
2.5
2
1.5
1
0.5
0
Water
quenching
Furnace
cooling
Rolled
{100}α′
{002}α′
{101}α′
{101}α
{100}α
{002}α
{110}β
{002}α
{100}α
{110}β
{101}α
3.2
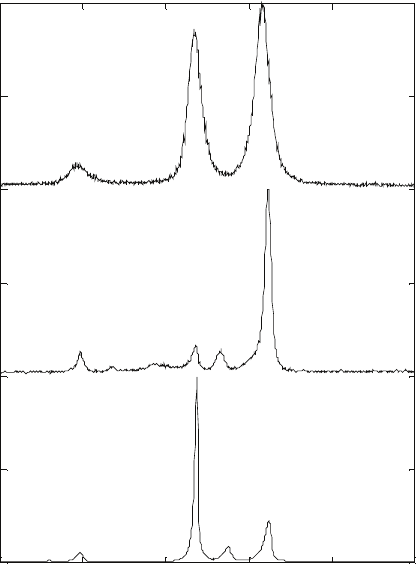
Diffraction patterns at room temperature in the range of 30–40°
2
θ
for Ti-6Al-2Sn-4Zr-2Mo-0.08Si under different heat treatment
conditions. The intensities are given in relative values. For clarity, the
diffraction patterns are shifted with respect to each other along the
vertical axis.
Titanium alloys: modelling of microstructure36
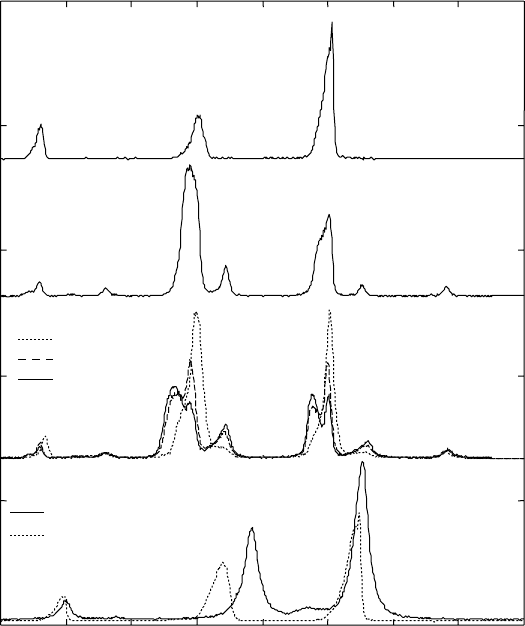
This most commonly used titanium alloy is classified as an α + β titanium
alloy. The phase composition of the Ti 6-4 alloy after different heat treatments
is mainly α phase (see Fig. 3.1), with a small amount of β phase in the
annealed condition, 5 wt.%.
There is also a small amount of retained β phase after furnace cooling
from the β-region (see the small peak at 35.6° in 2
θ
in Fig. 3.1). During slow
cooling, the diffusional redistribution of the alloying elements leads to
enrichment of the β phase with β stabiliser (vanadium in the case of Ti-6Al-
4V). As a result, a small amount of β phase remains stable at room temperature.
A similar observation for the alloy studied is shown in Chapter 7 for cooling
Beta21s Room temperature
Intensity (counts)
5000
4500
4000
3500
3000
2500
2000
1500
1000
500
0
30 35 40 45 50 55 60 65 70
2θ (°)
{110}β
{200}β
Water
quenching
{211}β
{110}β
{200}β
{211}β
Furnace
cooling
{100}α
{200}β+{002}α
{101}α
Aged
{102}α
{200}β
{211}β+
{103}α
{112}α
{201}α
3.3
Diffraction patterns at room temperature and profile fits (dotted
lines) in the range of 30–70° 2
θ
for β21s under different heat
treatment conditions. For clarity, the diffraction patterns are shifted
with respect to each other along the vertical axis.
Synchrotron radiation X-ray diffraction 37
from the β-region with different cooling rates. The amount of the β phase
after furnace cooling is about 7 wt.%.
An interesting observation is ascertained for the Ti 6-4 alloy regarding the
reflections at high 2
θ
angles. After furnace cooling, the reflection {103}α
consists of a number of subpeaks (see Fig. 3.4b, ‘Furnace cooling’). This
fact is also observed for {102}α and {112}α reflections, but not for hkl
reflections having l = 0 (see Fig. 3.4a). After slow furnace cooling, α phase
with different c and the same a lattice parameters is formed. The a lattice
parameter of the α phase after furnace cooling is 0.2938 nm, while the c
lattice parameter corresponding to the four subpeaks in Fig. 3.4b is 0.4701,
0.4684, 0.4668 and 0.4655 nm (see Table 3.1). The above observation can be
explained by considering the nature of the β to α transformation in titanium
alloys. For titanium alloys, the transformation from β to α is of monovariant
type. For different temperatures, different amounts of α and β phases are in
Intensity (counts)
1600
1400
1200
1000
800
600
400
200
0
Ti-6Al-4V Room temperature
Water quenching
Furnace cooling
Annealed
56 56.5 57 57.5 58
2θ (°)
(a)
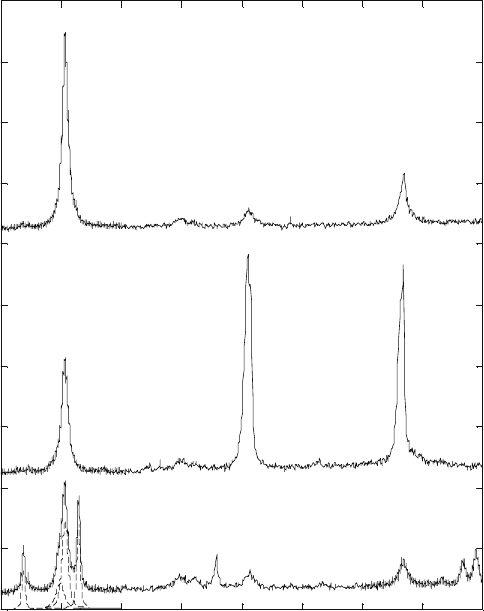
3.4
Reflections and profile fits (dotted lines) of (a) {110}α and
(b) {103}α for Ti-6Al-4V under different heat treatment conditions. For
clarity, the diffraction patterns are shifted with respect to each other
along the vertical axis.
Titanium alloys: modelling of microstructure38
equilibrium and the α phase can be precipitated at different stages
(temperatures) during slow continuous cooling. The α phase precipitated at
different temperatures has different morphology – finer lamellae are attributed
to lower temperatures of transformation (Chapter 6). This may result in
different levels of the residual stresses for α phase precipitated at different
temperatures. In addition, the composition in terms of alloying elements of
the α phase precipitated at different temperatures is different. The above two
reasons are the most plausible explanation for the α phase observed with
different c lattice parameters.
After water quenching from both β-region (1100 °C) and α + β region
(850 °C), the structure of the Ti 6-4 alloy consists only of hcp α phase. No
amounts of β phase are present. This structure is a product of diffusional
(small fraction in final structure) β to α and diffusionless (large fraction) β
to α′ transformations upon quenching. The diffusionless transformation occurs
at temperatures below 700–750 °C and results in the formation of the martensite
structure (α′). The evidence for the presence of martensite in these cases is
the much wider α reflections (see Fig. 3.5). The full-width half maximum
3.4
Cont’d
62.5 63 63.5 64 64.5 65
2θ (°)
(b)
Ti-6Al-4V Room temperature
Water quenching
Furnace cooling
Annealed
Intensity (counts)
3500
3000
2500
2000
1500
1000
500
0

Synchrotron radiation X-ray diffraction 39
Table 3.1
Lattice parameters in nanometers at room temperature for titanium alloys after different heat treatments
Alloy Phase Lattice Annealed Furnace cooled Water quenched Water quenched
parameter from β from β from α + β
Ti-6Al-4V α
a
0.2935 0.2938 0.2935 0.2939
c
0.4673 0.4701 0.4668 0.4673
0.4684
0.4668
0.4655
β
a
0.3226 0.3228
Ti-6Al-2Sn-4Zr-2Mo-0.08Si α
a
0.2937 0.2942 0.2949
c
0.4687 0.4698 0.4690
β
a
0.3254 0.3264
β21s α
a
0.2943
c
0.4686
β
a
0.3267 0.3272 0.3266

Titanium alloys: modelling of microstructure40
(FWHM) values of all α reflections are nearly twice those in the furnace
cooled and annealed alloy. It is suggested that, for Ti 6–4 alloy, quenching
from the 750–900 °C temperature range produces an orthorhombic martensite
(α″). This is not confirmed in the diffraction patterns shown here. The diffraction
patterns of Ti 6-4 quenched from 1100 and 850 °C are similar, showing the
presence of hcp martensite only. Ageing at 600 °C of the alloy quenched
from 850 °C leads to small changes in the diffraction pattern (Fig. 3.1, top).
A shoulder appears on the left side of the {101}α reflection. In addition, a
new reflection is observed at 32.75° (d = 0.2483 nm). There is a new phase
precipitated from the α′ phase. It is possible that the orthorhombic martensite
(α″) is precipitated upon ageing, but the reflections are weak.
3.2.2 Ti-6Al-2Sn-4Zr-2Mo-0.08Si
Ti 6-2-4-2 in three different heat treatment conditions is examined, namely
rolled (this is the starting state of the alloy, which was processed from an
ingot at a temperature above the β-transition and then successively α/β
rolled) and furnace cooling and water quenching after β-homogenisation at
1100 °C. The phase composition under all three conditions is mainly α phase
(see Fig. 3.2). Small amounts of β phase are present in the rolled and furnace
cooled alloy, 8 wt.% for both cases. The intensity of the {002}α reflection in
the rolled alloy is significantly higher than the intensities of {100}α and
{101}α reflections due to the presence of crystallographic texture. No retained
FWHM (∆2θ)
0.6
0.5
0.4
0.3
0.2
0.1
0
{100}α {101}α {100}α {101}α
Ti-6Al-4V Ti-6Al-2Sn-4Zr-2Mo-0.08Si
Reflections
Annealed
Furnace
cooling
WQ(1100)
WQ(850)
Ageing
3.5
Full-width half maximum for α reflections of Ti-6Al-4V and Ti-6Al-
2Sn-4Zr-2Mo-0.08Si under different heat treatment conditions.
Synchrotron radiation X-ray diffraction 41
β phase is present after water quenching. The only phase in the alloy after
water quenching is the hcp martensite (α′) phase (see Figs. 3.2 and 3.5). The
phase constitution of Ti 6-2-4-2 after different heat treatments is similar to
that of Ti 6-4 under the same heat treatment conditions. The lattice parameters
of the α phase in both alloys are similar. The lattice parameters of the β
phase for Ti 6-2-4-2 alloy are appreciably larger than those for Ti 6-4 alloy
(Table 3.1). This effect most probably is due to the different composition of
the β phase at room temperature. In Ti-6Al-4V alloy, the β phase is stabilised
at room temperature as a result of its enrichment with vanadium. More than
15 wt.% vanadium (corresponding to Mo-equivalent [Mo]
eq
of 11 wt.%) is
necessary to stabilise the β phase at room temperature. In Ti-6Al-2Sn-4Zr-
2Mo-0.08Si alloy, the β phase is stabilised at room temperature as a result of
its enrichment with molybdenum. The β phase for this alloy has more than
20 wt.% molybdenum at room temperature (Chapter 6). Since vanadium has
lower atomic radius as compared to titanium it causes a decrease in the
lattice parameter of the β phase in the substitutional solution. Molybdenum,
conversely, with its larger atomic radius, causes an increase in the lattice
parameter.
Considering the schematic pseudo binary phase diagram of titanium alloys,
the Ti 6-2-4-2 alloy is to the left-side of the Ti 6-4 alloy. This implies that the
amount of the residual β phase in Ti 6-4 should be larger than in Ti 6-2-4-2
alloy. The X-ray data indicate that the amount of the residual β phase in Ti
6-4 alloy is slightly lower when compared to the Ti 6-2-4-2 alloy. The reason
for this discrepancy most probably is due to the difference in the oxygen
levels in the two alloys. The amount of oxygen in the Ti 6-4 alloy (0.19
wt.%) is significantly higher than in the Ti 6-2-4-2 alloy (0.065 wt.%).
Oxygen stabilises the α phase. The β phase is therefore depressed in the Ti
6-4 alloy because of the high oxygen content, and promoted in the Ti 6-2-4-
2 alloy because of the low oxygen content.
3.2.3 β21s
β21s in three different heat treatment conditions is examined – aged (this is
the starting state of the alloy, which was processed through β solution treatment
followed by ageing) as well as furnace cooling and water quenching from
900 °C. In the aged alloy, a mixture of α + β crystal structures is present (see
Fig. 3.3). The amount of the α phase is 58 wt.%. The presence of α phase in
the aged condition is a result of the heat treatment, i.e. β solution treatment
followed by ageing. This is a typical condition for a commercial β21s alloy
because α precipitation provides strengthening.
Both water quenching and furnace cooling from 900 °C produce pure β
phase microstructure. α phase is not observed after heat treatment. The
martensite transus temperature in this alloy is lowered by the alloying elements
Titanium alloys: modelling of microstructure42
(15.75% [Mo]
eq
) to temperatures below room temperature. This means that
martensite phase (α′) cannot exist in this alloy. As a result, retention of the
β phase at room temperature is permitted if the β to α phase transformation
is suppressed upon cooling. The α phase does not form even after slow
furnace cooling at a cooling rate around 0.5 °C/s. Since a certain amount of
α phase should be in equilibrium, the reasons for the suspending of the α
phase are kinetic.
The lattice parameters of the β phase in the β21s alloy are larger than
those in Ti 6-4 and Ti 6-2-4-2 alloys (see Table 3.1). Again, the reason is in
the alloy composition (14.1 wt.% Mo, 3 wt.% Al and 3.48 wt.% Nb). Both
molybdenum and niobium have higher atomic radii than titanium, and therefore
they increase the lattice parameter when substituting titanium atoms in the
bcc β phase.
3.3 Measurements at elevated temperatures
Consecutive HR-XRD measurements at different high temperatures with a
number of scans at each temperature are needed in order to reveal the kinetics
of possible phase transitions, for alloys under different heat treatment
conditions. Some of the diffraction patterns obtained from the high temperature
measurements of Ti 6-4, Ti 6-2-4-2 and β21s alloys are shown in Figs. 3.6,
3.7 and 3.8.
The oxygen content of the alloys after high temperature HR-XRD
measurements is increased, to 0.6 wt.%, 0.5 wt.% and 0.8 wt.% for Ti 6-4
(annealed), Ti 6-2-4-2 (rolled) and β21s (furnace cooled), respectively. These
values are the average oxygen contents of the bulk samples. The vacuum
level (0.3 Pa) of the furnace chamber is not sufficient to completely prevent
oxidation.
An increase in the oxygen content raises the β-transus temperature. The
phase equilibria of the alloys at the elevated temperatures are calculated for
different oxygen contents (see Fig. 3.9), with Thermo-Calc software and
using the Ti-database, for oxygen contents in the range from 0 to 1.4 wt.%.
The Ti-database is validated for oxygen concentrations up to 0.3 wt.%.
Therefore, large errors above this value are possible. Indeed, a very significant
influence of the oxygen content on the equilibrium contents of α and β
phases is demonstrated. The increased oxygen content enhances and stabilises
the α phase in respect to the β phase.
The microstructure analysis of cross-sections of Ti 6-4 and Ti 6-2-4-2
after high-temperature HR-XRD measurements using scanning electron
microscopy shows the presence of a surface oxidised layer (see Fig. 3.10).
The microstructure of the surface layer consists of coarse α phase lamellae.
This is a well-defined layer with an obvious boundary between the layer and
the matrix. In addition, some coarse α phase colonies, grown from the surface
Synchrotron radiation X-ray diffraction 43
layer towards the core, are present. The microstructure of the core consists of
very fine α + β plates colonies. The microhardness of the surface layer for
Ti 6-4 alloy is 528 HV1 while the microhardness of the matrix is 382 HV1.
The difference in the microhardness of the surface layer and that of the
matrix is due mainly to the difference of the microstructure. The difference
in the oxygen level may also have an influence on the microhardness difference.
The measurements performed on the cross-sections using scanning electron
microscopy with energy dispersive X-ray and wavelength dispersive X-ray
with an oxygen detection capability of 0.8 wt.% did not detect any change in
the oxygen counts for the surface layer and the matrix. Hence, the difference
in the oxygen levels between the surface layer and the core is small.
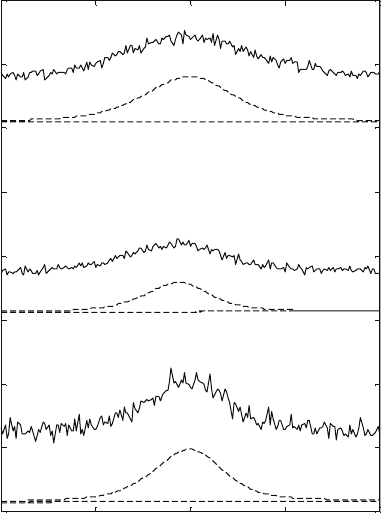
Before heating
After heating
Scan 1
Scan 2
Scan 3
Ti 6-4 annealed
× 10
4
Intensity (counts)
2.5
2
1.5
1
0.5
0
1000 °C
700 °C
600 °C
Room
temperature
{100}α
{002}α
{101}α
{100}α+
{110}α″
{002}α
{020}α″
{101}α
{111}α″
{021}α
Scan 1
Scan 3
Scan 6
{100}α+
{110}α″
{002}α
{020}α″
{101}α
{111}α″
{021}α″
{002}α
{101}α
{100}α
Before heating
After heating
31 32 33 34 35 36 37 38 39
2θ (°)
3.6
Diffraction patterns at different temperatures for Ti-6Al-4V alloy.
For clarity, the diffraction patterns are shifted with respect to each
other along the vertical axis.

Titanium alloys: modelling of microstructure44
The surface layers were formed during the entire cycle of high-temperature
measurements. The surface layer shown in Fig. 3.10 has a thickness of 145–
160 µm, and is a result of high temperature exposure as follows: 1.5 hours
at 600 °C, 1 hour at 700 °C, 0.5 hour at 800 °C, 0.5 hour at 900 °C and 0.5
hour at 1000 °C. Hence, the phase transformations observed at high temperature
are in conditions of concurrent oxidation.
The diffraction patterns are obtained from a surface layer the thickness of
which is limited by the depth of the X-ray beam penetration. The depth of
the X-ray penetration is in the range of 5–6.5 µm for a scan in the range of
30–40° in 2
θ
and 12 µm at 70° in 2
θ
. Hence, the diffraction patterns are
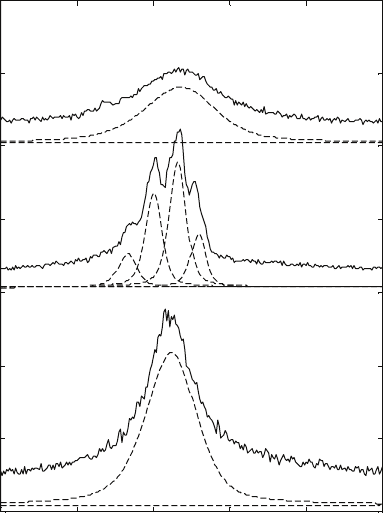
Before heating
After heating
Scan 1
Scan 3
Scan 6
Ti 6-2-4-2 rolled
× 10
4
Intensity (counts)
5
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
1000 °C
800 °C
600 °C
Room
temperature
{100}α
{002}α
{101}α
{100}α
{002}α
{101}α
Scan 1
Scan 3
Scan 6
{100}α
{002}α
{002}α
{101}α
{100}α
Before heating
After heating
31 32 33 34 35 36 37 38 39
2θ (°)
{110}β
{110}β
{101}α
3.7
Diffraction patterns at different temperatures for Ti-6Al-2Sn-4Zr-
2Mo-0.08Si alloy. For clarity, the diffraction patterns are shifted with
respect to each other along the vertical axis.
