Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.


Differential scanning calorimetry and property measurements 75
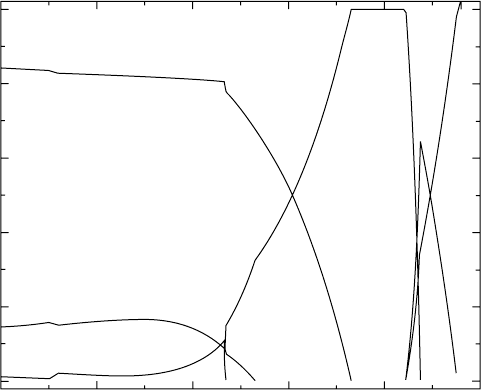
A polythermal section of the phase diagram (Fig. 4.5) traces the change of
the equilibrium phase composition of the alloy with temperature. In the
calculations, the chromium and niobium compositions are kept constant, and
the aluminium concentration and the temperature are varied. In the same
figure, we depict (see dashed line) the alloy composition. From these
calculations, one can suggest that the equilibrium phase compositions change
in the following sequence on increasing the temperature from room temperature
to 1600 °C:
α
2
+ γ + B2 → α + α
2
+ γ + B2 → α + γ + B2 →
α + γ → α → α + β + L → β + L → L. [4.1]
Further, the amounts of phases are shown (see Fig. 4.6). Though, usually, the
kind of sharp change of the equilibrium mole percent curves of phases around
700 °C in Fig. 4.6 is caused by the change of phase equilibrium, Fig. 4.5
T
(°C)
1600
1500
1400
1300
1200
1100
1000
900
800
700
600
40 42 44 46 48 50
Al (at.%)
L
L + β
β
α + β
α + β + L
L + α
α
α + γ
α + γ + B2
γ + B2
γ
α + α
2
+ γ + B2
α
2
+ γ
α
2
+ γ + B2
α
2
+ γ
4.5
Calculated polythermal sections of the quaternary Ti–Al–Cr–Nb
system with the composition of the alloy plotted on them. The
calculations are for Cr = 1.9 at.% and Nb = 3 at.%. For clarity, only
the range of compositional interest for gamma titanium aluminides is
plotted.
Titanium alloys: modelling of microstructure76
clearly shows that this is not the case in the present system. Based on the
above analysis we suggest that:
(i) The thermal effect TE5, which is the major peak in the calorimetry
curves (Fig. 4.2), is due to the γ to α phase transformation at continuous
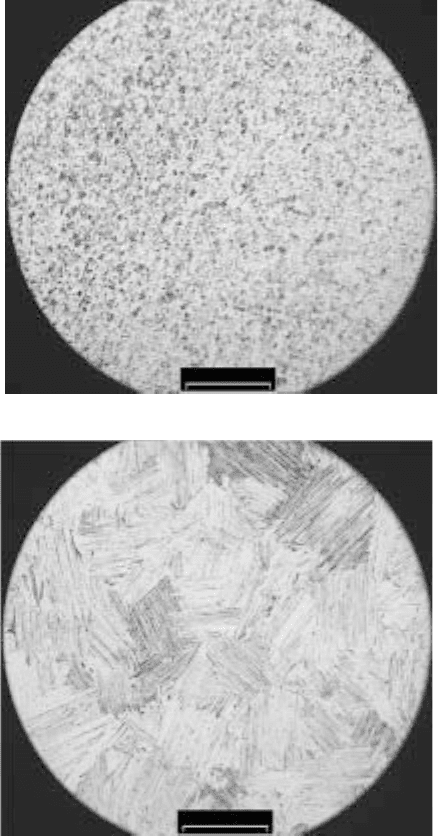
heating. Microstructures of the alloy heated to different characteristic
temperatures in the calorimetry curve, viz. 900, 1020, 1160, 1200,
1220 (Fig. 4.7a) and 1390 °C (Fig. 4.7b), prove this. These temperatures
are purposely chosen as the temperatures of the ends of the different
thermal effects. After heating to and cooling from 1220 °C, which is
about the start temperature of TE5 at heating with the rate of 20 °C/min
(see Fig. 4.2), the microstructure (Fig. 4.7a) remains similar to the
initial microstructure (Fig. 2.5) in respect to the grain morphology. We
might expect some changes, since thermal effects TE1–TE4 are passed.
However, these changes are possibly at a finer microstructure level,
and are not observable by optical microscopy. On the other hand, the
microstructure of the alloy heated to the next temperature (1390 °C),
which is about the end temperature of TE5 at heating with rate 20 °C/
min (see Fig. 4.2), is totally different. The microstructure is fully lamellar,
which is typical for the alloy after cooling from temperatures above α-
transus. This is an experimental proof that the thermal effect TE5 is a
result of the γ to α phase transformation in the alloy.
600 800 1000 1200 1400 1600
T
(°C)
Mole % phase
100
80
60
40
20
0
γ
α
Liq
β
B2
α
2
B2
4.6
Calculated equilibrium mole percentage of phases versus
temperature for Ti-46Al-1.9Cr-3Nb-0.17O alloy.
Differential scanning calorimetry and property measurements 77
(ii) The thermal effect TE4, which is just before TE5, is due to the
disappearance of the B2 phase, where the alloy moves from the α + γ
+ B2 to α + γ phase equilibrium ranges. This effect is small because of
the small amount of the B2 phase. The thermodynamic calculations
200 µm
(a)
200 µm
(b)
4.7
Microstructure of Ti-46Al-1.9Cr-3Nb alloy after heating with a rate
of 20 °C/min to (a) 1220 and (b) 1390 °C and cooling with a rate of
20 °C/min.
Titanium alloys: modelling of microstructure78
show that the equilibrium temperature for this transformation is 1130 °C,
but in the calorimetry curves, this effect is observed at about 1200 °C.
Probably, overheating above the equilibrium temperature is necessary
for this transformation to take place. The disappearance of the B2
phase at 1200 °C is also detected by high-temperature X-ray diffraction
in isothermal conditions at elevated temperatures (see Chapter 3 and
Fig. 4.8).
(iii) The thermal effect TE3 is due to the transition of α
2
+ γ + B2 to α + γ
+ B2 by crossing the four phase α + α
2
+ γ + B2 region. In the
quaternary Ti–Al–Cr–Nb system, this transition is in a narrow temperature
range. The effect is relatively small, because of the small amount of the
α
2
phase. The thermodynamic calculations show that the equilibrium
temperature range for this transformation is 1067–1070 °C. On the
calorimetry curves for continuous heating, it is observed at higher
temperatures – around 1170 to 1180 °C. Again, this difference can be
due to kinetics reasons. Appreciable increase in the amount of α phase
at temperatures higher than 1100 °C has been shown for the same alloy
(Chapter 3), which is in agreement with what is suggested above.
Finally, effects like TE4 and TE3 have not been observed in differential
33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 33.8
50
100
150
200
250
300
350
{011} B2
20 °C
1000 °C
1200 °C
Intensity (c.p.s.)
2θ (°)
20 °C
1000 °C
1200 °C
{111} γ
{002} α(α
2
)
31.5 32.0 32.5 33.0 33.5
2θ (°)
Intensity (c.p.s.)
10000
8000
6000
4000
2000
0
4.8
In situ
synchrotron radiation diffraction patterns of Ti-46Al-1.9Cr-
3Nb alloy at different temperatures.
Differential scanning calorimetry and property measurements 79
thermal analysis and differential scanning calorimetry curves of other
gamma titanium aluminides where the B2 phase does not exist (Ohnuma
et al., 2000).
(iv) The broad thermal effect TE2 is associated with gradual change of the
ratios between the α
2
, γ and B2 phases. The ratios between these phases
change upon heating to above 900 °C (Fig. 4.6), where the amount of
the B2 phase increases, while the amounts of α
2
and γ phases decrease.
Such an increase in the amount of B2 phase is experimentally validated
for the same alloy (see Fig. 4.8 and Chapter 3). The amount of B2
phase increases when the temperature is increased from room temperature
to 1000 °C (Fig. 4.8). Further increase of the temperature to 1100 and
1200 °C results in decrease and disappearance of the B2 phase. At
higher heating rates, as earlier stated, TE1 and TE2 are overlapped.
Even at low heating rates where these peaks are separated, the start
temperature of TE2 seems to be before the end of TE1.
(v) It is not possible to explain the appearance of TE1 on the basis of the
calculated phase equilibria. We therefore suggest that TE1 is due to
transformation towards equilibration of the initial thermomechanically
forged alloy. Though X-ray analysis shows the presence of α
2
, γ and
B2 phases in the forged state, the phase composition in terms of the
amounts of phases may differ from the equilibrium one. Heating to
above 900 °C would allow diffusion processes to take place that would
lead to equilibration of the phase constitution. In addition, in the
temperature range where TE1 and TE2 exist, homogenisation diffusion
processes in the γ phase occur. These are shown by X-ray measurements
(Chapter 3 and Fig. 4.8). An experiment with two heating cycles to a
temperature just above the TE1 further proves this. TE1 is clearly
present during the first heating, but it is not present during cooling or
during repeat heating. Hence, we suggest that this peak is related to, in
the main, the non-equilibrium structure after thermomechanical
processing.
The thermodynamic calculations suggest that, for the composition, the
homogeneous α phase (after completion of TE5) at heating would transform
directly through peritectic transformation of α to α + β + L without the α to
α + β phase transition before that. The α to α + β transition occurs in the
binary Ti–Al phase diagram. Our calorimetry data show that the thermal
effect TE6 could be assigned only to the solid state α to α + β phase transition,
and TE7 involves the liquid phase. Indications for this are the appearance
and shape of the TE6 peak as well as the temperature range where it appears.
TE6 is not completed for heating with rates of 30, 40 and 50 °C/min, probably
because of kinetic reasons. Next, TE7, which has just started at about
1440 °C for small heating rates (5 and 10 °C/min), most probably is due to

Titanium alloys: modelling of microstructure80
the beginning of partial melting (α + β to α + β + L). In these samples, after
the calorimetry experiments, there were partially melted parts around the
edges. The calorimetry measurement is not an equilibrium process. It is
possible for the calorimetry results to deviate from the thermodynamic
calculation. The alloy composition of each phase cannot reach uniformity at
any time due to the diffusion barrier. The transformations α → α + β → α
+ β + L may be interpreted according to the non-equilibrium reason.
Table 4.1 summarises these effects.
4.1.4 Kinetics of the γ to α phase transformation
As discussed in the previous section, a variety of phase transformations take
place during continuous heating. The different phase transformations appear
with different thermal effects, affected differently by the heating rate.
Since the γ to α phase transformation is the major phase transformation
and has practical importance in the heat treatment of the gamma titanium
aluminides, here, we pay a special attention to it. The transformation involving
γ at continuous heating starts at TE3, where the α
2
phase disappears. However,
in some temperature range, from TE3 to TE4, it proceeds with the presence
of another phase – B2. In its pure form, where only the two phases exist, the
γ to α phase transformation starts after TE4, when the B2 phase also disappears.
The pure γ to α phase transformation is represented by TE5. This phase
transformation does not start with 100% γ phase but with a mixture of about
66% γ + 34% α phases (at the end of TE4), and completes with homogeneous
100% α at the α-transus temperature.
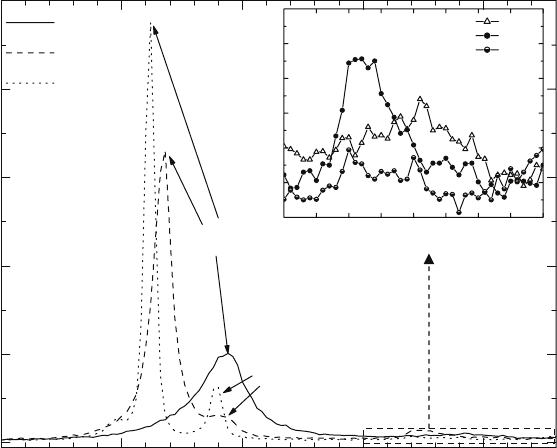
In order to reveal the kinetics of the γ to α phase transformation from the
calorimetry signal, we extract the signal corresponding to pure γ to α phase
transformation only (Fig. 4.9), without the presence of other phases. This is
done by baseline connecting the end of TE4, where the B2 phase disappears
Table 4.1
Thermal effects in the differential scanning calorimetry curves
at continuous heating and corresponding phase transformations of Ti-
46Al-1.9Cr-3Nb alloy
Thermal Phase transformation
effect
TE1 Equilibration and homogenisation of the alloy
TE2 Change of phase ratios between α
2
, γ and B2 phases. Increase
of B2 in respect to α
2
and γ
TE3 α
2
+ γ + B2 to α + γ + B2
TE4 α + γ + B2 to α + γ (disappearance of B2)
TE5 γ + α to α
TE6 α to α + β
TE7 α + β to α + β + L
Differential scanning calorimetry and property measurements 81
and pure γ to α phase transformation starts, and the end of TE5, where this
transformation completes with 100% of α phase. The calculated enthalpy
values using the processed calorimetry curves are similar for the different
heating rates (Table 4.2), confirming that these calorimetry signals correspond
to one same phase transformation at different heating rates.
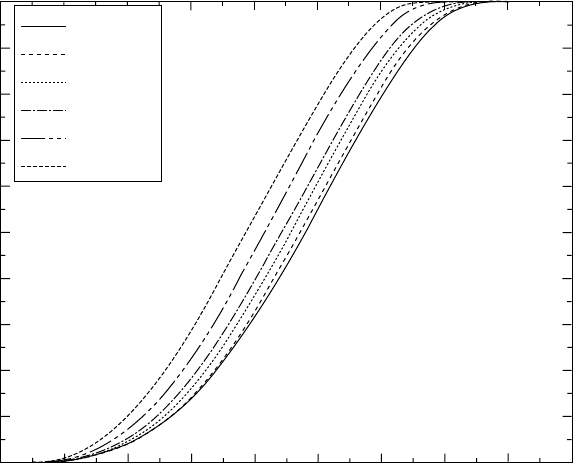
The degree of transformation is calculated (Fig. 4.10), using the method
detailed in Chapter 7. The calculated curves trace the course of the γ to α
phase transformation in the Ti-46A1-1.9Cr-3Nb alloy at continuous heating.
Transformed fraction does not mean the amount of the α phase. At zero
transformed fraction, the phase composition is 66% γ + 34% α, while at
50 °C/min
40 °C/min
30 °C/min
20 °C/min
10 °C/min
5 °C/min
endo. ⇒
Heat flow (W/g)
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
1200 1220 1240 1260 1280 1300 1320 1340 1360 1380
T
(°C)
4.9
Processed calorimetry curves for the γ to α phase transformation
in Ti-46Al-1.9Cr-3Nb alloy at different heating rates.
Table 4.2
Enthalpy of the γ + α to α phase transformation
at continuous heating with various heating rates
Heating rate (°C/min) Enthalpy (J/g)
50 65.9
40 69.6
30 68.1
20 70.6
10 72.0
5 78.2
Titanium alloys: modelling of microstructure82
transformed fraction = 1, the phase composition is 100% α phase. Based on
this, we calculate the amounts of the α phase at different temperatures and
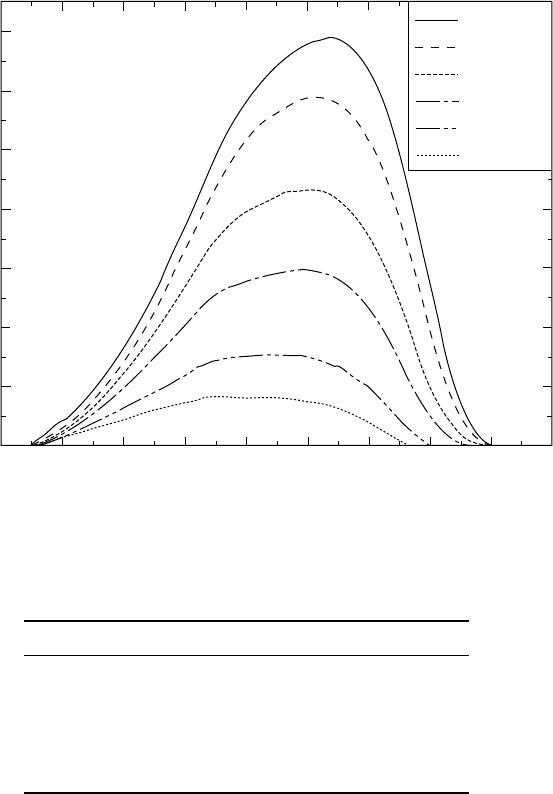
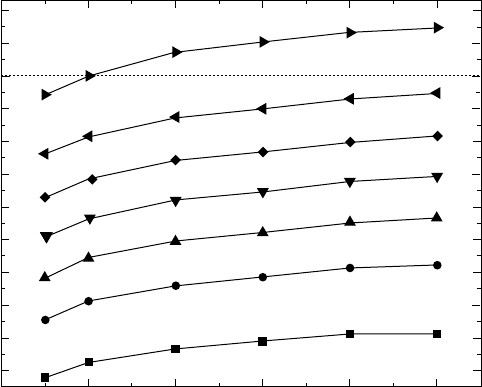
heating rates. The iso-lines in Fig. 4.11 show the dependence of the amount
of the α phase on the heating rate. Such a thermo-kinetic diagram can be
used to trace the course of transformation in real products, where the heating
rate varies significantly from the surface to the core. This is important for
titanium-based materials that have relatively low heat conduction coefficients.
4.1.5 Summary
The Ti-46Al-1.9Cr-3Nb alloy after being forged at 1200 °C without further
treatment has a duplex microstructure, consisting of fine equiaxed and lamellar
γ grains, with a small amount of α
2
plates and particles and about 1 wt.% B2
phase. Differential scanning calorimetry can reveal, reproducibly, several
thermal effects upon heating of the as-forged alloy. These thermal effects are
related to the equilibriation and homogenisation of the alloy, change of
phase ratios between α
2
, γ and B2 phases (in particular, the increase of B2 in
respect to α
2
and γ), and the following five phase transformations: α
2
+ γ +
50 °C/min
40 °C/min
30 °C/min
20 °C/min
10 °C/min
5 °C/min
Transformed fraction
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
1200 1220 1240 1260 1280 1300 1320 1340 1360 1380
T
(°C)
4.10
Calculated transformed fractions as a function of the
temperature for the γ to α phase transformation in Ti-46Al-1.9Cr-3Nb
alloy at different heating rates.
Differential scanning calorimetry and property measurements 83
B2 → α + γ + B2 → α + γ → α → α + β → α + β + L. The observation of
these transformations by differential scanning calorimetry is largely in
agreement with literature phase diagrams and thermodynamic calculations,
though care is needed to consider the different alloy compositions. Kinetics
of the γ + α to α phase transformation in the Ti-46Al-1.9Cr-3Nb alloy can be
quantitatively derived from the calorimetry data, giving phase compositions
at any point during the transformation upon continuous heating.
4.2 Mechanical properties of
ββ
ββ
β21s alloy
This section investigates and shows the effect of heat treatment parameters
with respect to the microstructure and mechanical properties of β-type titanium
alloys. β alloys are the most versatile class of titanium alloys. They offer
very attractive combinations of strength, toughness, and fatigue resistance,
at large cross-sections. The disadvantage compared to α + β alloys is an
increase in density and cost.
β21s (Ti-15Mo-3Al-2.7Nb-0.25Si) is a relatively recently developed
metastable β alloy. Molybdenum, as an alloy agent to titanium, up to 15%,
provides oxidation resistance and improves corrosion resistance. Aluminium
is an α stabiliser, but it is added to β21s to improve ductility and to reduce
the weight. Strip is the main product form, and the material can be economically
rolled to foil.
T
(°C)
1350
1340
1330
1320
1310
1300
1290
1280
1270
1260
1250
1240
01020304050
Heating rate (°C/min)
Alpha transus temperature
99 % alpha
90 % alpha
80 % alpha
70 % alpha
60 % alpha
50 % alpha
40 % alpha
4.11
Calculated thermo-kinetics diagram plotted as temperature
versus heating rate. Iso-lines show the amounts of the α phase.
Titanium alloys: modelling of microstructure84
β21s is metallurgically stable for at least 1000 hours at temperatures up to
615 °C. However, at elevated temperatures, oxygen absorption at the surface
degrades the tensile ductility of the material.
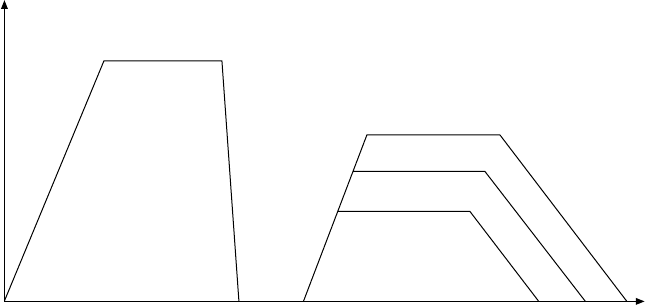
Five different thermal processing conditions are considered here (Fig.
4.12):
(i) aged (this is the starting state of the alloy, which was processed through
β solution treatment followed by ageing)
(ii) heat treated and then water quenched,
(iii) water quenched and ageing at 480 °C for 8 hours,
(iv) water quenched and ageing at 540 °C for 8 hours,
(v) water quenched and ageing at 595 °C for 8 hours.
The thermal processing has produced a fully equiaxed microstructure with α
phase precipitating at the grain boundaries of the β phase with varying grain
sizes (Fig. 4.13).
The Vickers hardness after water quenching decreases as compared to the
initial aged condition (see Fig. 4.14), due to the formation of mostly metastable
β microstructure during fast cooling from the β homogenisation field. Ageing
after quenching causes an increase of hardness, as compared to the quenched
condition. The reason for this is the formation of fine α precipitates during
ageing. There is a clear trend showing that as the ageing temperature is
increased, the hardness decreases. The reason for this decrease is the formation
of coarser precipitates during ageing at higher temperature, and possibly
relief of the micro-stresses.
The same trend, i.e. decrease at higher temperatures of ageing, is observed
with the ultimate tensile strength (Fig. 4.15), for the same reasons as for
hardness changes. The trend for change of the elongation, a measure of the
6 hours
810 °C
8 hours
Water quench
595 °C
540 °C
480 °C
4.12
Schematic presentation of heat treatments for β21s alloy.
