Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Titanium alloys: modelling of microstructure118
under isothermal conditions. An in-situ electrical resistivity technique is
introduced to experimentally study the course of the transformation.
Experimental procedures are described in original research papers (Malinov
et al., 2001, 2002, 2003; Markovsky et al., 2004). The kinetics are traced
within the theoretical frame of the JMA theory.
6.2 Resistivity experiments
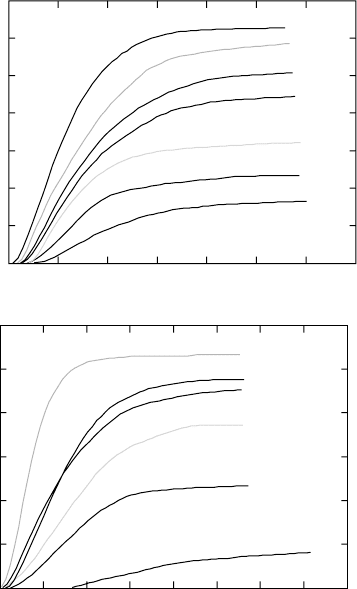
Following experiments, sets of curves showing the relative increase of the
resistivity during isothermal exposure (∆R/R
0
) versus time for different
temperatures can be recorded. Typical resistivity curves are shown in Fig.
6.1. Generally, for all these alloys, there is increase of resistometric signal
∆
R/R
0
(%)
3.5
3
2.5
2
1.5
1
0.5
0
(a) Ti-6Al-4V
0 10203040506070
Time (sec)
750 °C
800 °C
850 °C
870 °C
900 °C
920 °C
950 °C
∆
R/R
0
(%)
6
5
4
3
2
1
0
(b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si
0 10203040 50607080
Time (sec)
735 °C
750 °C
800 °C
850 °C
900 °C
930 °C
6.1
Resistivity data at different temperatures of isothermal exposure
(a) Ti-6Al-4V; (b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si; (c) Ti-8Al-1Mo-1V;
(d) β21s. For clarity, the resistivity curves are shifted with respect to
each other along the vertical axis.
The Johnson–Mehl–Avrami method 119
with lowering of temperature. Two factors may have influence on the resistivity:
(i) different amounts of the α and the β fractions, e.g. the increase of the α
fraction, and (ii) change of the compositions of the α and the β phases, e.g.
the enrichment of the β phase with vanadium in the case of Ti-6Al-4V. In
order to separate the above two effects, calibration curves are needed. The
results from the optical microscopy quantitative study of samples quenched
after heating to different temperatures for different times can be used to
build the calibration curves of the relative resistance (∆R/R
0
) versus the
∆
R/R
0
(%)
7
6
5
4
3
2
1
0
(c) Ti-8Al-1Mo-1V
0 10 203040 5060 70
Time (sec)
750 °C
800 °C
850 °C
900 °C
925 °C
950 °C
975 °C
∆
R/R
0
(%)
3
2.5
2
1.5
1
0.5
0
(d) β21s
0 20 40 60 80 100 120
Time (min)
750 °C
700 °C
650 °C
600 °C
500 °C
400 °C
6.1
Continued
Titanium alloys: modelling of microstructure120
amount of precipitated α phase (%) for each alloy. Such calibration curves
are necessary for converting resistivity data into α phase fraction. For Ti-
6Al-4V, Ti-6Al-2Sn-4Zr-2Mo-0.08Si, and Ti-8Al-1Mo-1V alloys, linear
relations between ∆R/R
0
and the amount of α are found. The linear relation
is well proved and very clear when the degree of transformation varies
between about 10 and 90%. The relative error of the calibration curve within
this range is about 5%. For the very early stages (<10% degree of
transformation) as well as for the final stages (>90% degree of transformation),
there are some deviations from the linear relation and larger errors are possible.
The following remarks on the resistivity effect are also significant:
• Only primary α phase has influence on the resistivity effect, because just
this phase is formed during the isothermal exposure. Secondary α phases,
i.e. α′ or α″ martensite, are formed as a result of residual β phase
decomposition on subsequent quenching or further cooling, which allows
one to evidently recognise and numerically evaluate the quantity of
phases formed at different stages of β decomposition. Their influence on
resistivity is not considered when studying the β phase decomposition
under isothermal conditions only.
• The effects of α, α′ and α″ phases on the resistivity differ mainly in the
kinetics and the temperature ranges when these transformations take
place on cooling. In the present case, resistivity is used for studying
stable α phase formation under isothermal conditions.
• The different morphology of the α phase in the initial state, before
heating to the single β-phase field, can influence the initial value of the
specimen resistivity (R
0
). However, there is no difference in the material’s
behaviour after solid solutioning at temperatures above β-transus, because
alloys with any starting microstructure become the same, consisting of
coarse grains of β phase.
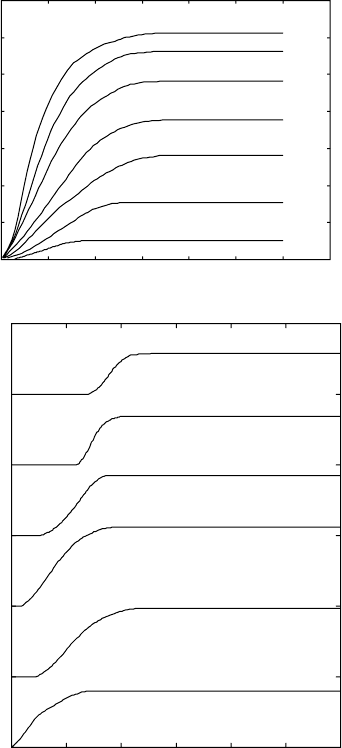
Using the aforementioned calibration relations, the experimental resistivity
curves can be recalculated or converted to give the amounts of α phase
versus time, for different holding temperatures (Fig. 6.2a–c), which trace the
kinetics of the β to α + β transformation for the Ti 6-4, the Ti 6-2-4-2 and the
Ti 8-1-1 alloys. These data can be further used for modelling and simulations.
At different temperatures of isothermal exposure, different final amounts of
the α phase are obtained. For titanium alloys, the transformation of β to α +
β is of monovariant type. At different temperatures, different amounts of α
and β phases are in equilibrium. There are different equilibrium compositions
of both phases at different temperatures. This observation will be discussed
in more detail in Section 6.6.
The composition of the alloy β21s corresponds to an aluminium equivalent,
[Al]
eq
, of 3 wt.%, and molybdenum equivalent, [Mo]
eq
, of 15.8 wt.%. This
composition determines the alloy as a metastable β titanium alloy (Boyer
The Johnson–Mehl–Avrami method 121
(a) Ti-6Al-4V
750 °C
800 °C
850 °C
870 °C
900 °C
920 °C
950 °C
010203040506070
Time (sec)
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
(b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si
735 °C
750 °C
800 °C
850 °C
900 °C
930 °C
010 20304050607080
Time (sec)
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
6.2
Kinetics of the β to α + β transformation at different temperatures
of isothermal exposure (a) Ti-6Al-4V; (b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si;
(c) Ti-8Al-1Mo-1V; (d) β21s.
Titanium alloys: modelling of microstructure122
et al., 1994). Hence, initial homogeneous β structure tends to precipitate α
phase upon isothermal exposure at temperatures in the α + β equilibrium
range. The alloy has α + β phase composition in the condition normally
supplied for commercial use. Before resistivity measurement, the samples
should be heated up to the β homogenisation field, e.g. 1000 °C, and held for
30 minutes. In order to check the completeness of the β homogenisation, one
can cool samples from this stage to room temperature. The results from
synchrotron X-ray study have shown that the phase composition in both air
(c) Ti-8Al-1Mo-1V
750 °C
800 °C
850 °C
900 °C
925 °C
950 °C
975 °C
0 10203040506070
Time (sec)
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
(d) β21s
0 102030405060
Time (min)
Amount of α phase (vol. %)
40
30
20
10
0
650 °C
600 °C
700 °C
500 °C
750 °C
6.2
Continued
The Johnson–Mehl–Avrami method 123
cooled and water quenched samples is homogeneous β phase, confirming
that at this stage the initial α + β structure has transformed to β. After the β
homogenisation, samples can be cooled rapidly to the chosen holding
temperature and then isothermally held at this temperature when the
resistometric signal is recorded. A set of curves of ∆R/R
0
versus time for
different temperatures of isothermal exposure can be recorded (Fig. 6.1d).
These curves trace the course of the α phase precipitation from the metastable
β phase at the temperatures studied. Some observations from the recorded
resistivity signals are discussed below.
The resistivity curve at 400 °C is inconsistent with the results for the other
temperatures. The signal shows immediate change of the resistivity from the
very beginning of the isothermal exposure. The difference between the initial
and the final values in ∆R/R
0
is smaller as compared to the other temperatures.
In addition, the curve shape is different. A possible reason for this difference
is that, at this temperature, the transformation is not purely diffusional β to
α transformation, but some precipitation of metastable phases, such as ω
phase, may be involved. This temperature is not included in the further
analysis.
Decomposition of the metastable β phase in the β21s alloy due to its
higher content of β stabilising elements is considerably slower, including a
pronounced incubation period (Fig. 6.1d). The longest incubation period
(about 28 min) is at 750 °C. This decreases when the temperature is lower.
The minimum value, i.e. the shortest, is for isothermal exposure at 600 °C.
Further decrease of the temperature to 500 °C results in increase of the
incubation time. These results indicate that the ‘nose’ point of the TTT
diagram for this alloy should be around 600 °C. The resistivity curves at all
temperatures tend to become horizontal after one hour isothermal exposure.
This means that the transformation is either completed or the rate of the
transformation is very slow and practically undetectable with the resistivity
method used.
In order to recalculate and transform the resistivity curves to curves showing
the amount of α phase versus time, we need to create calibration curves
between the resistivity signal and the amount of the α phase. Samples quenched
from different temperatures and after different times are studied by light
microscopy (Fig. 6.3). Large deviations in the estimated amounts of the α
phase are detected and the results from the metallography study are found to
be too statistically unreliable to be used for the calibration purpose. The
reason for this is the small amount and the fine microstructure of the α phase.
6.3 Metallography
The typical microstructures of Ti-6Al-2Sn-4Zr-2Mo-0.08Si and Ti-6Al-4V,
and Ti-8Al-1Mo-1V alloys after isothermal exposure at different temperatures
Titanium alloys: modelling of microstructure124
and subsequent quenching are presented in Figs. 6.4 and 6.5, respectively,
illustrating distinct difference in morphology observed between primary
and secondary α phases. First, it is necessary to point out that the α phase
covering the β grain boundary is observed in all cases of isothermal exposure
for both Ti-6Al-2Sn-4Zr-2Mo-0.08Si and Ti-6Al-4V alloys. This may be
explained by the comparatively low (300 °C/s) cooling rate after β solution
treatment. For the Ti 6-4 alloy, the critical cooling rate that depresses
completely all diffusion processes of alloying element redistribution should
be about 400 °C/s or higher. For the more diluted Ti 6-2-4-2 alloy, the critical
cooling rate is even higher (≥500 °C/s). Hence, some diffusion processes
may take place during the cooling at an insufficient rate from the β field to
the temperature of isothermal exposure. These processes cause grain boundary
α formation.
6.3.1 Ti 6-2-4-2 alloy
The as-quenched microstructure after isothermal exposure at 950 °C is
characterised by the presence of a fine grain boundary α phase (Fig. 6.4a).
The total amount of diffusionally grown α phase is about 8%. The remaining
volume fraction is occupied by a martensitic phase, which is formed from
the β phase on subsequent cooling. The matrix, martensite, microstructure is
not homogeneous. This is due to the complex mechanism of the transformation,
including partial diffusional redistribution of β alloying elements and
diffusionless martensitic transformation. (Note that the cooling rate after
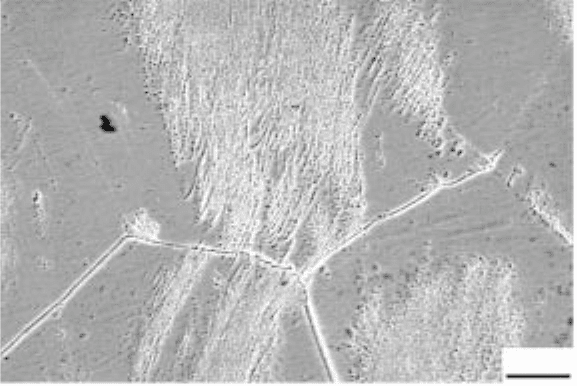
20 µm
6.3
Microstructure of β21s alloy after isothermal exposure at 650
°C
(dark field optical image).

The Johnson–Mehl–Avrami method 125
50 µm
50 µm
50 µm
(d) (e) (f)
100 µm
50 µm
20 µm
(a) (b) (c)
25 µm
(g)
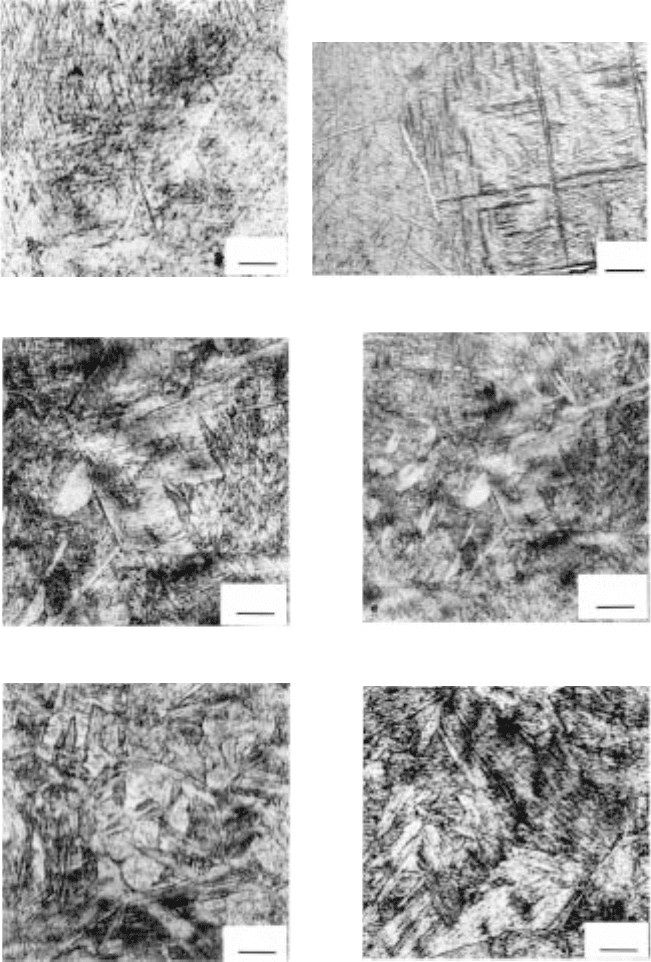
6.4
Microstructures of (a–c) Ti-6Al-2Sn-4Zr-2Mo-0.08Si and (d–g) Ti-
6Al-4V alloys after different temperatures of isothermal exposure and
subsequent quenching. (a) 950 °C; (b) 900 °C; (c) 850 °C; (d) 920 °C;
(e) 870 °C; (f) and (g) 850 °C.
Titanium alloys: modelling of microstructure126
25 µm
(a)
25 µm
(b)
25 µm
(c)
40 µm
(d)
(e)
25 µm
(f)
25 µm
6.5
Microstructures of Ti-8Al-1Mo-1V alloy after different
temperatures of isothermal exposure and subsequent quenching.
(a) 925 °C; (b) 900 °C; (c) and (d) 850 °C; (e) 800 °C; (f) 750 °C.
The Johnson–Mehl–Avrami method 127
isothermal exposure, 300 °C/s, is not high enough for pure martensitic
transformation).
A decrease of the temperature of isothermal exposure down to 900 °C
causes formation of a coarse α phase covering β grain boundaries. In addition,
some amount of α phase has diffusionally nucleated and grown from the
boundaries (Fig. 6.4b). The amount of the α phase is higher in small β grains
as compared to in big ones. This observation may be associated with the
influence of the grain boundary energy, which is proportional to the grain
boundary curvature. The total amount of diffusionally formed α phase at
900 °C is 38%.
Lower temperatures of isothermal exposure lead to a complete change of
the mechanism of α phase formation. In addition to the grain boundary α
phase, formed on cooling from the β field to the temperature of isothermal
exposure, an α phase nucleated and grown homogeneously inside the former
β grains is observed (Fig. 6.4c). The total amounts of α phase are 69 and
80% for 850 and 800 °C, respectively. The main difference between specimens
exposed at 850 and 800 °C is the different dispersion of the α lamellae –
finer lamellae are attributed to lower temperatures. This may be explained
by the higher contribution from the nucleation process in comparison to the
growth when the temperature of isothermal exposure is lower. Nevertheless,
in both cases, the morphology of the α phase allows one to suppose that it is
formed with partial participation of diffusionless (shifting) processes.
6.3.2 Ti 6-4 alloy
In the specimen exposed at 950 °C and then quenched, a diffusionally formed
α phase is found on some β grain boundaries, as a thin layer covering the
boundaries. The volume fraction of the α phase is about 16%. After exposure
at 920 °C (Fig. 6.4d), the morphology of the α phase, formed as a result of
diffusional decomposition of the β phase, remains the same. Its volume
fraction is slightly increased, to 20%. At 900 °C, a small portion of grain
boundary α phase, as well as α plates diffusionally grown from them, are
observed. The volume fraction of the α phase is about 38%.
At a lower temperature of 870 °C, the microstructure is changed principally.
Some portions of the α phase nucleated and grown within the β grains
appear, in addition to the grain boundary α phase (Fig. 6.4e). The volume
fraction of the diffusional α phase is about 60%. A further decrease of the
temperature down to 850 °C leads to an increase in the amount of
homogeneously nucleated α phase within the former β grains (Fig. 6.4f and
g). Some martensite is found in the regions close to the β grain boundaries.
A decrease of the temperature down to 800 °C and, especially, to 750 °C
causes further increase of the amount of diffusionally formed α phase, to 72
and 82%, respectively.
