Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Titanium alloys: modelling of microstructure138
relatively low for commercial titanium alloys dictating that the β to α
transformation takes place at lower temperatures (Chapter 14). It should also
be mentioned that the heat treatment conditions of the samples used by
Boyer et al. (1994) were different. In Boyer et al. (1994) the samples were
studied after heating at different temperatures. In this chapter, the alloys
were after β homogenisation and subsequent isothermal holding at different
temperatures. The different heat treatment may also have an influence on the
amount of α phase.
A comparison of the α phase amounts obtained from resistivity studies at
different temperatures for Ti-8Al-1Mo-1V, Ti-6Al-4V and Ti-6Al-2Sn-4Zr-
2Mo-0.08Si alloys is given in Table 6.3. It is obvious that the amounts of the
α phase are higher in Ti-8Al-1Mo-1V alloy as compared to the α phase
amounts at the same temperatures in Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-
0.08Si alloys, in agreement with the pseudo binary phase diagram of titanium
alloys. In this diagram, the Ti-8Al-1Mo-1V alloy is to the left-side of the Ti-
6Al-4V and the Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys, implying that the amount
of α phase in the Ti-8Al-1Mo-1V alloy should be higher.
6.6.3 β21s
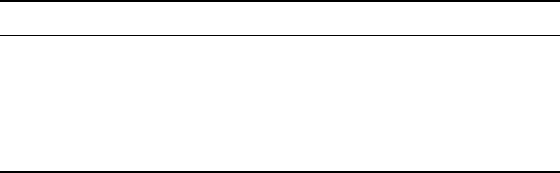
The calculated equilibrium amount of the α phase increases when the
temperature decreases (Fig. 6.10). This tendency is in agreement with the
behaviour of the phase transformation in titanium alloys. However, the large
difference between the calculated and the experimentally observed values,
especially at low temperatures, is apparent. The amounts of the α phase after
resistivity experiments at low temperatures, as discussed earlier in this chapter,
are not the actual equilibrium amounts. Nevertheless, even the amounts of
the α phase after additional ageing, where in the case of 600 °C a phase
equilibrium is believed to be reached, are much lower than the calculated
equilibrium amounts of the α phase. The experimental amounts of the α
phase are believed to be the correct ones and the calculated, especially at low
Table 6.3
Volume fractions of the α phase for Ti-8Al-1Mo-1V, Ti-6Al-2Sn-
4Zr-2Mo and Ti-6Al-4V alloys at various temperatures from resistivity
data
T
(°C) Ti-8Al-1Mo-1V Ti-6Al-2Sn-4Zr-2Mo Ti-6Al-4V
950 25 – 22
920–930 45 15 32
900 60 43 44
850 77 68 69
800 90 83 79
750 98 87 85
The Johnson–Mehl–Avrami method 139
temperatures are unrealistically high. The calculated amount of the α phase
at 500 °C, for example, is larger than 80% and the tendency is for further
increase of the amount of the α phase as the temperature decreases. Such
high equilibrium amount of α phase in β21s alloy is in contradiction with its
position in the pseudo binary β-isomorphous phase diagram (Boyer et al.,
1994) and the practical knowledge of this alloy. The deviations in the calculated
equilibrium α amount are most probably because this alloy was not used in
the Ti database validation. There is similar discrepancy between calculated
and experimental amounts of the α phase for other titanium alloys (Sections
6.6.1 and 6.6.2).
The major difference in the compositions at different temperatures is the
molybdenum and the niobium contents in the β phase (Fig. 5.7). There is a
tendency for diffusional enrichment of the β phase with both elements when
the temperature decreases. According to the phase diagram, such enrichment
of β phase by β alloying elements is necessary for α phase to be formed.
These calculations show the general characteristics of the phase transformation
in titanium alloys. It is the enrichment with molybdenum and niobium that
stabilises the β phase at room temperature. It should be noted that since the
calculated equilibrium amount of the α phase is deemed too high (Fig. 6.10),
Calculated
Experimental – 2 hours
Experimental – additional ageing
450 500 550 600 650 700 750 800
T
(°C)
Amount of α phase (%)
100
80
60
40
20
0
6.10
Calculated equilibrium amounts of the α phase versus
temperature in the β21s alloy compared with experimentally
observed. The calculations are performed for composition Al = 3.00,
Mo = 14.12, Nb = 3.48, Si = 0.14, Fe = 0.32, C = 0.016, N = 0.024, and
O = 0.15, all in wt.%.

Titanium alloys: modelling of microstructure140
considering the materials balance, the curves tracing the equilibrium
concentrations of alloying elements in the phases (Fig. 5.7b) should also be
shifted. However, the tendency should remain the same. One can conclude
that the β to α + β phase transformation in β21s alloy is connected with
diffusional redistribution of molybdenum and niobium between the α and
the β phases.
6.7 Kinetics of the transformation
In this section, we trace the kinetics of the diffusional β to α + β transformation.
The martensitic (β to α′) transformation is not involved. It was suggested
before on the phase relationship of Ti 8-1-1 that the β phase transforms to
martensite at temperatures in the α + β field from the transus down to about
900 °C. This is not observed in the Ti 8-1-1 alloy as described above. For all
the temperatures ranging from 750 to 975 °C, diffusional β to α transformation
is observed. The martensitic transformation has different kinetics and a distinctly
different resistivity response.
The analysis of the resistivity experimental data can be made within the
framework of the Avrami theory by means of the Johnson–Mehl–Avrami
(JMA) equation. The theory and equation trace the kinetics of the
transformation, giving a relation between the volume fraction of transformed
material and the time. For isothermal transformations, this equation has the
following popular and basic form:
f = 1 – exp(–kt
n
) [6.1]
where f is the product volume fraction which varies with time t, k is the
reaction rate constant and n is the Avrami index that describes the nucleation
and growth mechanisms.
In its general form, Eq. [6.1] applies to many real transformations. Under
isothermal conditions, it describes a variety of phase transformations including
polymorphic changes, discontinuous precipitation, eutectoid reactions, interface
controlled growth, and diffusion controlled growth. The kinetics of the β to
α + β phase transformation can be modelled by adapting the classic JMA
theory. The above equation can be written in the form:
ft
f
ykt
n
α
α
()
= = 1– exp(– )
max
[6.2]
where f
α
(t) is the amount of α phase after a time t,
f
α
max
is the maximum
(equilibrium) volume fraction of the α phase at the temperature of the
transformation, and y is the degree of the transformation.
It must be pointed out that the JMA equation describes the transformation
from its start and does not consider the pre-processing stages and the incubation
time. Hence, the time should be treated not as an absolute time but as relative
The Johnson–Mehl–Avrami method 141
to the start of the transformation. This can be taken into account by simple
subtraction of the incubation time.
Equation [6.2] can be used to analyse the experimental data by means of
logarithmic plots, where ln(ln(1/(1 – y))) is plotted versus ln(t). The slope of
the linear regression line is the Avrami exponent n, while from the intercept
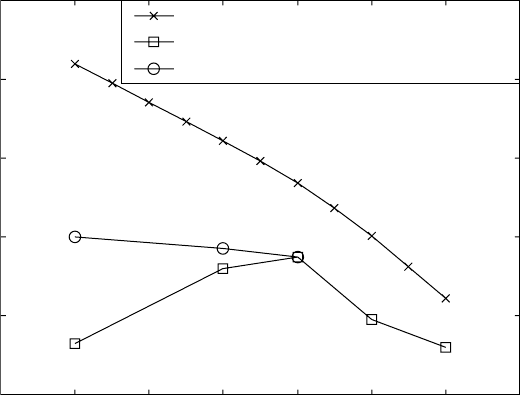
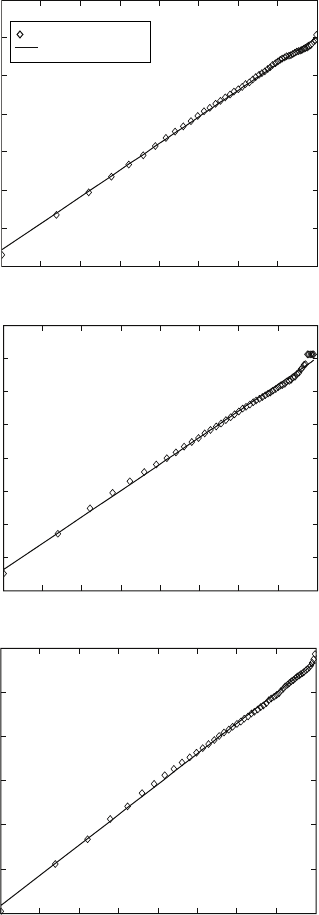
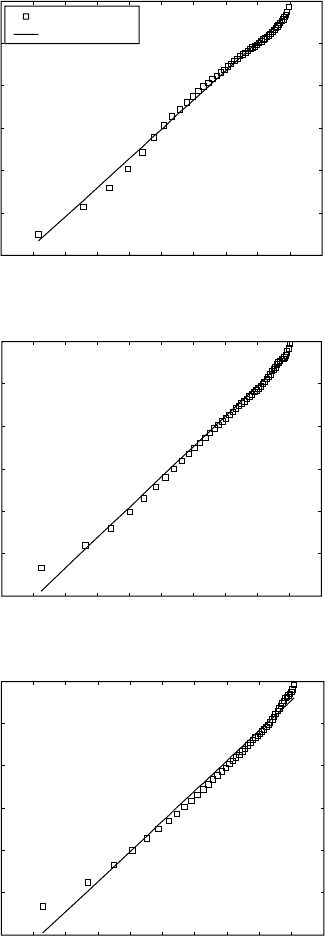
the k value can be calculated. Such plots are presented in Figs. 6.11 and 6.12
for some of the temperatures applied in the resistivity work.
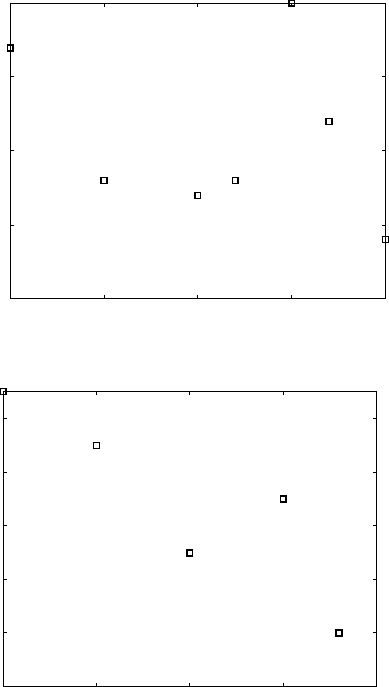
Using the above plots, n and k values for all applied temperatures can be
derived (Tables 6.4 and 6.5).
The Avrami index n obtained from these plots for all temperatures is
within the narrow range of 1.15–1.60 for Ti 6-4 and Ti 6-2-4-2 alloys and
1.26–1.49 for Ti 8-1-1. This corresponds to the mechanism of the β to α +
β transformation in titanium alloys, namely that β-grain boundaries are the
nucleation sites and the α phase has a plate-like morphology.
6.7.1 Ti 6-4 and Ti 6-2-4-2
For all the temperatures, the experimental measurements are described very
well by single straight lines when the plots are in the above coordinate
system. This confirms that the JMA theory can be applied to describe the
kinetics of the β to α + β phase transformation in the titanium alloys under
isothermal conditions. Moreover, the mechanism of the transformation does
not change during the course of the transformation. In near-β titanium alloys
(Ti 10-2-3 and β-CEZ), the mechanism of the transformation changes during
the course of the transformation when the transformation takes place at low
temperatures (350 °C), indicated by obvious changes of the line slope. Such
effects are not observed here.
Note, however, that although the above plots are the most usual means of
deriving the JMA kinetics parameters, such a free approach may be misleading.
The reader will find that acceptable good fittings between the experimental
and the calculated fractions are possible for any n value ranging within 1.2–
1.5 and for all curves in Fig. 6.2a and b. Since n is correlated with the
nucleation and growth mechanisms (the geometry of the transformation), the
next conclusion is that the mechanism of the transformation does not change
in the temperature ranges concerned. This conclusion is valid for most
transformations over appreciable temperature ranges. Thereby, the next step
is to fit the experimental data in Fig. 6.2a and b to the JMA equation, Eq.
[6.2], by setting the n value free but constant for all the temperatures used.
This can be performed by an optimisation computer program, trying to minimise
the sum square error between the experimental and calculated fractions for
all temperatures. From such calculations, the values of n = 1.31 for Ti 6-4
and n = 1.44 for Ti 6-2-4-2 are obtained.
The temperature dependence of the rate constant is not monotonic in the
Titanium alloys: modelling of microstructure142
Data
Best linear fit
In(In(1/(1–
y
)))
3
2
1
0
–1
–2
–3
–4
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(a) Ti-6Al-4V (750 °C)
In(In(1/(1–
y
)))
3
2
1
0
–1
–2
–3
–4
–5
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si (750 °C)
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(c) Ti-6Al-4V (850 °C)
6.11
Plots of ln(ln(1/(1–
y
))) against ln(
t
) for deriving the Johnson–
Mehl–Avrami parameters for (a,c,e) Ti-6Al-4V and (b,d,f)
Ti-6Al-2Sn-4Zr-2Mo-0.08Si at different temperatures. (a) Ti-6Al-4V
(750 °C); (b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si (750 °C); (c) Ti-6Al-4V (850 °C);
(d) Ti-6Al-2Sn-4Zr-2Mo-0.08Si (850 °C); (e) Ti-6Al-4V (950 °C); (f) Ti-
6Al-2Sn-4Zr-2Mo-0.08Si (930 °C).
The Johnson–Mehl–Avrami method 143
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–5
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(d) Ti-6Al-2Sn-4Zr-2Mo-0.08Si (850 °C)
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–5
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(e) Ti-6Al-4V (950 °C)
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(f) Ti-6Al-2Sn-4Zr-2Mo-0.08Si (930 °C)
6.11
Continued
Titanium alloys: modelling of microstructure144
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
Data
Best linear fit
750 °C
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(a)
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(b)
800 °C
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
850 °C
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(c)
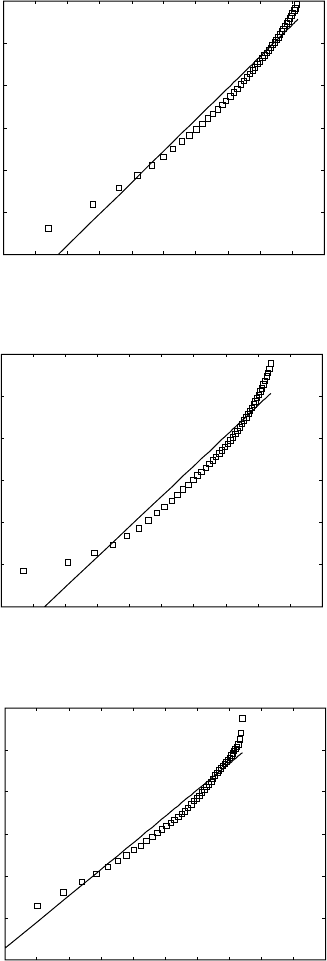
6.12
Plots of ln(ln(1/(1–
y
))) against ln(
t
) for deriving the Johnson–Mehl–
Avrami parameters for Ti-8Al-1Mo-1V alloy at different temperatures.
(a) 750 °C; (b) 800 °C; (c) 850 °C; (d) 900 °C; (e) 950 °C; (f) 975 °C.
The Johnson–Mehl–Avrami method 145
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(d)
900 °C
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
950 °C
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(e)
In(In(1/(1–
y
)))
2
1
0
–1
–2
–3
–4
–1 –0.5 0 0.5 1 1.5 2 2.5 3 3.5 4
In(
t
)
(f)
975 °C
6.12
Continued
Titanium alloys: modelling of microstructure146
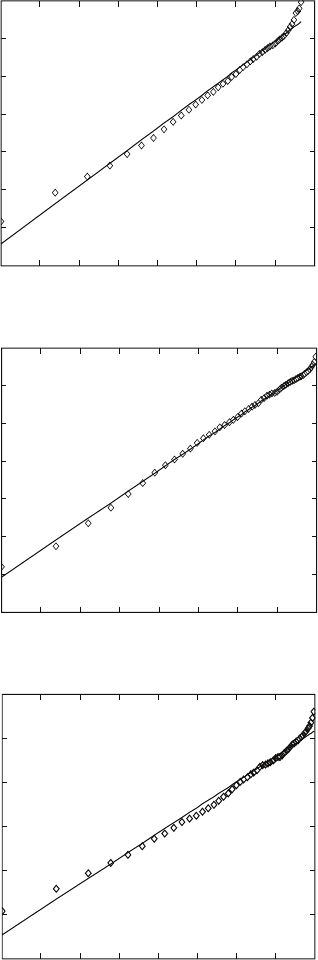
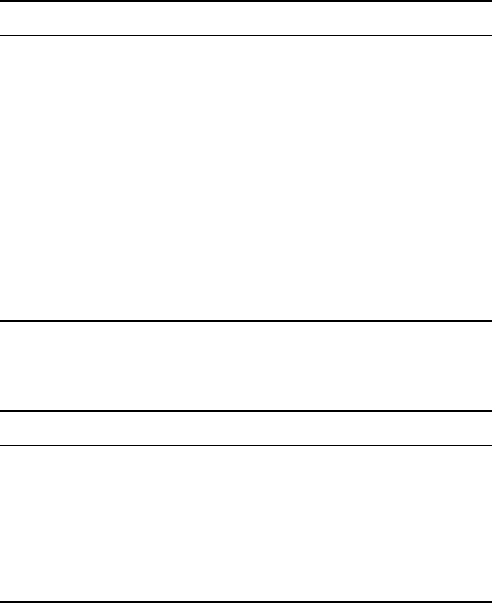
above cases (Fig. 6.13). For both alloys, there is an abnormality at about
900 °C. Apparently, the transformation can be considered as having two
somewhat different kinetics above and below 900 °C. Within both temperature
intervals and in both alloys, the rate constant increases with decreasing
temperature, i.e. increasing undercooling. Such a characteristic is usual for
phase transformations taking place on cooling. These results are in agreement
with the results from the metallography study of samples after isothermal
exposure at different temperatures (see Sections 6.3.1 and 6.3.2). The
microstructures of the α phase produced above and below 900 °C are different.
For the temperatures above 900 °C, the α phase is mainly grain boundary
nucleated and grown (Fig. 6.4a,b and d). For a high degree of undercooling
(temperatures below 900 °C) (‘Undercooling’ refers to the interval between
the temperature of isothermal exposure and the β transus), mixed mechanisms
of the transformation are observed. The main part is homogeneously nucleated
Table 6.4
Johnson–Mehl–Avrami kinetic parameters for Ti-6Al-
4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys obtained by ln(ln(1/(1–
y
))) versus ln(
t
) plots
Alloy
T
(°C)
nk
Ti-6Al-4V 950 1.41 0.017
920 1.39 0.024
900 1.21 0.046
870 1.34 0.025
850 1.38 0.022
800 1.34 0.026
750 1.40 0.028
Ti-6Al-2Sn-4Zr-2Mo-0.08Si 930 1.15 0.032
900 1.53 0.014
850 1.53 0.012
800 1.36 0.024
750 1.60 0.013
735 1.30 0.059
Table 6.5
Johnson–Mehl–Avrami kinetic parameters for Ti-8Al-
1Mo-1V alloy obtained by ln(ln(1/(1–
y
))) versus ln(
t
) plots
T
(°C)
nk
750 1.38 0.046
800 1.43 0.036
850 1.43 0.032
900 1.49 0.023
925 1.40 0.025
950 1.44 0.029
975 1.26 0.085
The Johnson–Mehl–Avrami method 147
(within the former β grains) and plate-like grown α phase (Fig. 6.4c,e,f and
g). Small amounts of the grain boundary α are observed as well.
These observations imply that the Avrami index (n values) is different for
the kinetics of the β to α + β transformations above and below 900 °C. As
examples, for grain boundary nucleation after saturation, n = 1, and for
diffusion-controlled growth of plates under the assumption that all particles
are present at t = 0 and have negligible initial dimensions, n = 1.5.
Ti-6Al-4V
kE
, JMA rate constant
0.04
0.035
0.03
0.025
0.02
750 800 850 900 950
T
(°C)
(a)
Ti-6Al-2Sn-4Zr-2Mo-0.8Si
kE
, JMA rate constant
0.02
0.018
0.016
0.014
0.012
0.01
750 800 850 900 950
T
(°C)
(b)
6.13
Calculated Johnson–Mehl–Avrami rate constants for β to α + β
transformation in (a) Ti-6Al-4V and (b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si
alloys assuming constant Avrami index (
n
).
