Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.


Differential scanning calorimetry and property measurements 85
material’s ductility, is the opposite, namely the elongation increases, with
higher temperature of ageing (Fig. 4.16). Obviously, the ageing temperature
is a very significant parameter for control of the microstructure and properties
in β-titanium alloys. By altering this temperature, the desirable combination
of strength and ductility can be achieved.
100 µm
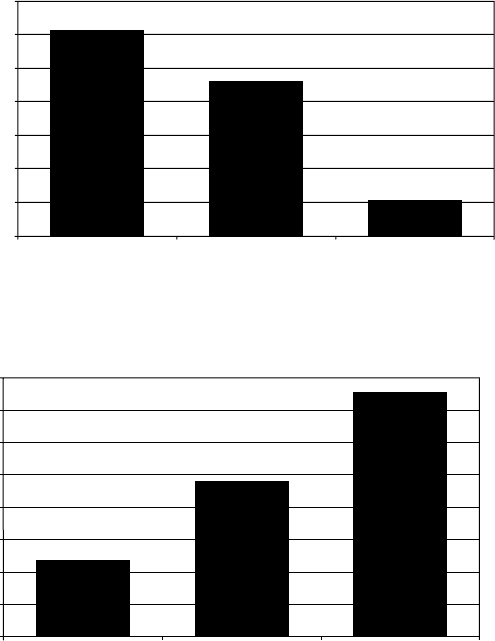
4.13
Microstructure of β21s alloy after water quenching and ageing at
480 °C for 8 hours.
Aged Water Ageing at Ageing at Ageing at
(starting state) quenched 480 °C 540 °C 595 °C
Vickers hardness (HV)
370
360
350
340
330
320
310
300
290
280
270
4.14
Vickers hardness of β21s alloy with different processing
conditions.
Titanium alloys: modelling of microstructure86
4.3 Effects of hydrogen penetration
Titanium alloys are susceptible to hydrogen, which causes embrittlement,
leading to the deterioration of the properties of the alloys. For a Ti-42Al-
11Nb (at.%) alloy, the yield strength increases with increasing amount of
hydride, but the ultimate tensile strength, ductility and fracture toughness
decrease. Therefore, the amount of hydrogen that a titanium alloy can absorb
during service is a major measure of the ability of the alloy to retain good
properties. Much effort has been made to quantify the hydrogen susceptibility
of titanium alloys. This section describes the effect of hydrogen.
Confusion exists concerning the form of hydrogen after entering titanium
alloys. It was suggested that a hydride with the structure (TiAl)H
x
might
form. Samples of Ti-49.9Al (at.%) were ground, polished and cathodically
480 540 595
Ageing temperature (°C)
Tensile strength (MPa)
1350
1300
1250
1200
1150
1100
1050
1000
4.15
Tensile strength of β21s alloy after ageing at different
temperatures.
480 540 595
Ageing temperature (°C)
Elongation (%)
20
19
18
17
16
15
14
13
12
4.16
Elongation of β21s alloy after ageing at different temperatures.
Differential scanning calorimetry and property measurements 87
charged in a 1 mol/l NaOH and 250 mg/l As
2
O
3
solution at room temperature
with a current density of 300 A/m
2
. X-ray diffraction showed that for a
sample with a single γ phase before charging, a hydride phase was formed
after 4 hours of charging. The hydride phase was determined to be (TiAl)H
0.5
,
with tetragonal lattice parameters a = 0.450 nm and c = 0.327 nm (c/a =
0.727).
The surface of a γ and α
2
two-phase titanium aluminide sample was
covered with a black layer after prolonged cathodic hydrogen charging.
Analytical transmission electron microscopy showed that the surface layer
was a hydride based on (TiAl)H
x
. It was determined that the hydride had a
tetragonal crystal structure with lattice parameters a = 0.452 nm and c =
0.326 nm (c/a = 0.721), and it was present up to 25 µm in depth from the
surface of the sample after charging for 2 hours. The charging condition was
a 5% H
2
SO
4
solution, and current density equal to 5000 A/m
2
. The weight
change of the sample with increasing charging time was also measured. The
weight decreased with increasing charging time after 1 hour.
Hydrogen charging of Ti-42Al, Ti-45Al and Ti-50Al induced crack formation
after a short charging time, while additional charging produced pits within
the γ phase in the γ and α
2
two-phase coexisting grains. However, no damage
was seen in the α
2
phase or equiaxed single α grains.
Cast Ti-48Al-2Cr and wrought Ti-46.5Al-4(Cr,Nb,Ta,B) gamma titanium
aluminides were cathodically charged with hydrogen for various times to
study possible hydrogen trap sites and the nature of the hydride being formed
(Sundaram et al., 2000). The charging solution was 1
N H
2
SO
4
solution with
1 g/l thiourea. The charging time varied between 1 and 24 hours and the
current density was 1 A/m
2
. No visible change was noted on the charged
surface after 8 hours as compared with the uncharged surface. However,
after 16 hours, small black particles, presumably hydrides, were observed on
the surface. After 24 hours, a black hydride layer covered the surface. After
X-ray diffraction patterns were obtained, Sundaram et al. (2000) stated that
the result proved to be similar to the previous findings.
Sundaram et al. (2000) determined that a hydride was formed when hydrogen
entered their samples. However, this does not discount the possibility that
some hydrogen may occupy the interstitial sites in the alloys. Both could
take place simultaneously.
The following summarises the further investigation into the hydrogen
susceptibility of β21s and Ti-46Al-1.9Cr-3Nb alloys, by measuring the amount
of hydrogen penetrating into the sample within a specific charging time.
Establishing a rate of penetration can give an indication of the ability of the
alloy to resist hydrogen.
Immediately after charging, some of the samples were placed in a solution
of glycerine for 48 hours at room temperature. This technique had been used
before, and the quantity of hydrogen released during 48 hours at room
Titanium alloys: modelling of microstructure88
temperature was used as a measure for the quantity of hydrogen absorbed
during permeation. After such experiments, no hydrogen was released and
collected after 48 hours. The hydrogen remained within the samples.
The average Rockwell hardness numbers before and after charging were
exactly the same. Some of the samples cracked, however, on using the Rockwell
hardness indentation after charging.
The effects of hydrogen on the samples could be clearly seen after the
experiment, especially after severe charging, such as 4 hours at 85 °C in
2.8% H
2
SO
4
at 250 A/m
2
. The samples had suffered extensive corrosion
while the remainder was covered with a black layer. Sundaram et al. (2000)
reported similar damage.
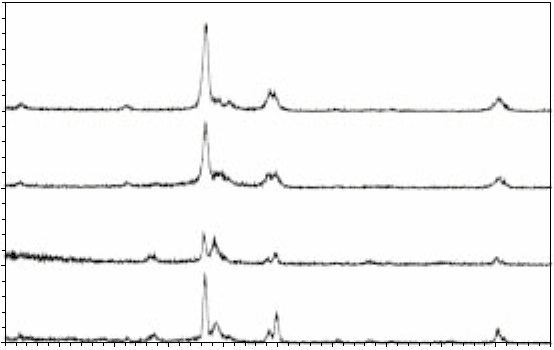
The additional peaks present in the X-ray diffraction patterns for charged
samples indicate the presence of a hydride (Fig. 4.17). The plane index
values corresponding to the peaks in the diffraction patterns were determined
by comparison with data by Sundaram et al. (2000), as the hydride peaks
established here are similar to XRD data of hydrides available from these
authors. The intensity of the peaks is summarised in Table 4.3. The fluctuation
in the total integrated intensities of the peaks in the XRD patterns is, most
likely, due to the inevitable positional variation in the experimental set-up
for different samples.
4.17
X-ray diffraction patterns for Ti-46Al-1.9Cr-3Nb alloy. (a)
Uncharged; (b) charged for 1 hour at room temperature, 250 A/m
2
,
5% H
2
SO
4
solution; (c) charged for 4 hours at 85 °C, 250 A/m
2
, 2.8%
H
2
SO
4
solution; (d) charged for 2 hours at room temperature,
5000 A/m
2
, 5% H
2
SO
4
solution.
Intensity (c.p.s.)
1000
750
500
250
0
20 25 30 35 40 45 50 55 60 65 70
2θ (°)
(a)
(b)
(c)
(d)
001γ
110γ
111γ
201α
2
002γ
200γ
201γ
220γ
101H
111H
121H
102H
Differential scanning calorimetry and property measurements 89
There is a small amount of α
2
phase present in the uncharged Ti-46Al-
1.9Cr-3Nb alloy. However, the small amount of α
2
phase disappears with
charging (see Fig. 4.17).
Ti-46Al-1.9Cr-3Nb alloy subject to cathodic charging has shown cracking.
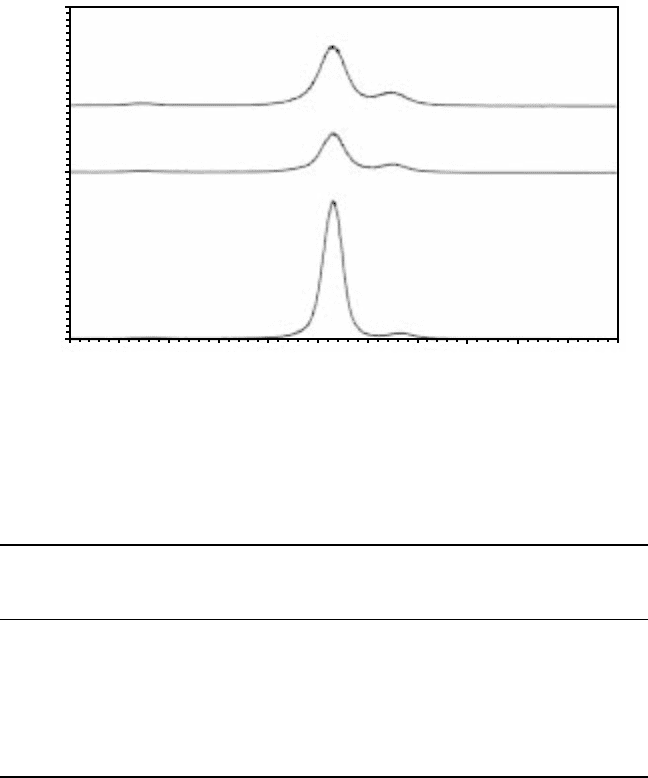
The XRD pattern for the β21s alloy charged in the solution of H
2
SO
4
with
0.2 g/l As
2
O
3
produces no additional peak in the 2
θ
range between 20 and
70°, when compared with the XRD pattern for the uncharged alloy (Fig.
4.18). No hydride has formed. Comparison of the XRD patterns for uncharged
alloy shows the possible heterogeneity of the material, concerning the shift
of peaks, although there might be a contribution from experimental factors.
Both intensity and 2
θ
can be altered, depending on the level at which the
sample is placed within the XRD apparatus. Therefore, if all peaks move in
the one direction, or if all peaks drop in intensity, it is possible that this is due
to samples being placed at different levels during the XRD experiments.
However, if the intensity of one peak increases while that of another peak
decreases in relation to the uncharged XRD pattern of the sample in question,
this is not because of an experimental factor. There is an increase in the
amount of β phase while the amount of α phase decreases.
In spite of the absence of hydride, the surface of β21s still displays damage
caused by hydrogen charging, and this could be seen even with the naked
eye. Charging for 2 hours at room temperature, 5000 A/m
2
in 5% H
2
SO
4
solution produced extensive cracking, while the surfaces of the samples
charged for 2 hours at 85 °C and 250 or 5000 A/m
2
were blackened when
compared to their original appearance.
Table 4.3
X-ray diffraction peak relative height of titanium aluminide after
hydrogen charging
Phase
hkl
Uncharged 5% H
2
SO
4
, 2.8% H
2
SO
4
, 5% H
2
SO
4
,
0.2 g/l As
2
O
3
, 1 g/l thiourea, 0.2 g/l As
2
O
3
,
250 A/m
2
, 250 A/m
2
, 5000 A/m
2
,
1 h, room 4 h, 85 °C 2 h, room
temperature temperature
γ 001 11 12 – 17
110 8 12 22 11
111 100 100 100 100
002 26 26 25 21
200 25 26 35 46
201 4 5 13 8
220 18 20 25 24
α
2
201 13 –––
(TiAl)H
x
101 –– 40 16
111 – 26 85 30
121 –– 18 8
102 –– 11 4
Titanium alloys: modelling of microstructure90
Table 4.4 shows the hydrogen analysis results.
It has already been established that a hydride phase based on (TiAl)H
x
forms in Ti-46Al-1.9Cr-3Nb alloy during cathodic charging, but no hydride
forms in β21s during cathodic charging. Table 4.4 shows that hydrogen does
enter β21s, and this leads to the conclusion that the hydrogen occupies the
interstitial sites in β21s.
From Table 4.4, the amount of hydrogen present before charging is
approximately 40 times greater in β21s than Ti-46Al-1.9Cr-3Nb alloy. However,
4.18
X-ray diffraction patterns for β21s alloy. (a) Uncharged; (b)
charged for 2 hours at room temperature, 5000 A/m
2
, 5% H
2
SO
4
solution; (c) charged for 2 hours at 85 °C, 5000 A/m
2
, 5% H
2
SO
4
solution.
Intensity (c.p.s.)
20000
18000
16000
14000
12000
10000
8000
6000
4000
2000
0
34 35 36 37 38 39 40 41 42 43 44 45
2θ (°)
(a)
(b)
(c)
100α
110β
101α
Table 4.4
Rates of hydrogen penetration
Material Current Temperature Time Hydrogen Hydrogen Rate of
density (°C) (h) (ppm) entered penetration
(A/m
2
) (ppm) (ppm/h )
Ti-46Al- Uncharged ––24 ––
1.9Cr-3Nb 250 room 1 99 75 75
250
(a)
85 4 638
(b)
614 154
5000 room 2 643 619 310
β21s Uncharged ––963 ––
250 85 2 1132 169 85
5000 85 2 1293 330 165
(a)
The charging solution was 2.8% H
2
SO
4
. A 5% H
2
SO
4
solution was used for all
other samples.
(b)
This corresponds to 2.5 at.% of hydrogen.
Differential scanning calorimetry and property measurements 91
calculating the rates of hydrogen penetration for each of the samples has
demonstrated that β21s is less susceptible to hydrogen penetration. Both an
increase in the current density and an increase in the temperature of the
solution cause an increase in the rate of hydrogen penetration.
In summary, the small amount of α
2
phase in the Ti-46Al-1.9Cr-3Nb
alloy becomes undetectable after hydrogen charging. A hydride based on
(TiAl)H
x
, which has tetragonal lattice parameters of a = 0.452 nm and c =
0.326 nm (c/a = 0.721), forms in Ti-46Al-1.9Cr-3Nb alloy after cathodic
charging. Hydrogen charging induces crack formation on both Ti-46Al-1.9Cr-
3Nb alloy and β21s. No hydride is formed in β21s by cathodic charging. The
hydrogen enters the alloy and occupies the interstitial sites between the
atoms. During charging, there is an increase in the amount of the β phase and
a decrease in the amount of the α phase on the β21s surface. β21s is less
susceptible to hydrogen penetration than Ti-46Al-1.9Cr-3Nb alloy. The ability
of β21s to resist hydrogen penetration, when compared with the ability of Ti-
46Al-1.9Cr-3Nb alloy, is approximately greater by a factor of two.
4.4 References
Ohnuma I, Fujita Y, Mitsui H, Ishikawa K, Kainuma R and Ishida K (2000), ‘Phase
equilibria in the Ti–Al binary system’, Acta Mater, 48 (12), 3113–23.
Sundaram P A, Quadakkers W J and Singheiser L (2000), ‘Hydrogen effusion in cathodically
charged gamma titanium aluminides’, J Alloys Compd, 298 (1–2), 274–78.

95
5
Thermodynamic modelling
Abstract: Thermodynamic calculations using Thermo-Calc are used to
quantify the phase fraction and element partition in a number of
conventional titanium alloys and γ-TiAl based alloys, chosen from all of the
titanium alloy groups and including both well investigated alloys and those
that are relatively new. Calculations of the phase constitution and element
distribution in these alloys show a good agreement with the experimental
measurement. In addition, Thermo-Calc calculation can help identify the
existence of some phases that are not readily observed experimentally. It can
be used as a guide in alloy design.
Key words: titanium aluminides based on TiAl, phase transformation,
precipitates, phase stability, thermodynamics.
5.1 Introduction
The thermodynamics modelling is usually based on the Gibbs energy
calculation. Two most commonly used calculation packages are Thermo-
Calc (Andersson et al., 2002) and MTDATA (Davies et al., 2002). These
packages have been successfully applied for thermodynamic calculations of
different materials systems (Dore et al., 2000; Ekroth et al., 2000; Eskin,
2002; Gorsse and Shiflet, 2002; Guo and Sha, 2000; Jarvis et al., 2000; Sha,
2000; Zackrisson et al., 2000) (Chapters 3 and 6).
Thermodynamic calculation can supplement experimental characterisation,
and allows the prediction of phase type, and fraction and element distribution
in different phases. Thermo-Calc is a computer package developed particularly
for thermodynamic calculations of multi-component equilibrium as a function
of pressure, temperature and the combined effect of alloying elements, using
a databank of assessed thermodynamic data and models for the phases in the
system that are as good an approximation of the nature as possible. It employs
rigorous thermodynamic expressions and numerical methods of minimising
the chemical energy of the system, so that interpolation between the available
experimental data can be made. Good agreement has been obtained in the
past between the calculated phase compositions and experimental
measurements.
In this chapter, thermodynamic calculations of the phase equilibria in
different titanium alloys will be shown, including Ti-6Al-4V (Ti 6-4), Ti-
6Al-2Sn-4Zr-2Mo (Ti 6-2-4-2), Ti-6Al-2Sn-4Zr-6Mo (Ti 6-2-4-6), Ti-8Al-
1Mo-1V (Ti 8-1-1), Ti-5.8Al-4Sn-3.5Zr-0.7Nb-0.5Mo-0.35Si (IMI 834), Ti-
6Al-7Nb (IMI 367), Ti-10V-2Fe-3Al (Ti 10-2-3) and TIMETAL β21s. These
alloys are chosen from all of the titanium alloy groups and include both well-
Titanium alloys: modelling of microstructure96
investigated alloys and those which are relatively new. The calculations are
performed for the actual alloy composition taking into account all major
alloying elements. Further thermodynamic calculation procedures are given
in Chapters 3 and 6. The results from the thermodynamic calculations and
their coupling with the models for simulation of the microstructure evolution
in titanium alloys are discussed in several chapters in this part of the book.
5.2 Conventional titanium alloys
In conventional titanium alloys, there are different equilibrium amounts of α
and β phases as well as equilibrium compositions of both phases at different
temperatures. We shall calculate the phase equilibria for different titanium
alloys in wide temperature ranges. The calculated data are compared with
experimentally obtained data (Chapter 6).
5.2.1 Ti-6Al-4V (Ti 6-4)
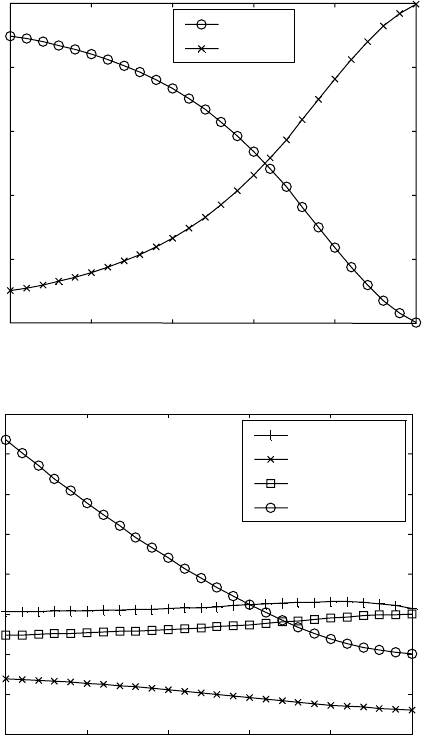
The calculated amounts of the α and the β phases for the Ti-6Al-4V alloy are
given in Fig. 5.1a, showing increased equilibrium amount of the α phase
when the temperature decreases. The tendency is in agreement with the
behaviour of the α → β phase transformation in titanium alloys.
Further, we calculate the equilibrium chemical compositions of both α
and β phases as functions of the temperature (Fig. 5.1b). Such calculation is
important for further modelling work because it gives information for the
diffusion redistributions between the two phases during the course of β to α
transformation.
5.2.2 Ti-6Al-2Sn-4Zr-2Mo (Ti 6-2-4-2)
The calculation for the Ti-6Al-2Sn-4Zr-2Mo alloy also shows increased
amounts of the α phase and decreased amounts of the β phase with temperature
decrease (Fig. 5.2a). Compared to the Ti-6Al-4V alloy, the α phase in the Ti-
6Al-2Sn-4Zr-2Mo alloy is more stable in the entire temperature range. The
amount of the α phase at lower temperature for this alloy approaches 100%,
while in the Ti-6Al-4V alloy, there is some amount of the β phase remaining.
On the other hand, for the Ti-6Al-4V alloy, the phase composition of 50%α
+ 50%β is at about 905 °C, while in the Ti-6Al-2Sn-4Zr-2Mo alloy, the
50%α + 50%β phase composition is at around 955 °C. This is why the
Ti-6Al-2Sn-4Zr-2Mo alloy is considered as a high-temperature titanium
alloy, and can work at higher temperatures as compared to the Ti-6Al-4V
alloy.
The calculations of the equilibrium concentrations of the alloying elements
in the α and the β phases for this alloy suggest that the β to α transformation
Thermodynamic modelling 97
at cooling is in conditions of diffusional redistribution and enrichment of
molybdenum in the β phase (Fig. 5.2b).
The Ti-6Al-2Sn-4Zr-6Mo alloy is similar to the Ti-6Al-2Sn-4Zr-2Mo alloy
but with a higher amount of molybdenum, and is classified as an α + β alloy.
The thermodynamics of the two alloys is similar.
Alpha
Beta
Phase amounts (%)
100
80
60
40
20
0
750 800 850 900 950 1000
T
(°C)
(a)
750 800 850 900 950 1000
T
(°C)
(b)
Content (wt.%)
16
14
12
10
8
6
4
2
0
Al in alpha
V in alpha
Al in beta
V in beta
5.1
Calculated equilibria versus temperature in the Ti-6Al-4V alloy:
(a) equilibrium amounts of the α and the β phases; (b) equilibrium
concentrations of aluminium and vanadium in the α and the β
phases. The calculations are for composition of Al = 6 wt.%; V = 4
wt.%; Fe = 0.2 wt.%; O = 0.15 wt.%; N = 0.03 wt.%; and C = 0.01
wt.%.
