Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Titanium alloys: modelling of microstructure148
Following the above reasoning and fitting the resistivity data to the JMA
equation assuming different n values for β to α + β transformation for
temperatures above and below 900 °C, the derived kinetics parameters as
well as the different mechanisms of the α phase nucleation and growth for
both alloys are summarised in Table 6.6.
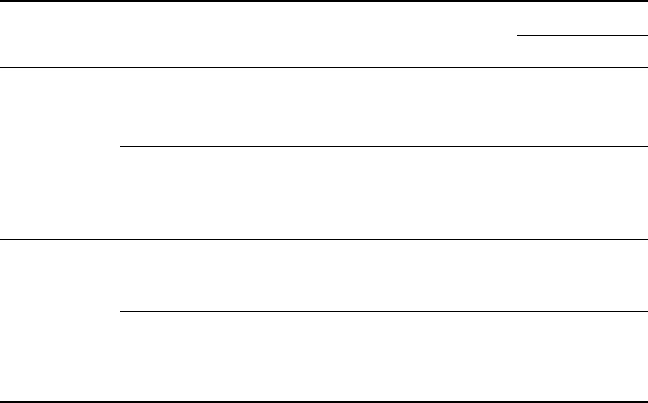
In Fig. 6.14, the calculated (from the JMA theory) and the experimental
(from the resistivity measurements) transformation kinetics are compared
for different constant temperatures. Calculated fractions are based on Eq.
[6.2], using the n and k values given in Table 6.6. A very good agreement
between the calculated and the experimental curves is evident. This agreement
supports the validity of the obtained JMA kinetic parameters, which can be
used in the heat treatment practice of the titanium alloys to trace and predict
the course of β to α + β transformation under different isothermal conditions.
The approach described above using two different mechanisms and,
respectively, two different n values of the β to α + β transformation is
different from the approach used in Chapter 7: in that chapter the kinetics of
the same transformation are modelled during continuous cooling based on
differential scanning calorimetry data. A single n value for the entire temperature
interval of the transformation is attributed to continuous cooling conditions.
In the following, some explanation of the different approaches is given.
The mechanisms of the transformations are different for continuous cooling
and isothermal conditions with respect to the nucleation and growth conditions.
This difference is significant for phase transformations taking place on cooling
Table 6.6
Johnson–Mehl–Avrami kinetic parameters for two different mechanisms
of the β to α + β transformations in Ti 6-4 and Ti 6-2-4-2 alloys at various
temperatures of isothermal transformations
Alloy
T
(°C) α phase morphology JMA parameters
nk
Ti-6Al-4V 950 Grain boundary α phase, and some 1.1 0.045
920 amounts of α plates nucleated and 0.055
900 grown from the grain boundaries 0.068
870 Mixed: Homogeneously nucleated 1.35 0.025
850 and plate-like grown α structure, 0.024
800 and grain boundaries nucleated and 0.025
750 grown α phase (Fig. 6.4c) 0.033
Ti-6Al-2Sn- 930 Grain boundary α phase, and some 1.15 0.035
4Zr-2Mo- 900 amounts of α plates nucleated and 0.043
0.08Si grown from the grain boundaries
850 Mixed: Homogeneously nucleated 1.48 0.013
800 and plate-like grown α structure, 0.017
750 and grain boundaries nucleated 0.018
735 and grown α phase (Fig. 6.4f) 0.044
The Johnson–Mehl–Avrami method 149
Ti-6Al-4V
Experimental
Calculated
0 10203040506070
Time (sec)
(a)
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
750 °C
800 °C
850 °C
870 °C
900 °C
920 °C
950 °C
Ti-6Al-2Sn-4Zr-2Mo-0.08Si
0 10 20304050 607080
Time (sec)
(b)
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
735 °C
750 °C
800 °C
850 °C
900 °C
930 °C
6.14
Experimental and calculated kinetics of the β to α + β
transformation at different temperatures for (a) Ti-6Al-4V and
(b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys.
Titanium alloys: modelling of microstructure150
in titanium alloys. In the case of continuous cooling, at each temperature, the
transformation starts and proceeds in conditions of microstructure that has
been formed at previous stage(s). For example, at 850 °C, the transformation
on continuous cooling is in conditions of nucleation sites that already exist
(grain boundary α formed on cooling from β-transition to the current
temperatures), which are preferable sites for further growth. In the case of
isothermal exposure, the sample is cooled rapidly from the β-field to the
temperature of isothermal exposure (e.g. 850 °C). At this temperature,
spontaneous nucleation (mainly homogeneous) and growth take place. In
other words, in the cases of isothermal exposure, when the undercooling is
high, the amount of the α phase formed and grown within the former
β grains will be higher as compared to the grain boundary α (see Fig. 6.4f
and g). These two different mechanisms result in a somewhat different
morphology of the α phase formed and imply a difference in the Avrami
index derived from continuous cooling and isothermal experiments.
Nevertheless, both approaches and models have their own importance and
area of application.
6.7.2 Ti 8-1-1
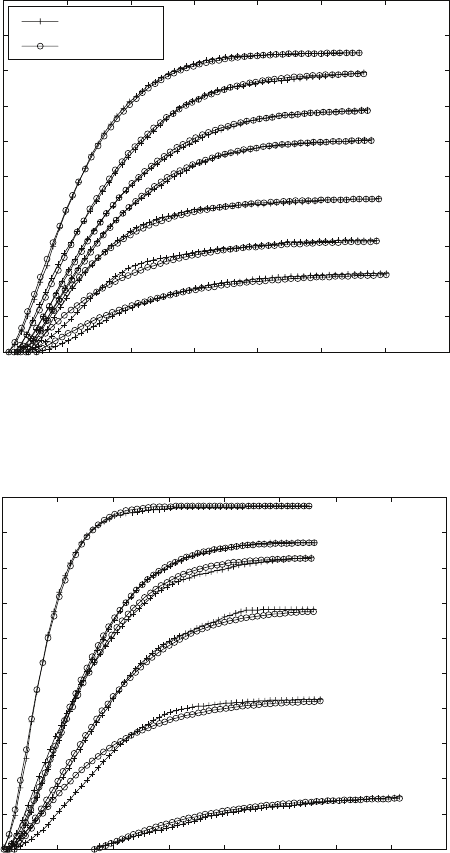
For lower temperatures (750, 800 and 850 °C), the experimental measurements
are described well by single straight lines (see Figs. 6.12 and 6.15). However,
at higher temperatures (900 °C and above), there is an obvious tendency for
change of the line slope (see Fig. 6.12d–f). The data apparently consist of
two parts which can be fitted with two separate lines (see Fig. 6.15b). This
observation implies that the mechanism of the transformation alters during
the course of the transformation.
The above data are in agreement with metallography of the alloy quenched
from different temperatures (see Section 6.3.3). There are different mechanisms
for the β to α + β phase transformation in Ti 8-1-1 alloy, depending on the
temperature of the isothermal transformation. At lower temperatures
(<900 °C), the main part transforms by homogeneous nucleation (within the
former β grains) and grows into plate-like or lamellar-like α phase. At higher
temperatures (≥900 °C), firstly, grain boundary α phase is formed.
Subsequently, the α phase fraction increases in conditions of decreasing
nucleation rate (exhausting of the grain boundaries of the former β phase).
Concurrently or subsequently, there is some amount (depending on the
temperature) of α plates nucleating and growing from the grain boundaries.
These different stages of the transformations correspond to the two separate
lines in Fig. 6.15b. The first stage takes place within the first 8–15 s, depending
on the temperature of isothermal transformation. Finally, it should be
remembered that the mechanism of the transformation alters gradually in the
time.
The Johnson–Mehl–Avrami method 151
Acceptable good fittings are possible for any n value ranging within 1.25–
1.5 and for all curves in Fig. 6.2c. Different mechanisms of the transformation
give different Avrami index (n values) for the kinetics of the β to α + β
transformation.
Considering all of the above, the experimental data in Fig. 6.2c can be
800 °C
n
= 1.42
Homogeneous
nucleations and
plate-like growth
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(a)
900 °C
n
2
= 2.01
Grain boundary α phase
formation with decreasing
nucleation rate and
α plates nucleated and
grown from the grain
boundaries
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(b)
n
1
= 1.09
Grain boundary
α phase formation
6.15
Plots of ln(ln(1/(1–
y
))) against ln(
t
) showing the different
mechanisms of the β to α + β transformation for Ti-8Al-1Mo-1V alloy
at different temperatures. (a) 800 °C; (b) 900 °C
Titanium alloys: modelling of microstructure152
fitted to the JMA equation, Eq. [6.2], by setting the n value free but constant
for the different mechanisms observed and discussed above. The derived
parameters, as well as the different mechanisms of the α phase nucleation
and growth, are summarised in Table 6.7. These are in agreement with the
general theory of phase transformations. At lower temperatures, an n value
of 1.42 is derived, corresponding to the transformation mechanism of
homogeneous nucleation and plate-like growth. Similar n values have been
derived for the β to α + β transformation at the same temperatures for Ti-
6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys (Section 6.7.1). The rate
constant k increases with decreasing temperature (see Table 6.7), implying
that the transformation is controlled by the rate of nucleation. Lower temperature
of the transformation corresponds to higher degree of undercooling, therefore
higher driving force of the transformation. Under these conditions, the rate
of nucleation (mainly homogeneous) controls the overall transformation rate.
The Ti-6Al-4V and the Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys have similar
tendencies in the k values (Section 6.7.1).
At higher temperatures, as stated above, the mechanism of the transformation
alters during the course of the transformation. Firstly, grain boundary α
phase is formed. This corresponds to an n value of 1.01 (see Table 6.7). At
this stage, clear temperature dependence of the rate constant k is not apparent.
Subsequently, the mechanisms of the transformation smoothly alter to grain
boundary α phase formation in conditions of exhausting grain boundaries
and α-plates nucleated and grown from the grain boundaries, corresponding
to an n value of 1.96. The rate constant k at this stage increases with increasing
temperature (see Table 6.7). This implies that the overall transformation rate
at this stage is controlled by diffusion.
The change of the mechanism of the β to α + β transformation for the Ti-
8Al-1Mo-1V alloy at high temperatures is not observed in Section 6.7.1 for
Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys. The reasons for this
difference are mainly the difference in the composition and the microstructure
of the alloys. The Ti-8Al-1Mo-1V alloy has a lower molybdenum equivalent
compared to the Ti-6Al-4V and the Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys, so,
at the same temperature of isothermal exposure, a larger amount of the α
phase is in equilibrium and needs to be transformed. The transformation
continues until the equilibrium amount of α is reached, even if all grain
boundaries of the former β phase are exhausted. Moreover, the Ti-6Al-4V
and the Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys in general (Boyer et al., 1994),
and in these particular cases, have finer microstructure of both the low
temperature α and the high temperature β phases than the Ti-8Al-1Mo-1V
alloy, implying larger amount of phase boundaries. Finally, the difference in
the β-transus temperature (1000 °C for Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-
0.08Si alloys and 1040 °C for Ti-8Al-1Mo-1V alloy) may also have an
influence on the change of the kinetics during the course of the transformation.
The Johnson–Mehl–Avrami method 153
Table 6.7
Johnson–Mehl–Avrami kinetic parameters and different mechanisms of the β to α + β transformation in Ti-8Al-1Mo-1V alloy at
various temperatures of isothermal transformations
T
(°C) First stage Second stage
Mechanism of transformation JMA parameters Mechanism of transformation JMA parameters
nk nk
975 Grain boundary α 1.01 0.043 Grain boundary α phase formation 1.96 0.0085
950 phase formation 0.043 in conditions of exhausting 0.0065
925 0.043 grain boundaries and α plates 0.0052
900 0.043 nucleated and grown from 0.0050
the grain boundaries
850 Homogeneously nucleated 1.42 0.029
800 and plate-like grown 0.035
750 α structure 0.044

Titanium alloys: modelling of microstructure154
In Section 6.7.1, there is also weak evidence for altering of the mechanism
of the transformation at high temperature.
It should be pointed out that the mechanisms of the β to α + β transformation
in Ti 8-1-1 alloy depend on the temperature but they do not change abruptly
with the temperature. The change is gradual (around 900 °C) from one
mechanism to another. So, the different mechanisms suggested (Table 6.7)
mean ‘the main mechanism of the transformation at the corresponding
temperature’.
In Fig. 6.16, the calculated and the experimental transformation kinetics
are compared.
6.7.3 β21s
The experimental resistivity curves can be recalculated to give the amount of
α phase versus time (Fig. 6.2d). The recalculation is based on the quantitative
X-ray analysis after the resistivity experiments and assuming, not verified
strictly for this alloy, a linear relation between the resistivity signal and the
amount of the α phase at constant temperature. Remember that the curves
Amount of α phase (vol. %)
100
90
80
70
60
50
40
30
20
10
0
Experimental
Calculated
01020304050
Time (sec)
750 °C
800 °C
850 °C
900 °C
925 °C
950 °C
975 °C
6.16
Experimental and calculated kinetics of the β to α + β
transformation at different temperatures for Ti-8Al-1Mo-1V alloy.

The Johnson–Mehl–Avrami method 155
for 600 and 500 °C trace only the first stages of the transformation and do
not present the entire transformation until phase equilibria are achieved.
The diffusion mobility of molybdenum and niobium in the β phase,
especially at low temperatures, should have influence or the control of the
overall transformation rate. In β titanium, the diffusion coefficient of
molybdenum is slightly lower than the diffusion coefficient of niobium.
Since larger redistribution of molybdenum is required (see Fig. 5.7b) and
because of the lower diffusion coefficient of molybdenum in the β phase,
one may suggest that the molybdenum diffusion in the β phase controls the
transformation. The diffusion distances of molybdenum in the β phase are
calculated for the different ageing treatments (see Table 6.8). The calculated
diffusion distances can be used only for approximate estimations of the
degree of transformation, because the amount of molybdenum that needs to
diffuse increases as the temperature decreases. The diffusion distance over
two hours at 650 °C is 1.22 µm. The experiments show that the β to α + β
phase transformation is completed after two hours at 650 °C. However, two
hours are not enough for the completion of the phase transformation at 600
and 500 °C. This may well be explained with the much shorter diffusion
distances after two hours at these temperatures (see Table 6.8). In simple
terms, the diffusional mobility is low, and therefore longer times are required.
The diffusion distance over 54 hours at 500 °C (1.07 µm) is similar to the
diffusion distance over two hours at 650 °C. Hence, these two ageing regimes
are equivalent in terms of the diffusion distance for the alloy. However, at
the lower temperature of 500 °C, a higher amount of molybdenum needs to
diffuse. It is therefore not surprising that, while at 650 °C the transformation
is completed after two hours, at 500 °C at least 54 hours are necessary. Ten
hours at 600 °C give a diffusion distance of 1.61 µm and so the experimental
results show that the phase transformation is completed. The correlation of
the calculated diffusion distances with the experimental results obtained
indicates that, at temperatures below 650 °C, the β to α + β phase transformation
is controlled mainly by the diffusion of molybdenum in the β phase.
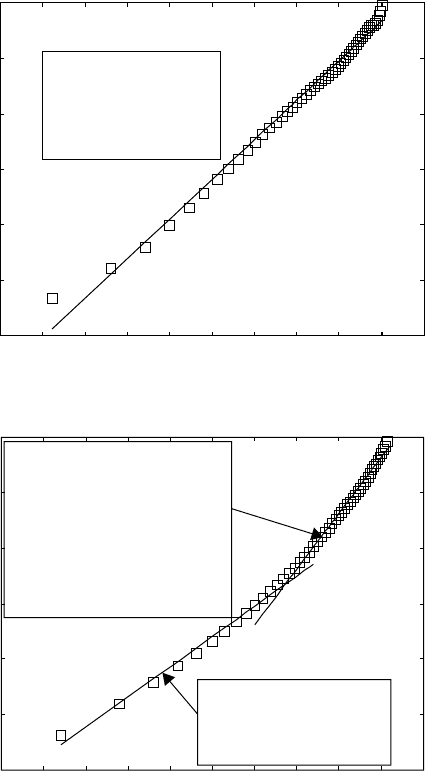
For 750, 700 and 650 °C, the experimental measurements are described
very well by single straight lines (see the example ln(ln(1/(1 – y))) plot in
Table 6.8
Calculated values of the diffusion distance of molybdenum in β21s
Ageing Ageing time Diffusion coefficient,
Dt
(µm)
temperature (°C) (hours)
D
(m
2
·s
–1
)
650 2 2.07×10
–16
1.22
600 2 7.20×10
–17
0.72
600 10 7.20×10
–17
1.61
500 2 5.86×10
–18
0.21
500 18 5.86×10
–18
0.62
500 54 5.86×10
–18
1.07
Titanium alloys: modelling of microstructure156
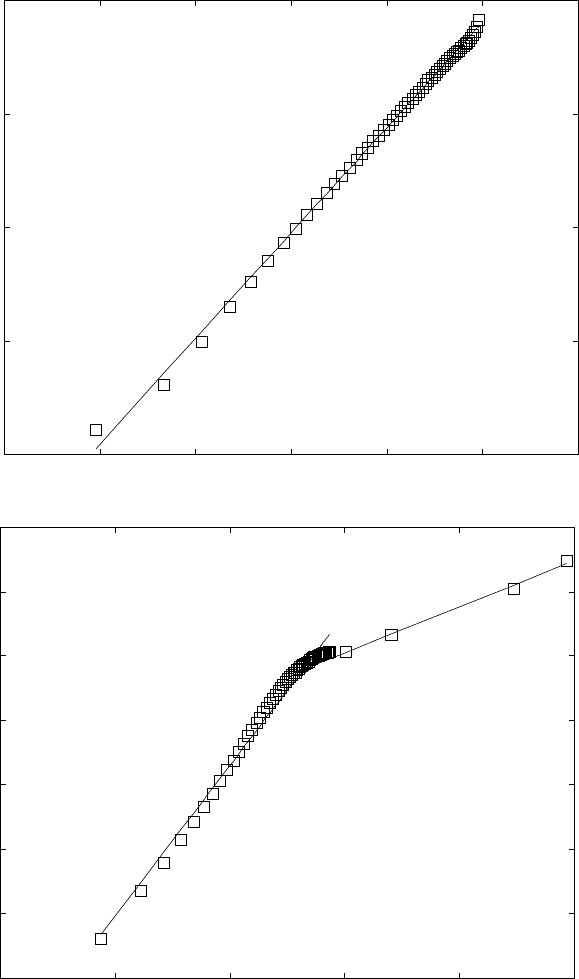
Fig. 6.17a). The Avrami index, n, for the temperatures of 750, 700 and
650 °C, is 1.78, 1.86 and 1.86, respectively. These characterise the mechanism
of the transformation when β grain boundaries are preferable nucleation
sites for α phase, which has a lamellar morphology.
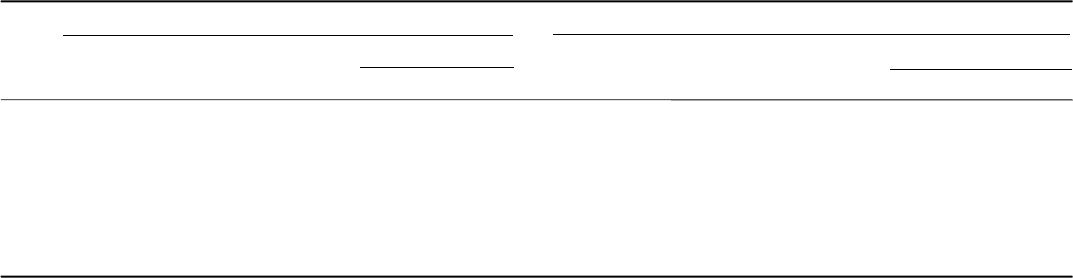
For lower temperatures (600 and 500 °C), data from both resistivity and
additional ageing are traced within the framework of JMA theory. The data
for these temperatures apparently consist of two parts, which can be fitted
with two separate lines (see Fig. 6.17b). In the first stage of the transformation,
the data fit corresponds to straight lines with slopes 1.20 and 1.17 for 600
and 500 °C, respectively. However, with time increase, there is an obvious
tendency for change of the line slope (see Fig. 6.17b) to lower values of
about 0.4 and 0.36 for 600 and 500 °C, respectively. Other near-β titanium
alloys (Ti 10-2-3 and β-CEZ) have similar changes. At these low temperatures,
the β to α + β transformation is in conditions of (i) nucleation including
homogeneous nucleation (mainly at the first stages), and further (ii) slow
diffusion controlled growth of very fine α plates. The change of the
transformation mechanisms is gradual, at around 650 °C.
The derived JMA parameters allow the transformation kinetics to be
calculated (Fig. 6.18).
6.8 Time–temperature–transformation diagrams
6.8.1 Ti 6-4 and Ti 6-2-4-2
Using the resistivity data on the kinetics of β to α + β transformation at
different constant temperatures, complete isothermal transformation diagrams
can be designed. These diagrams, also known as time–temperature–
transformation (TTT) diagrams, give the relationship between the temperature
(plotted linearly) and the time (plotted logarithmically) for a fixed fractional
amount of transformation to be attained. Such diagrams are usually used in
alloy heat-treatment practice. In literature, usually, only the ‘start’ and the
‘end’ of the transformation are plotted in these diagrams. However, times
corresponding to the very start and the very end of the transformation are
difficult to be measured experimentally. For this reason, in calculations, we
can use transformed fractions of 5 and 95% for the start and the end of the
transformation, respectively (Fig. 6.19). For these titanium alloys, the ‘end
of transformation’ does not correspond to when the initial phase (β phase)
completely transforms to the new phase (α phase). At each temperature, a
different phase composition (α + β mixture) is the final product (see Section
6.6). Hence, the start and the end of the transformation should not be read as
5 and 95% of the α phase but as 5 and 95% degree of transformation. Iso-
lines are calculated and plotted on the same diagrams (Fig. 6.19), showing
the real amounts of the α phase.
The Johnson–Mehl–Avrami method 157
n
= 1.86
234 5678
In(
t
)
(a)
In(In(1/(1–
y
)))
2
0
–2
–4
–6
2 4 6 8 10 12
In(
t
)
(b)
In(In(1/(1–
y
)))
1
0
–1
–2
–3
–4
–5
–6
n
= 0.36
n
= 1.17
6.17
Plots of ln(ln(1/(1–
y
))) against ln(
t
) for deriving the Johnson–
Mehl–Avrami parameters for β21s alloy at (a) 700 and (b) 500 °C.
700 °C
500 °C
