Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


752
KLEIN
2.0
ABRASION-RESISTANT COATINGS
Alumina coatings have been developed because they provide higher hardness
on
the sur-
faces
of
many substrates,
in
particular stainless steel. In porous and dense conditions, a
simple application of alumina on a surface can provide scuff resistance. This means that
ceramic coatings have less tendency to show scratches, because ceramics in general have
higher hardness than metals and plastics. Some technological problems
in
the application
and adherence
of
sol-gel alumina coatings come up from thermal expansion mismatch
on
a large scale.
Oxynitride films have been prepared by nitriding silica gel films. These films are
harder than silica alone because they incorporate nitrogen. Gel-prepared oxynitride films
incorporate nitrogen in larger proportions than thermally nitrided conventional films. These
films offer both mechanical durability and chemical durability in hermetic packages for
microelectronic devices, where there is a large demand for dielectric films.
3.0
CORROSION-RESISTANT COATINGS
Early on, phosphate coatings were applied to silicate glass panels to improve their chemical
resistance to attack by water. This improvement was accomplished in
0.2
p.m. This process
made
it
possible
to
make a cheaper window. which showed the chemical durability
of
a
more expensive window with the use
of
a small amount of materials. This points out one
of the primary advantages
of
the sol-gel process in the form of films. The material use
is efficient. Any excess material is recovered and can be used again. resulting
in
little
waste. Even relatively costly components such as zirconia can be incorporated
in
the
coating. The added durability of the coating protects the bulk
of
the material without
requiring incorporation of the costly component throughout the material.
4.0
OPTICAL THIN FILMS
There are a number of optical thin films. First. there are rear-view mirrors for cars. These
coatings consist of titania-silica-titania interference filters that give the effect of total
reflectance. The coatings are applied successively with a backing
on
in between. Second,
there are solar reflecting films for windows. These coatings consist of palladium-containing
titania
films
that show selective absorption (e.g., Irox by Schott Glaswerk). Third, there
are antireflective coatings
of
several types. Both borosilicates and titania silicates have
been developed for this application. The index of refraction in this case can be graded by
changes in the composition, changes in the microstructure. and changes
in
the porosity.
In comparison to Vycor, the process developed at Sandia National Laboratories,
in
Ah-
querque, New Mexico. shows better long-term behavior, structural stability, and resistance
to
abrasion in accomplishing antireflection on solar cells and flat plate or tubular solar
collectors. Antireflection in the ultraviolet range can be achieved with thoria and hafnia
films. Broadband antireflection properties have been used in laser optics applications.
Other selective wavelength films have been developed with transition metal oxides.
5.0
ELECTRONIC AND MAGNETIC FILMS
Thin films have been applied from solution for numerous electronic and magnetic applica-
tions. Indium tin oxide (ITO) has been developed for electrically conducting transparent

SOL-GEL COATINGS 753
films for displays. Cadmium stannate is another transparent conductor. Opto-electronics
such as lead-lanthanum zirconate titanate (PLZT) have been prepared. Iron oxide layers
have been used for magnetic films. Vanadia layers have been used for antistatic films.
Barium titanate has been prepared in thin layers for multilayer capacitors. Potassium
tantalate has been use for capacitors as well. The advantage of thin films from gels for a
dielectric is that the thinner the layer, the higher the capacitance or the higher the density
of
devices possible on a chip.
Tungstate films have been prepared for electrochromic displays. Titania films have
been made for photoelectrodes. The area of transition metal oxide films from solutions
grows as the filed
of
microelectronics comes up with more and more devices.
Recently, the interest in supercomputers has spilled over into the sol-gel processing
community. The rare earth-transition metal oxide compounds, which show superconductiv-
ity at liquid nitrogen temperatures, are difficult to process
in
practical geometries with
the exception of thin films. The sol-gel process, which allows for molecular-scale mixing
of
the components
in
these compounds. is especially promising for depositing thin films.
6.0
ADVANTAGES
OF
SOL-GEL COATINGS
Acceptance of the sol-gel process for thin films is mostly a question of educating the
user of thin films and coatings on how to apply these films. The number
of
commercial
products available to the general public in this area is small. There are the spin-on coatings
for doping silicon wafers (Accuspin from Allied-Signal Electronic Chemicals and Liqui-
coat from-Chemicals). There are the liquid oxides for various applications (Atolon from
Nippon Soda). Many industries may have internal uses for what can be classified as sol-gel
coatings or sputtered coatings.
To summarize sol-gel coatings, there are limitations. The main limitation is that
only films around
1
pm thick can be applied with ease by a sol-gel process. Thinner
films can be applied, but thicker films cannot. Thick films suffer from the same problems
of drying, shrinkage, and cracking that plague bulk samples. However, for thin films there
are the applications cited above and probably others that have been overlooked.
An important feature to emphasize is the microporous nature of the films in the
early stages. It appears that microporosity has not been exploited for applications in which
a high surface area coating is desired. Some applications to look for in sol-gel coatings
are catalytic surfaces. membrane properties, and self-lubricating surfaces.
BIBLIOGRAPHY
Brinkcr,
C.
J.
and M.
S.
HalTington, Soltrr
Errc,r,qy
Mlrter..
5.
159 (1981).
Dislich,
H.
and P. Him,
J.
Non-C,yt.
Soliris,
4,
11
(1982).
Gruningcr, M..
J.
B. Wachtman, and R. Haber,
MRS
Syp.
Proc..
54,
X23 (1986).
Klein, L.
C..
Atrrlrr.
Rev.
Mtrrer.
Sci.,
15.
227
(
1985).
Klein, L.
C.,
Ccrtrrir.
Ens.
Sci.
Proc..
5.
379
(1984).
Klein, L.
C..
Ed.,
Sol-Gel
Techrrolog>jfor
Tlrir~
Films,
Fibers.
Prefr,rms, Electror~ics
mrl
Specirrln,
Livage,
J.,
and
J.
Lcmerie,
Amur.
Rev.
Muter.
Sci.,
12,
103
(1982).
Mukherjcc.
S.
P..
and W.
H.
Lowdcrmilk,
J.
Norl-Cryst.
Solids.
48, 177 (1982).
Pantano,
C.,
and P. Glaser,
J.
Norr-Cr:\..st.
Solids.
63.
201 (1984).
Shrrps.
Park Ridge,
NJ:
Noyes Publications,
1988, 407
pp.
This Page Intentionally Left Blank

Radiation-Cured Coatings
Joseph
V.
Koleske
Clzrrrlestorz.
West
Virgirzirr
1
.O
INTRODUCTION
Curing coatings by means of radiation represents one of the new techniques that is replacing
the use of conventional or low solids, solvent-borne coatings. Radiation-cured coatings
offer
a
manufacturer several important features. These include:
High solids-usually
100%
solids.
Low capital investment (with certain specific exceptions).
Low energy curing costs-low power requirements and elimination of solvent costs.
Rapid cure speeds.
Ability to cure a variety of substrates, including heat-sensitive substrates such
as
Increased productivity.
Shorter curing lines and decreased floor space requirements for operating line and
A variety of different chemistries from which to select, and thus broad formulating
plastics and parts for the electronics industry.
for liquid coating storage.
latitude from the wide variety of formulation ingredients available.
The main sources
of
actinic energy for curing coatings by radiation are electron beam and
ultraviolet light.* In 1984. Pincus’ indicated that there were four suppliers
of
electron
beam
(EB)
equipment and more than
40
suppliers of ultraviolet light (UV) equipment.
The ninth edition (1987) of the
Radiation Curing
Bqer’s
Guide
lists the same number
of
EB
suppliers and about
50
suppliers
of
UV equipment. In the United States, there were
*
It is realized that other radiation processes such
as
microwave, Infrared. and gamma rays can be used
to
cure
coatings. However, this chapter
IS
only concerned with electron beam and ultraviolet light radiatlon. which
arc
the most important commerclal processes.
755

756
KOLESKE
about 100 electron beam units and about
25,000
ultraviolet light units operational in
1983- 1984.’ These figures include laboratory, pilot, and production units. With the indus-
try growing at about 10-1576 per year,”‘ it is very reasonable to expect that these numbers
had increased by the end
of
the decade. Equipment
for
EB curing is significantly more
expensive than that for UV curing, and it represents the exception listed above in the “low
capital requirement” advantage of radiation curing. Even a laboratory-sized EB unit will
run into six figures, whereas simple conveyorized UV systems can be obtained for a few
thousand dollars. Furthermore, energy consumption is high with EB units and low
UV
systems.
In 1980 about 80 million pounds of formulated radiation-curable product were sold
in the United States. This number increased
to
127 million pounds in 1985 and was
expected to increase to 216 million pounds in 1990! About 88%
of
the 1985 total was
cured by ultraviolet light and the remainder by electron beam. Thus, it is readily apparent
that radiation curing is a specialty area, but it is one that is growing much faster than the
overall coatings market. A recent study’ indicates that the market will be 336 million
pounds by 1995, which represents a 9.2% compounded growth rate from the prediction
for 1990.
There are two basic technologies involved in the radiation-cured coatings market.
The older and more well-known technology involves free radical chemistry, and the cure
of compounds containing unsaturation (i.e. acrylates, styrenehnsaturated polyester, and
the like). The other technology involves cation chemistry and the cure of cycloaliphatic
epoxides and compounds that will copolymerize with them under the influence
of
Bransted
or Lewis acids.
2.0 EQUIPMENT
2.1
Electron Beam
With electron bean1 curing, energy transfer is caused by energetic or accelerated elec-
trons.”-” Therefore, photoinitiators usually are not used in
EB
cure formulations and there
are no photoinitiators fragments, which might have an odor or otherwise detract from
properties in the cured coating. The electron source is a filament that is heated inside a
vacuum tube. The electrons are accelerated
to
a high energy (-los
V)
by an impressed
electrical field with the degree
of
acceleration increasing with increasing applied voltage.
High voltages (150,000-300.000 V) are involved. The accelerated electrons pass through
a
metallic foil window and are sent on to the compound, which is capable of absorbing
their energy and thus undergoing polymerization. The ability
of
the electrons to be absorbed
by the compound (i.e., the radiation-curable fornlulation) depends
on
the material’s density,
and therefore depth of penetration is inversely proportional
to
density. The electron bom-
bardment of the formulated coating abstracts hydrogen atoms from some of the molecules,
thereby generating short-lived free radicals that can initiate the polymerization of acrylates
and similar compounds that will interact and react with such species. Clear coatings of
up
to
20
mils
(500
pm) and pigmented coatings of about 15 mils (400 pm) can be cured
with EB equipment.
2.2
Ultraviolet Light
In general, ultraviolet light units operate with electromagnetic radiation that is in the optical
region
of
200-760 nm. They do produce infrared radiation of 760 nm to
1
.0
mm. but this

RADIATION-CURED COATINGS
757
energy is thermal and acts either
to
anneal the cured coating and relieve internal stresses
and strains or
to
enhance cure rate in cationic cure systems. Of course, in certain formula-
tions this thermal energy can have a deletorious effect by causing volatilization
of
reactants,
and it is minimized in some equipment. In other cases, such as cationic UV curing, the
thermal energy can be highly beneficial by kinetically enhancing the cure rate
of
the
compounds.
Basically, in the case of UV curing, compounds susceptible to rapid polymerization
are contacted with initiating species obtained by photolysis
of
a
photoinitiator. In the UV
cure* of such compounds, a photoinitiator that is capable of photolyzing or degrading
to
an active species is added to the formulation. When light of the proper wavelength strikes
the photoinitiator, the active species is generated and polymerization rapidly takes place.
Certain photoinitiators generate free radicals, and these are used
to
cure acrylates. Other
photinitiators generate cations, which are used
to
cure cycloalipathic epoxide-based sys-
tems. It should be readily apparent that matching the output of the ultraviolet light source
with the absorption spectra of the photinitiator is an important aspect of this technology.
There are four different ultraviolet light curing technologies. These are
as
follows.
Medium pressure mercury vapor lamps
(in
certain countries, low pressure and high
Electrodeless vapor lamps.
Pulsed xenon lamps.
Lasers.
pressure mercury vapor lamps are also used).
Medium pressure mercury vapor (MPH) lamps have been used commercially for
about
20
years. The bulb is an evacuated quartz tube that contains metallic mercury and
has electrodes at each end. Electrical energy is supplied through the electodes, and an arc
is struck between them. This heats mercury in the tube
to
a plasma, which emits UV,
visible. and infrared radiation. Output ranges from about
100
to
400
in., and
a
warm-up
time
of
10-
15
minutes is often recommended for the bulb to be fully operational. The
systems can be doped
to
alter the emission spectrum, but usually only mercury is used.
Electrodeless lamps have been in use for about a decade and currently are very
popular
in
the industry.“’.” The vacuum the tube UV bulb is manufactured from quartz.
which is invisible to the UV radiation. The bulb contains either mercury or other proprietary
metals and gases. The systems is activated by microwave or radiofrequency energy (i.e.
it does not have electrodes). Because of the nature
of
this activation system,
it
essentially
has instant on and off operation. There are available a variety of doped bulbs with various
output spectra that can be “matched”
to
that
of
different photoinitiators. Bulb lifetime is
usually
5-10
times longer than that of standard MPH lamps.
Xenon lamps are quartz tubes filled with doped xenon. The lamp is powered by
pulsed electrical current.“’.” These units offer very low heat output along with short time,
extremely high peak intensity output (some types have as high as
8000-10,000
W output.
although most operate at much lower intensities). The output spectrum is continuous with
this source rather than that
of
the discrete line types
as
from the MPH lamps. However,
in certain instances mercury is added to the tube contents to enhance curing in the region

758
KOLESKE
of
mercury's spectral lines. The output has also been modified with other metals such as
iron and beryllium.
Argon ion and nitrogen lasers in combination with specific photinitiators that have
a strong absorbance at the emission line of the laser have been used
to
cure multifunctional
acrylates.'3.'4 Although the studies are interesting and may hold promise for the future,
at present this is considered
to
be a research area of potential interest for the electronics
industry.
3.0
CHEMISTRY
Most of the following discussion deals with ultraviolet light technology. The reason for
this is that photinitiator breakdown is important
to
cure. In a general sense, the same
compounds that will cure with the free radical photoinitators will cure with electron beam.
3.1
Photoinitiators
3.1.
l
Free
Radical
Type
Free radical generating photoinitiators are of two general functional types.'5.'" The first
type involves
a
mechanism known as
honzol~~tic~agr~~entation,
in which a compound such
as a benzoin alkyl ether undergoes
a
photochemically induced fragmentation into highly
active free radicals as described in Figure
1.
The second type of free radical generation functions through
a
mechanism that is
termed
electron
tramfer..
This mechanism involves
a
photolytic excitation of the photoiniti-
ator from a found state singlet to an excited triplet state. This is followed by electron transfer
to
a
hydrogen atom doner, such
as
dimethylethanolamine (DMEA), and the formation of
highly excited free radicals as described in Figure 2.
Typical commercial photinitiators include compounds such as 2,2-diethyoxy-aceto-
phenone, 2,2-dimethoxy-2-phenyl acetophenone, hydroxycyclohexylphenyl ketone,
benzophenone.-triethylamine, 2-methyl-
l-[4-(methylthio)phenyl]-2-morpholino-propane-
1.
l-phenyl- 1,2-propane dione-2-( o-ethoxycarbonyl)oxime, and benzoin methyl, isopro-
pyl, isobutyl, and other alkyl ethers.
Free radical generating photoinitiators
of
the foregoing types are inhibited or inacti-
vated by oxygen as a result of a complex that forms between the light-activated photoinitia-
tors and molecular oxygen. This effect can be overcome by inerting the coating with
nitrogen during cure, by adding waxes to the system, or by use of excess photoinitiator.
Air that has been dispersed in the coating system during formulation contains oxygen,
and
it
acts as a stabilizer. However, formulations containing very active photoinitiators
Benzoin Alkyl
Ether
Benzoyl Radical Alkoxybenzyl Radical
Figure 1
Homolytic
fragmentation.
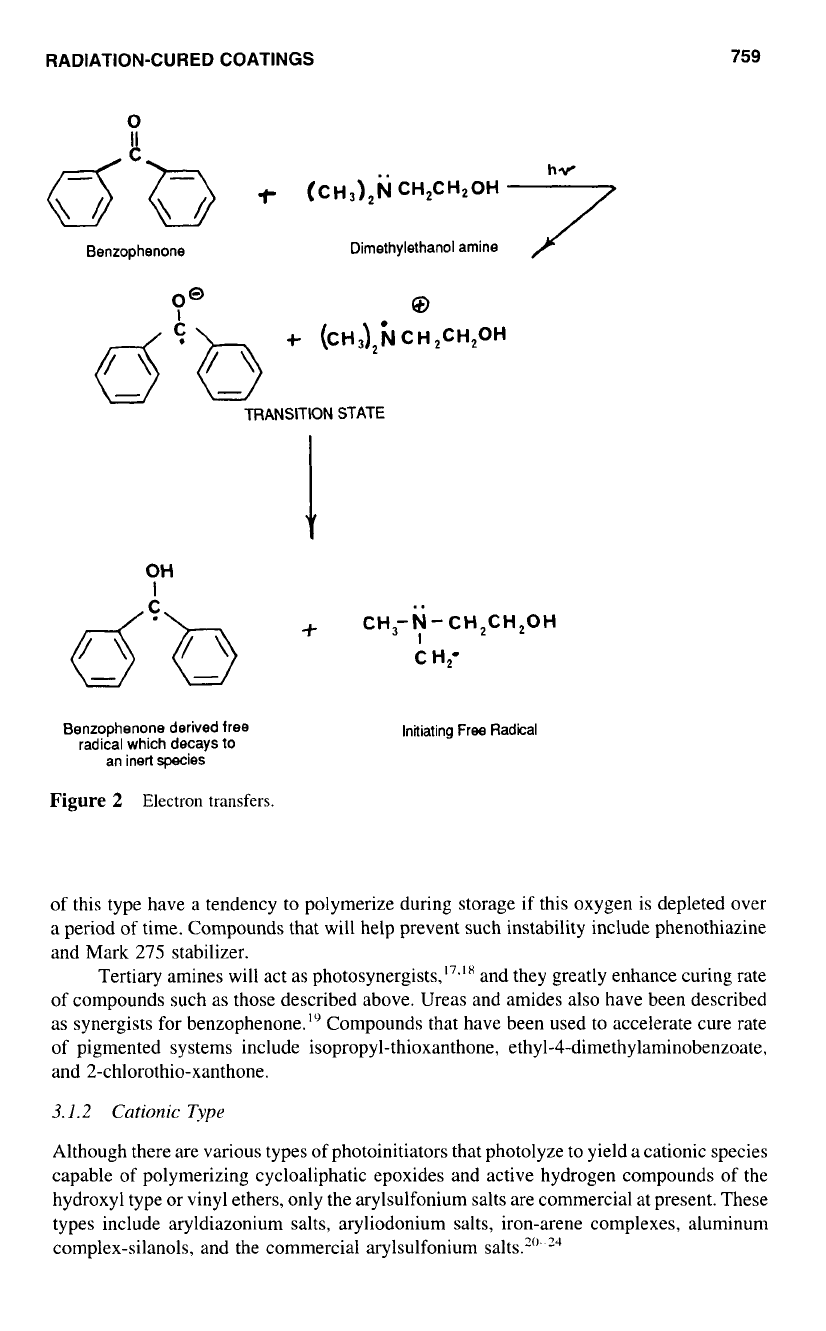
RADIATION-CURED COATINGS
759
0
I
Benzophenone Dimethylethanol amine
/
OH
i
Benzophenone derived free
radical which decays
to
an inert species
+
CH,-N-CH,CH,OH
..
I
C
H,-
lnliating
Free
Radical
Figure
2
Electron transfers.
of
this type have
a
tendency to polymerize during storage if this oxygen is depleted over
a
period of time. Compounds that will help prevent such instability include phenothiazine
and Mark 275 stabilizer.
Tertiary amines will act
as
photo synergist^,'^.^^
and they greatly enhance curing rate
of
compounds such
as
those described above. Ureas and amides also have been described
as
synergists for benzophenone.'" Compounds that have been used
to
accelerate cure rate
of pigmented systems include isopropyl-thioxanthone,
ethyl-4-dimethylaminobenzoate,
and 2-chlorothio-xanthone.
3.1.2
Cationic
Type
Although there are various types of photoinitiators that photolyze to yield
a
cationic species
capable of polymerizing cycloaliphatic epoxides and active hydrogen compounds of the
hydroxyl type or vinyl ethers, only the arylsulfonium salts are commercial at present. These
types include aryldiazonium salts, aryliodonium salts, iron-arene complexes, aluminum
complex-silanols, and the commercial arylsulfonium salts.""

760
KOLESKE
Aryldiazonium hexafluorophosphates and tetrafluoroborates decompose under the
action of UV light and yield Lewis acids such as BF3 and PFS, nitrogen, and other frag-
ments.?5-?7
These photoinitiators were used in the infancy of cationic UV cure of cycloali-
phatic epoxides. Although they were quite active for first-generation products, the disad-
vantages
of
thermal instability, which led to short shelf life, and
of
nitrogen evolution,
which led to pinholes and bubbles in films thicker than about
0.2
mil, inhibited commercial
use and led
to
their replacement by the onium salts in the marketplace.
The polymerization
of
epoxides with aluminum complex-silanol photoinitiators has
been described.'x.'" The technology is not being practiced in the United States, but it may
be in use in Japan. The iron-arene complexes represent a new type of cationic photoinitiator
that was recently described.3".3' When photolyzed, these compounds degrade to yield both
Lewis acid type catalysts and free radicals. Since these compounds are relatively new,
detailed information about them is not available.
Various investigators studied the onium salts of iodine
or
the Group VI
element^.^'"^'
Currently, the arylsulfonium salts are commercially used as photoinitiators. These com-
pounds do not have the deficiencies of the diazonium salts because there is no nitrogen
evolution
on
photolysis and,
if
protected from ultraviolet light, the systems can have
ambient-condition shelf lives in excess of
2
years. When UV light interacts with the onium
salts, an excited species is formed. This species undergoes homolytic bond cleavage to
yield a radical cation, which extracts
a
hydrogen atom from a suitable donor and generates
another free radical species. The new compound then gives up the proton for formation
of a strong Bransted acid. The Bransted or protic acid that is the polymerization catalyst
is of the form
HMF(,
where
M
is a metal such
as
antimony, arsenic, or phosphorus. This
catalyst is long-lived, and the cationic polymerization
of
the epoxide system can continue
in the "dark" after initial exposure to UV light until the available epoxide is exhausted
or the polymerization is terminated by some other mechanism. Thus, the onium salts
generate both cationic species and free radicals and can be used in radiation-activated,
dualmechanism systems.
Note that the onium salt photoinitiator is
a
blocked or latent photochemical source
of the strong BrQnsted acid that acts as
a
catalystlinitiator for the formulated system.
Because of the acidity of the UV-generated catalyst or initiator, it is necessary
to
keep
the formulated system (substrate, coating equipment, etc.) free from basic compounds that
would neutralize the acid and either negate or slow down cure rate. Even very weak basic
compounds will react or interact with the strong acidic species.
3.1.3
Dual-Mechanism Curing
Since the cationic photoinitiators generate both free radicals and Bransted acids when
exposed to ultraviolet light, it is possible
to
combine acrylates that will cure with free
radicals and epoxides that cure with the protic acids. Free radical generating photoinitiators
such as
2,2-diethoxyacetophenone
can be added, if an additional source of free radicals
is necessary. Experience has shown that this usually is not necessary. Of course, the
benzophenoneamine systems described earlier should not be used. Little can be found in
the about this interesting topic, but dual-mechanism curing should prove to
be
a
useful technique in the future and merits further study.
Dual-mechanism systems that involve free radical chemistry coupled with thermal
chemistry are also known. Dual-cure plastisols'" and dual-cure pigmented'? coatings have
been reported. The combination
of
ultraviolet and infrared radiation for curing coatings,

RADIATION-CURED COATINGS
761
which can be beneficial for either of the dual-mechanism systems, has also been dis-
3.2
Formulation
The subject of formulation involves both free-radical-curable ingredients and how they
are used, and cationic-curable ingredients and how they are used. If one takes an extremely
simplistic approach, only a single species that will cure under the influence of radiation
is needed for a coating. However, most formulations contain a variety of ingredients to
achieve the property balance that is needed to meet a given set of performance criteria.
Most formulations contain a base oligometric or multifunctional compound, a flexibi-
lizer (which may be the base compound) a low viscosity, reactive diluent (which may be
mono- or multifunctional), for viscosity control, a multifunctional acrylate for high cross-
link density, a photoinitiator (unless electron beam cure is used), and usually a surfactant
or flow and leveling agent. Combinations
of
more than one material of each type are
usually used to optimize properties. The performance characteristics of specific end uses
may also require use of a slip agent, nonreactive organic or inorganic fillers and/or pig-
ments, adhesion promoters, flattening agents, an augmenting flow and leveling aid, or
some other additive-type ingredient.
3.2.1
Free
Radical
System
In general, systems based on acrylates are used
in
free radical cure systems.4S“’X The base
material is usually an epoxy acrylate or a urethane acrylate. Epoxy acrylates are the reaction
products
of
acrylic acid with various diglycidyl ethers of bisphenol
A.
The epoxy acrylates
contain acrylate and hydroxyl functionality, but they do not contain epoxide functionality
and should not be confused with the epoxides
to
be discussed in the cationic cure systems
section. The term “epoxy acrylate” is a widely used and accepted misnomer; as can be
seen by the structural formula in Figure
3,
“acrylated epoxy” would be a better term to
use for these compounds.
Urethane acrylates are often prepared by end capping a polyether, polyester. or
caprolactone poly01 with a diisocyanate and then reacting this isocyanate prepolymer with
an hydroxyalkyl acrylate.‘”-’’ The chemistry is more complex than this simple description,
and in order of component addition is important to minimizing viscosity. Both epoxy
acrylates and urethane acrylates have a high viscosity
(2
IO”
cp) at ambient temperatures.5’
To
facilitate manufacture, handling, and later formulation, the compounds are often made
in a low viscosity mono- or multifunctional acrylate, which will later serve as a reactive
diluent and/or cross-linking agent in a formulated system.
In either the neat or the diluted form, these base compounds are diluted to application
viscosity with more mono- or polyfunctional acrylate. Polyfunctional acrylates increase
cross-link density and improve solvent resistance. increase hardness, and increase glass
Figure
3
An
“epoxy
acrylate.”
