Sha W., Malinov S. Titanium Alloys: Modelling of Microstructure, Properties and Applications
Подождите немного. Документ загружается.

Titanium alloys: modelling of microstructure128
6.3.3 Ti 8-1-1 alloy
First, a very small amount, about 9%, of separate α phase crystals is found
in the alloy exposed at 950 °C and then quenched. At 925 °C, the amount of
the α phase increases to about 29%. In both cases, the α phase is observed
as thin layers, covering β grain boundaries (Fig. 6.5a). Decreasing the
temperature of isothermal holding, down to 900 °C, causes formation of
coarser grain-boundary α phase and appearance of the first small portions of
α crystals growing from this grain-boundary phase using it as a nucleating
site (Fig. 6.5b). The total amount of the α phase at 900 °C is about 46%.
Further decrease of the holding temperature down to 850 °C leads to a
change of the mechanism of the α phase formation. An α phase nucleated
and grown homogeneously inside the former β grains is observed (Fig. 6.5c
and d). A substantial increase in the total amount of the α phase (up to 70%)
is detected. The grain-boundary α phase at 850 °C (Fig. 6.5c) is thinner as
compared to the grain-boundary α phase at 900 °C (Fig. 6.5b). It is possible
that the thin α layer covering the β grain boundary at 850 °C is formed on
cooling from the β solution temperature to the temperature of isothermal
exposure (note that the cooling rate was not high enough to depress completely
the β phase decomposition).
A decrease of the temperature down to 800 and 750 °C (Figs. 6.5e and f,
respectively) causes further increase of the amount of α phase up to 88 and
98%, respectively. The mechanism of the α phase formation remains the
same. The main part of the α phase is homogeneously formed within the
former β grains as packets of lamellar α phase. Additionally, a small amount
of α phase covering β grain boundary is observed which is probably precipitated
on cooling from solid solutioning to the holding temperatures.
In summary, at temperatures of exposure above 900 °C, the predominant
place for α precipitation is the β grain boundary, whereas at lower temperatures,
most α lamellae precipitate rather homogeneously inside the β grains. Optical
microscopy quantitative data are in good agreement with the contents of α
phase calculated from resistivity work.
6.4 X-ray diffraction
X-ray diffraction is not usually used for quantitative study of α and α + β
titanium alloys. The reason for this is that in these alloys a martensite
transformation may take place and it is difficult to distinguish between the α
and the α′ (martensite) phases. In the β21s alloy, the martensite start temperature
is below room temperature. Hence, mainly α and β phases in different ratios,
depending on the processing and heat treatment conditions, exist. These two
phases are easily detectable and distinguishable by X-ray diffraction. The
equilibrium amount of the α phase should increase when the temperature is
lower (see also Section 6.6).
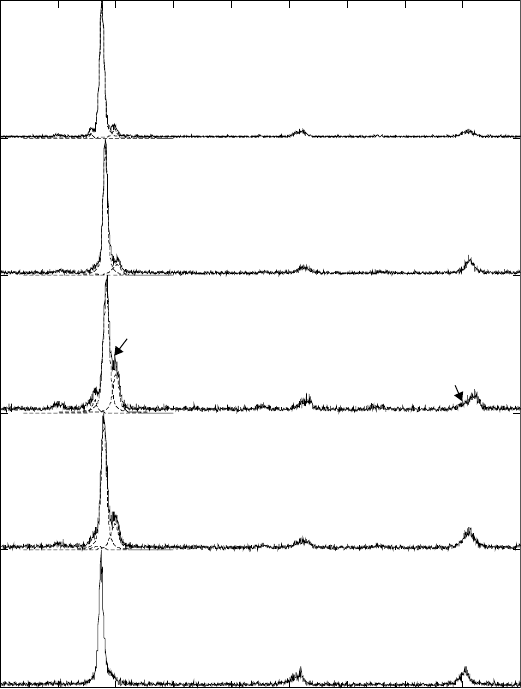
The Johnson–Mehl–Avrami method 129
After the resistivity experiments, β21s samples can be cooled to room
temperature and studied by X-ray diffraction. The results from the samples
after isothermal exposure for two hours at different temperatures are given in
Fig. 6.6. The amount of the α phase can be calculated from the fitted diffraction
patterns using the direct comparison method. In order to avoid error due to
preferred orientation (crystallographic texture that usually is present in
Relative intensity
5
4
3
2
1
0
30 35 40 45 50 55 60 65 70 75
2θ (°)
750 °C
700 °C
650 °C
600 °C
500 °C
{110}β
{200}β
{211}β
{101}α
{100}α
{002}α
{102}α
{110}α
{103}α
6.6
X-ray diffraction patterns and profile fits (dotted lines) in the
range of 30 to 75° 2
θ
(Cu K
α
radiation) of β21s after isothermal
exposure at different temperatures for two hours. The intensities are
given relative to the maximum, {110}β reflection. For clarity, the
diffraction patterns are shifted with respect to each other along the
vertical axis.
Titanium alloys: modelling of microstructure130
thermomechanically processed titanium alloys), the quantitative analysis should
be based on the entire diffraction pattern instead of using single reflections.
The principle of ‘averaging the integral intensities’ should be applied. All α
and β reflections observed should be used.
X-ray analysis of the alloy after different temperatures of isothermal exposure
shows different quantities of the α phase. The total amount of the α phase
precipitated after isothermal exposure at 750 °C is estimated at about 12%.
Decreasing of the temperature of isothermal holding down to 700 and
650 °C results in an increase of the α phase fraction (see Fig. 6.6). The
amounts of the α phase at 700 and 650 °C are 19 and 35%, respectively.
Further decrease of the temperature of isothermal exposure down to 600 and
500 °C leads to decrease of the total amount of the α phase to 32 and 13%,
respectively.
The increased total amount of the α phase when the temperature is decreased
from 750 to 650 °C is in agreement with what is expected from the
thermodynamics of this phase transformation. However, the lower amounts
of the α phase after isothermal exposure at lower temperatures contradict the
thermodynamic equilibria for titanium alloys. It is likely that, at lower
temperatures (600 and 500 °C), the β to α + β transformation has slow
kinetics, and phase equilibria are not reached during the two-hour exposure.
In order to check this assumption, experiments involving additional prolonged
ageing are necessary.
6.5 Additional ageing
In order to check the completeness of the phase transformation after the
resistivity experiments at 650, 600 and 500 °C, the β21s samples were re-
heated and held at the same temperatures for longer times. Additional ageing
for two hours was applied to the sample after resistivity experiments at 650
°C. The diffraction patterns after resistivity and after the additional ageing
were identical. The identical amounts of α and β phases before and after this
additional ageing mean that the phase transformation was completed before
the additional ageing and a phase equilibrium had been reached.
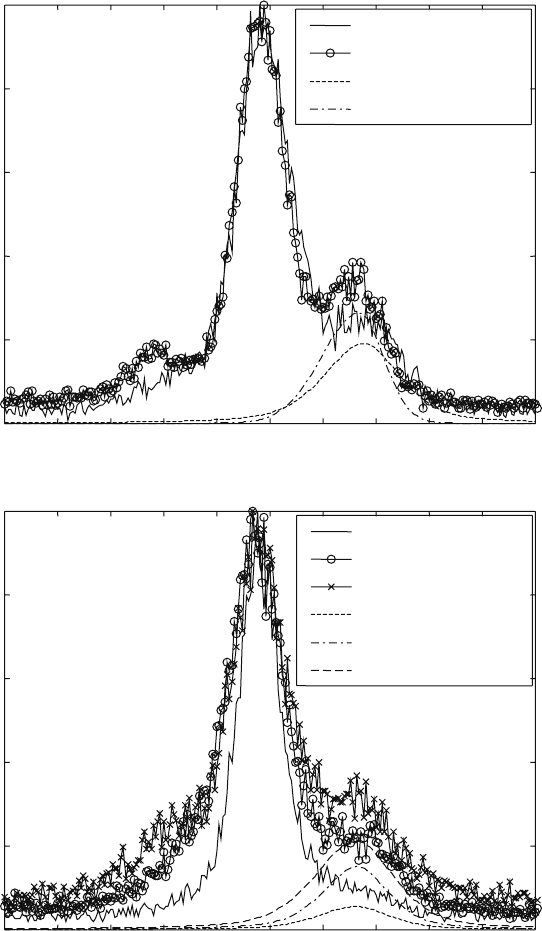
Additional ageing of 8 and 14 hours was applied for the sample at 600 °C
(note that the total time of isothermal exposure was 10 and 16 hours,
respectively). The diffraction pattern after additional ageing for 8 hours (10
hours in total) showed an increased amount of α phase (see Fig. 6.7a). The
amount of the α phase was increased from 32% after the resistivity experiment
to 36% after the additional ageing. The phase transformation at this temperature
was incomplete after the resistivity experiments. A slow transformation process
was still in progress upon additional ageing, but it was undetectable with the
resistivity technique used. Further increase of the ageing time to 16 hours
did not show any change in the diffraction pattern. So, the β to α + β
The Johnson–Mehl–Avrami method 131
600 °C
2 h
10 h
Profile fit 2 h
Profile fit 10 h
500 °C
36.5 37 37.5 38 38.5 39 39.5 40 40.5 41 41.5
2θ (°)
(a)
Relative intensity
1
0.8
0.6
0.4
0.2
0
2 h
18 h
54 h
Profile fit 2 h
Profile fit 18 h
Profile fit 54 h
36.5 37 37.5 38 38.5 39 39.5 40 40.5 41 41.5
2θ (°)
(b)
Relative intensity
1
0.8
0.6
0.4
0.2
0
6.7
X-ray diffraction patterns in the range of 36.5 to 41.5° 2
θ
and
profile fits of the {101}α reflection for β21s after isothermal exposure
at (a) 600 and (b) 500 °C for different periods of time. The intensities
are given relative to the maximum, {110}β reflection.
Titanium alloys: modelling of microstructure132
transformation at this temperature has completed at or before 10 hours of
isothermal exposure.
After the resistivity experiments at 500 °C, the sample was re-heated and
aged for additional times of 16, 40 and 52 hours (note that these correspond
to total times of isothermal exposure of 18, 42 and 54 hours). The additional
ageing at this temperature also resulted in increase of the amount of α phase
(see Fig. 6.7b). The amount of α phase increased from 13% after the resistivity
experiments for two hours to 25% after additional ageing for 16 hours (18
hours in total). The ageing time prolongation resulted in further increase of
the amount of α phase to 33 and 40% after additional ageing for 40 and 52
hours (42 and 54 hours in total), respectively. At this temperature, the β to α
+ β phase transformation may still not have completed even after 52 hours
additional ageing.
In addition, the X-ray diffraction patterns after exposure at different
temperatures and times showed a tendency in the full width half maximum
(FWHM) values of the α reflections. The FWHM values were higher when
the temperature was lower, implying that the precipitated α phase at lower
isothermal temperatures possesses a larger degree of inhomogeneity. On the
other hand, the time prolongation resulted in decrease of the FWHM values,
indicating α phase homogenisation. The change in FWHM could also be
related to α grain or precipitate size, with large values indicating small sizes.
In summary, for the β alloy β21s, more dependable data can be obtained
using quantitative X-ray analysis, giving linear dependency between resistivity
signal and α volume fraction. This can then be employed to convert resistivity
data into curves for α phase amount versus time of isothermal exposure (Fig.
6.2d). At the same time, it is established that samples exposed at 500 and
600 °C do not reach equilibrium α + β condition after two hours resistivity
experiments. Equilibrium is not reached at 500 °C even after 52 hours additional
ageing.
6.6 Thermodynamic equilibria
From the fundamental theory of the phase transformations, when an alloy is
held at a certain temperature for a long time it tends towards and possibly
reaches a thermodynamic equilibrium. If this principle is applied here, it can
be assumed that in the final stage, when the transformation has been completed,
phase equilibria at the corresponding temperatures have been achieved. This
means equilibrium amounts of α and β phases as well as equilibrium
composition of both phases.
A Ti database which allows phase equilibria calculations to be performed
for multi-component, conventional titanium alloys is available. The phase
equilibria can be calculated for the compositions and the temperature range
used. The calculations are carried out with Thermo-Calc software and using
The Johnson–Mehl–Avrami method 133
the above-mentioned Ti database, for the actual alloy compositions, taking
into account the Al, V, Mo, Nb, Si, Fe and O contents. The C, N and H
contents are not taken into account in the calculations, except in the case of
β21s when C and N are taken into account. The experimental (from the
resistivity study) and calculated results are compared in Table 6.1 for
Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si and Table 6.2 for Ti-8Al-1Mo-
1V, respectively.
6.6.1 Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si
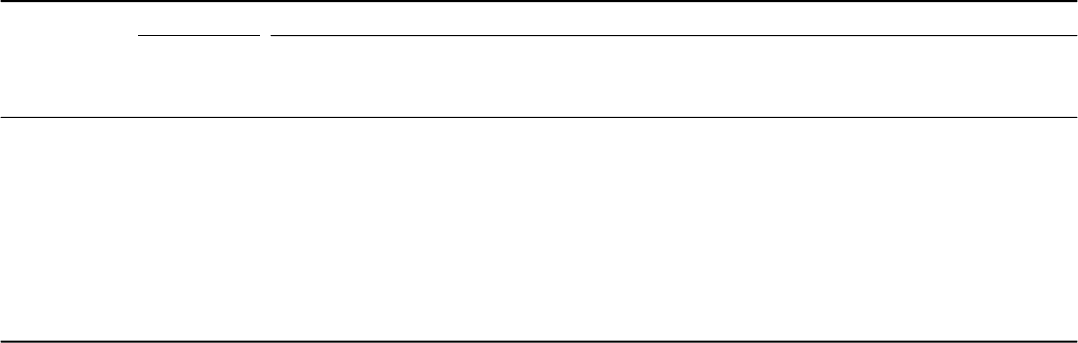
A good agreement between the experimental and calculated phase compositions
is found for the Ti 6-4 alloy (Table 6.1). The correspondence is better at
lower temperatures (750–800 °C). At higher temperatures (850–900 °C) the
calculated equilibrium α amount is slightly lower than that experimentally
observed. The differences between the experimental and calculated phase
compositions for Ti 6-4 are within the acceptable error range.
The differences between the calculated amount of equilibrium α phase
and the amount experimentally detected by the resistivity experiments are
significant for the Ti 6-2-4-2 alloy, which has a more complicated composition
than Ti 6-4 (Table 6.1). No reasonable explanation for this observation could
be found. In order to clarify this discrepancy, additional experiments were
carried out. Samples from both alloys were treated according to the same
heat treatment process. Thereafter, the samples were studied by means of
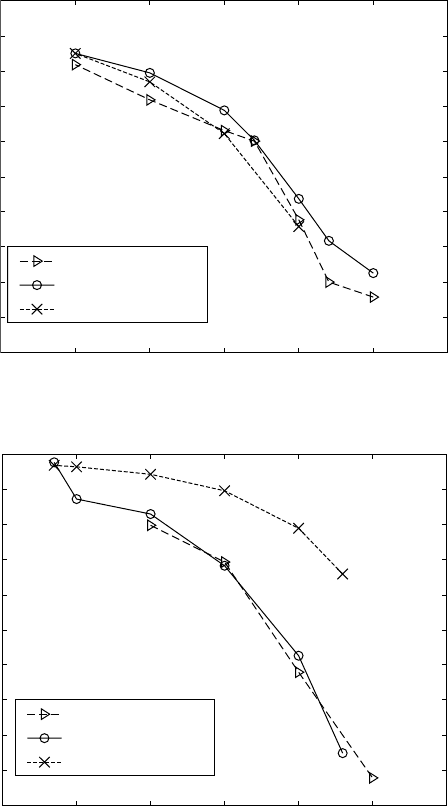
quantitative metallography (Fig. 6.8). Again, acceptable correspondence for
the Ti 6-4 alloy was observed (Fig. 6.8a). For the Ti 6-2-4-2 alloy the
experimental results from the metallography were in agreement with the
experimental results of the resistivity and in disagreement with the calculated
phase equilibria (Fig. 6.8b). Thus, it is reasonable to believe that the
experimentally observed α-amounts were the correct ones and the calculated
values for Ti 6-2-4-2 were unrealistically high. It should be noted that the
curve tracing the kinetics at a high temperature (see curve corresponding to
930 °C in Fig. 6.2b) has a tendency to increase with the time prolongation.
This may indicate that the transformation at this temperature is still incomplete.
Possibly, a very slow transformation process is still in progress, but it is
undetectable with the experimental technique used. Nevertheless, it is very
unlikely that even when the time is significantly prolonged, the experimental
amount of α phase (current data value 15%) will reach the amount calculated
(65%). One may conclude that for both Ti-6Al-4V and Ti-6Al-2Sn-4Zr-
2Mo-0.08Si alloys, there is a better correspondence between the calculated
and experimentally observed α amounts at lower temperatures.
Titanium alloys: modelling of microstructure134
Table 6.1
Experimental and calculated phase compositions for Ti-6Al-4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys at various temperatures
Alloy
T
Experimental Calculated
(°C) αβα β Al in Al in V in V in Sn in Sn in Zr in Zr in Mo in Mo in
αβαβαβαβαβ
(vol.%) (vol.%) (mol.%) (mol.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%)
Ti-6Al-4V 950 22.5 77.5 – – – – – – – – – – – –
900 43.7 56.3 35.9 64.1 6.9 5.8 2.0 6.7 – – – – – –
850 68.9 31.1 62.2 37.8 6.6 5.5 2.4 9.2 – – – – – –
800 79.5 20.5 76.9 23.1 6.5 5.3 2.8 12.2 – – – – – –
750 85.1 14.9 85.0 15.0 6.4 5.1 3.1 15.8 – – – – – –
Ti-6Al-2Sn- 930 14.9 85.1 65.8 34.2 6.7 5.1 – – 1.8 2.1 3.8 4.3 0.56 4.5
4Zr-2Mo- 900 42.7 57.3 78.9 21.1 6.5 4.9 – – 1.9 1.9 3.9 4.3 0.71 6.4
0.08Si 850 68.2 31.8 89.5 10.5 6.3 4.8 – – 2.0 1.3 3.9 4.2 0.95 10.0
800 82.9 17.1 94.2 5.8 6.3 4.6 – – 2.0 0.81 4.0 4.0 1.1 14.2
750 87.3 12.7 96.4 3.6 6.2 4.4 – – 2.0 0.45 4.0 3.6 1.2 18.9
735 97.7 2.3 96.8 3.2 6.2 4.3 – – 2.0 0.37 4.0 3.44 1.3 20.4
The Johnson–Mehl–Avrami method 135
6.6.2 Ti-8Al-1Mo-1V
Significant and unacceptable discrepancy between the experimental and
calculated phase compositions is found, especially at high temperatures. The
agreement is better at lower temperatures (750–800 °C). At higher temperatures,
Ti-6Al-4V
Metallography
Resistivity
Calculated
700 750 800 850 900 950 1000
T
(°C)
(a)
Amount of α phase (%)
100
90
80
70
60
50
40
30
20
10
0
Ti-6Al-2Sn-4Zr-2Mo-0.08Si
Metallography
Resistivity
Calculated
700 750 800 850 900 950 1000
T
(°C)
(b)
100
90
80
70
60
50
40
30
20
10
0
6.8
Experimental and calculated α phase amounts for (a) Ti-6Al-4V
and (b) Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys versus temperature.
Amount of α phase (%)
Titanium alloys: modelling of microstructure136
the calculated equilibrium α amount is significantly higher than that
experimentally observed. In order to clarify this discrepancy, samples quenched
from different temperatures were studied by means of quantitative
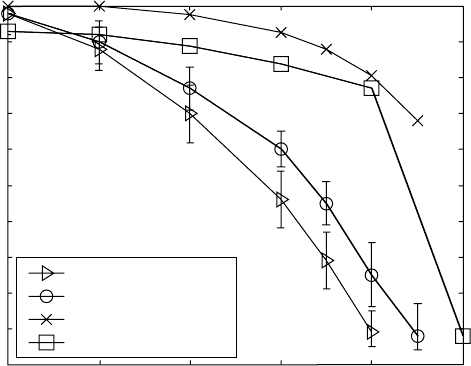
metallography. The data obtained from the different ways are plotted in Fig.
6.9, together with the data published by Boyer et al. (1994).
The β-transus temperature for Ti 8-1-1 alloy with normal element contents
is approximately 1040 °C (Boyer et al., 1994). Using Thermo-Calc and the
Ti database, a β-transus temperature of 1039 °C for the composition of the
alloy used is obtained. The results from the calculation show that, at 975 °C,
nearly 70% of the β phase should transform to α phase (see Table 6.2), so the
major part of the transformation is between 1039 and 975 °C. Such an
amount of equilibrium α phase at a temperature so close to the β-transus is
probably unrealistically high. The β to α transformation in titanium alloys is
in a much wider temperature range. Thus, there is reason to believe that the
experimental amounts of the α phase at higher temperatures are the correct
ones.
There is also a difference between the amounts of the α phase in Boyer
et al. (1994) and in the resistivity and metallographic data here. The difference
can be due to different composition of the alloys used. For example, oxygen
level has a dramatic influence on the thermodynamics and kinetics of the
phase transformations in titanium alloy (Chapter 14). The oxygen level of
the Ti-8Al-1Mo-1V alloy used in this chapter was 0.085 wt.%, which is
750 800 850 900 950 1000
T
(°C)
Amount of α phase (%)
100
90
80
70
60
50
40
30
20
10
0
Metallography
Resistivity
Calculated
Experimental (Boyer)
6.9
Experimental and calculated α phase amounts for Ti-8Al-1Mo-1V
alloy versus temperature.
The Johnson–Mehl–Avrami method 137
Table 6.2
Experimental and calculated phase compositions for Ti-8Al-1Mo-1V alloy at various temperatures
T
(°C) Experimental Calculated
αβαβAl in α Al in β V in α V in β Mo in α Mo in β Fe in α Fe in β O in α O in β
(vol.%) (vol.%) (mol.%) (mol.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%) (wt.%)
975 8 92 67.9 32.1 8.47 7.09 0.65 1.67 0.33 2.51 0.012 0.220 0.109 0.036
950 25 75 80.5 19.5 8.31 6.84 0.74 1.96 0.44 3.46 0.018 0.331 0.098 0.031
925 45 55 88.1 11.9 8.21 6.64 0.81 2.21 0.55 4.53 0.025 0.473 0.093 0.028
900 60 40 92.8 7.2 8.14 6.47 0.86 2.43 0.66 5.70 0.034 0.648 0.090 0.025
850 77 23 97.7 2.3 8.07 6.16 0.93 2.80 0.86 8.16 0.055 1.079 0.086 0.021
800 90 10 99.9 0.1 8.02 5.86 0.98 3.13 1.03 10.80 0.078 1.560 0.085 0.016
750 98 2 100 – 8.02 – 0.98 – 1.04 – 0.080 – 0.085 –
