Russell I. (ed.) Whisky. Technology, Production and Marketing
Подождите немного. Документ загружается.

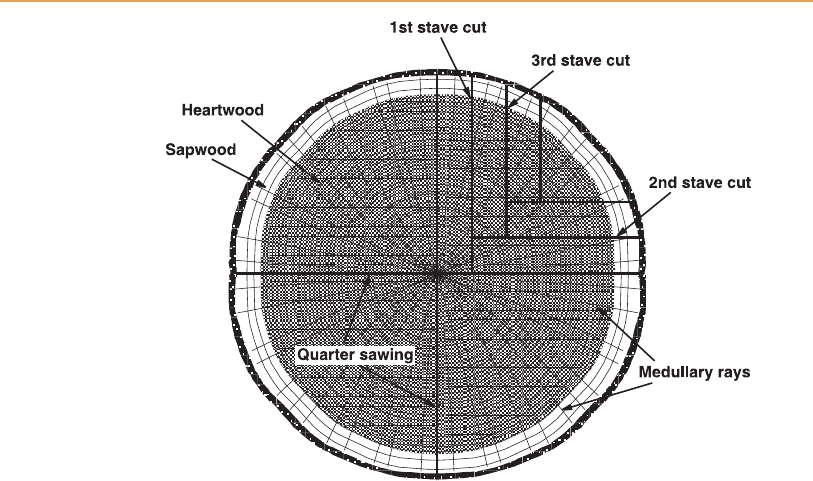
[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 214 208-241
From a log quarter, the first saw cut is removed from one of the flat surfaces
parallel to the radius of the tree. The quarter is then turned 908 and the second
cut is removed from the other flat surface. This is repeated until the amount of
timber left in the quarter is too small to be of any use. The sapwood and any
dead heartwoo d are then removed from the sawn timber to produce the fin-
ished stave and heading blanks.
The next stage of the process is the drying or seasoning of the timber. In the
USA, all of the oak for the production of bourbon barrels is dried in a drying
kiln over a period of approximately one month. This reduces the moisture
content of the wood to a wo rkable level of approximately 12 per cent.
Throughout the drying process the timber is held under specific temperature
and humidity conditions to ensure that the drying procedure is efficient and
that wood damage, such as the appearance of splits or cracks in the stave ends,
is minimized.
In Spain the seasoning of timber for the production of sherry casks is quite
different. The stave and heading blanks are initially air dried in the growing
regions of northern Spain for a period of approximately nine months, which
reduces the moisture content of the wood to approximately 20 per cent. The
timber is then shipped to the warmer sherry-producing regions in the south of
Spain, where it is further air seasoned for a period of approximately six to nine
months, or until its moisture content is redu ced to a workable level of approxi-
mately 14–16 per cent.
214 Whisky: Technology, Production and Marketing
Figure 7.1
Stave cuts in timber processing.

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 215 208-241
Bourbon cask construction
In the manufacture of bourbon barrels, the dried, rough-cut stave blanks are
initially jointed to produce staves that have smooth angled edges and are
slightly wider in the middle compared to the ends. This is essential for
forming the desired barrel shape. The assembly of the barrel begins by
arranging the straight staves into a circular structure that is closed at one
end. This is then steamed for a period of between ten and twenty minutes at
a temperature of approximately 958C to soften the fibres of the wood and
enable the staves to be bent. The staves are then drawn into the conventional
barrel shape using a windlass, and a temporary iron hoop is used to hold
them in place.
The next part of the process is to heat treat the inside of the cask shell. In
terms of whisky maturation this is probably the mos t important stage of
barrel production, as it defines the cask’s ability to matur e spirit. The cask
shell, which is still wet from the steaming process, is initially heated to
between 230 and 2608C for a period of approximately fifteen minutes.
This drives off the surface water and sets the staves in the shape of a barrel
(Hankerson, 1947). The shell is then charred, which involves setting the
inside on fire and allowing it to burn until the required degree of char
has been obtained. The role of the formed char layer during maturation,
and the toasted layer that lies beneath, is described in detail in the follow-
ing section.
Barrel ends are produced by pinning together pieces of oak wood of appro-
priate thickness using wooden dowels. This is then cut into the circular head
and is given a bevelled edge. The ends, which are also charred, are then
inserted into the cask shell. Their bevelled edges locate into grooves, known
as the croze, cut into each end of the shell. Finally, the hoops are driven on to
produce the finished bourbon barrel.
Sherry cask construction
The staves for a 500-l sherry butt are tapered in the same way as in the
production of a bourbon barrel, but are longer and thicker. Where the 180-l
barrel requires around 30 staves for its construction, the larger butt will con-
tain approximately 50 staves. These are initially raised into a circular structure,
as in bourbon barrel production, which is held in shape using temporary
hoops. This is then placed over an open fire at approximately 2008C, which
both heat treats the wood, turning it brown in colour, and makes it more
pliable, allowing it to be slowly pulled into the conventional butt shape
using a windlass. No steaming is involved in the construction of a sherry
cask, but water is applied to the outside of the staves during heating to prevent
cracking. As in the production of a bourbon barrel, the ends are initially pre-
pared by pinning together heading timber using wire dowels. These are then
cut into the circular head and inserted into the cask shell to produce the
finished sherry butt.
Chapter 7 Maturation and blending 215

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 216 208-241
Heat treatment chemistry
Heat treatment has always played an important role in the manufacture of
casks for maturing distilled spirits. Two distinct methods of heating are used:
toasting is a milder but more prolonged form of heat treatment, while charring
is more rapid and involves heating the inner face of the cask with a gas burner
until the inner face catches fire and becomes carbonized. Despite these differ-
ences, the objectives of the treatments are the same:
. Degradation of wood polymers to yield flavour compounds
. Destruction of resinous or unpleasant aroma compounds present in the wood
. Production of a layer of ‘active’ carbo n on the inner surface of casks (charring
only).
Degradation of wood polymers to yield flavour compounds
The main effect of heat treatment is the degradation of wood polymers to form
colour and flavour compounds. Most studies in the past have focused on the
degradation of lignin to produce aromatic aldehydes and acids, such as vanil-
lin and vanillic acid. However, recent research has highlighted the importance
of polysaccharide brea kdown in the formation of a number of flavour com-
pounds. Thermal degradation of polysaccharides (cellulose and hemicellulose)
produces large quantities of furaldehydes, of which furfural predominates
because of the preferential deterioration of the hemicellulose. Furaldehydes
themselves have little sensory impact, but their formation is accompanied by
that of many other mo lecules with sweet, caramel and toasted aromas. Maltol
and 2-hydroxy-3-methyl-2-cyclopentenone were identified in toasted oak after
heating (Nishimura et al., 1983), but comparison of the amounts present with
their odour thresholds suggests that their sensory impact may be limited
(Cutzach et al., 1997). More recently, furylhydroxymethyl ketone and 2,5-fur-
andicarbaldehyde have been identified in extracts of toasted oak, being
formed by the direct pyrolysis of sugars (Cutzach et al., 1999). Extracts also
contained 2,3-dihydromaltol, furaneol, and 2,3-dihydro-3,5-dihydroxy-6-
methyl-4H-pyran- 4-one, which are the products of the Maillard reaction
between amino acids and sugars (Cutzach et al., 1997).
The products of lignin degr adation, such as vanillin, syringaldehyde, con-
iferaldehyde and sinapaldehyde (Nishimura et al., 1983; Reazin, 1983) undergo
further oxidation, either in the wood or in the maturing spirit, to give vanillic
and syringic acids. Of these, vanillin is of greatest sensory importance on
account of its low odour threshold. Analysis of whisky matured in new
toasted casks shows that vanillin exceeds its odour threshold within the first
six months of maturation (Con ner et al., 2001). Synergism has been reported to
decrease the odour threshold of mixtures of lignin breakdown products
(Maga, 1985). The intensity of heat treatment can affect the levels of aromatic
aldehydes and acids generated. Studies using oak chips have shown that
toasting temperatures up to approximately 2008C increase levels of aromatic
aldehydes and acids (Nishimura et al., 1983). Higher temperatures and char-
216 Whisky: Technology, Production and Marketing

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 217 208-241
ring decrease levels, due to the formation of volatile phenols such as guaiacol
and syringol, or carbonization of the aromatics. In casks however, charring
increases the levels of lignin breakdown products extracted by the spirit.
Although the char layer contains few aromatics, heat penetration to sub-sur-
face layers promotes thermal degradation reactions, increasing aromatic alde-
hydes and acids, over a depth of 6 mm (Perry et al., 1990). Consequently, with
increasing charring time there is increased thermal degradation of wood con-
stituents behind the char layer. Although deeper in the stave, behind a thicker
char layer, this does not hinde r the extraction of these comp onents because the
disruption of the wood structure during charring increases the penetration of
the maturing spirit.
Other constituents of wood are affected by heat treatment. Complex ellagi-
tannins, such as vescalagin and castalagin, are greatly reduced by heating and
charring. Levels are generally low in distilled spirits (Mosedale, 1995), possi-
bly as a result of conversion to ellagic acid (Hale et al., 1999). Two aroma
compounds present in untreated oak are eugenol (clove-like aroma) and oak
lactone (coconut). While some studies show that toasting and charring
increase levels, others suggest there is little effect (Mosedale, 1995).
Although heat treatment may increase the formation of these compounds,
their low boiling points result in volatilization from the wood surface.
Heat treatment also increases the level of coloured compounds extracted
from the wood by the spirit. However, the exact chemical nature of cask colour
has not been elucidated. Heat treatment of model compounds suggests that
colour formation is mostly the result of hemicellulose and lignin degradation,
with little or no contribution from cellulose. The level of extractable colour
generally increases with the intensity of toasting and charring. However, as for
the aromatic aldehydes and acids, the char layer contributes little colour.
Destruction of resinous or unpleasant aroma compounds present in the
wood
Maturation of wines and spirits in new barrels can result in a rancid, sawdust
aroma. The major compound responsible for this aroma has been identified as
trans-2-nonenal, though other unsaturated aldehydes and ketones such as
trans-2-octenal and 1-octen-3-one may enhance this character (Chatonnet and
Dubourdieu, 1998). The levels present varied greatly between samples of
wood and their most likely route of formation is by chemical auto-oxidation
of linoleic acid during seasoning. The amount of trans-2-nonenal could be
markedly decreased, and the off-flavour completely eliminated, by increasing
toasting intensity during cask manufacture.
Production of a layer of ‘active’ carbon on the inner surface of casks
The formation of the ‘active’ carbon layer on the inner surface of the cask is the
result of carbonization of the polymeric constituents. This layer contributes
little in the way of colour or extractives to the maturing whisky. It does,
however, play an important role in the removal of immature character.
Chapter 7 Maturation and blending 217

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 218 208-241
Experiments have shown that it promotes the oxidation of dimethyl sulphide
(Fujii et al., 1992) and may reduce the concentration of other sulphur com-
pounds by a combination of adsorption and oxidation (Philp, 1986). Also, the
break up of the wood structure near the surface may allow easier penetration
by the spirit and increase the extraction of degradation components from sub-
surface layers (Mosedale, 1995).
Control of heat treatment
The control of toasting and charring of casks has a major effect on the sensory
properties of the matured spirits. The intensity of toasting is generally con-
trolled by time, with light, medium and heavy toasts the result of five to ten,
ten to fifteen, and fifteen to twenty minutes’ heating respectively (Mosedale
and Puech, 1998). Toasting normally uses oak-chip fires maintained by indi-
vidual coopers, and consequently there are large variations between different
cooperages and from one cooper to another. New methods are being investi-
gated to provide a better classification of toasted casks, and these are based on
either chromatographic analysis of the volatiles produced during toas ting or
their assessment using metal oxide-based odour sensors (Chatonnet, 1999).
The degree of char ring is also controlled by the burn time, with 15 seconds
used for a light char, 30 seconds for medium char and 45 seconds for a heavy
char (Mosedale and Puech, 1998).
Cask regeneration
Casks that fail to produce a satisfactory maturation, because continued re-use
has depleted the level of available extractives, can undergo regeneration.
These casks are first de-charred, using a rotating brush or flail system, and
the de-charred casks are then re-charred using a gas burner. When casks are
re-charred thermal degradation of lignin and polysaccharide occurs, yielding
similar flavour compounds to those produced in a new charred cask.
However, other constituents of oak are not regenerated, such as oak lactones
and hydrolysable tannins. Consequently the balan ce of wood extra ctives in
regenerated casks is very different from that in a new charred cask.
Re-charring is controlled either manually or by a timer. Reliance sole ly on
time can produce variable results, because casks from different sources have
variable moisture and spirit contents. This affects the drying time of the wood
before ignition, and as a result the degree of charring and the levels of colour
and extractives produced are not controlled. This problem can be overcome by
control measu res based on the surface temperature of the cask wood, or by the
colour of the flame. Initially the cask burns with a blue flame partly fuelled by
the spirit volatilizing from the cask wood; when dry the wood ignites and
burns with a strong yellow flame (Perry et al., 1990). Ch arring time s of 30 to 40
seconds are generally employed, although longer burn times can generate
higher levels of cask extractives and colour.
218 Whisky: Technology, Production and Marketing

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 219 208-241
Scotch whisky maturation
Maturation can be viewed as the specific combination of one type of distillate
with any one type of cask leading to the development of a flavour profile
relative to time (Philp, 1986). Modern analytical techniques have been used
to identify an increasing number of reactions that take place during whisky
maturation. Most of these reactions are identified by chemical changes, and
their influence on the sensory properties of a mature spirit has not been clar-
ified. This lack of sensory understanding is due to the complex nature of
whisky flavour. Overall flavour is the result of an interaction of a large number
of different aromas, some of which have yet to be identified. Frequently wood
or spirit treatments change more than one compound (or group of com-
pounds), and this can compromise the modelling, and therefore the prediction,
of the sensory impact of a single aroma compound.
Another drawback is that the research has been fragmented, with different
researchers looking at different products in different parts of the world. As the
number of reactions increases, maturation can no longer be thought of as a
homogeneous process with the same reactions occurring irrespective of the
cask type. Most probably maturation is a mixture of different reactions, and
the nature and extent of each is determined by the type of cask used.
Consequently, this description of maturation is in two sections; the first part
outlines the different reactions that have been identified, and the sec ond
relates these reactions to different cask types.
Maturation reactions
The reactions that occur during maturati on can be separated into additive and
subtractive activities:
. Additive activity includes reactions that introduce or form new aroma com-
pounds
. Subtractive activity includes reactions that remove or alter constituents of
new-make spirit.
The main example of additive activity during maturation is extraction of cask-
derived congeners. These can originate from the unprocessed heartwood, the
thermal degradation of wood polymers during cask manufacture, and carry-
over from previous use of the cask. These extractives can also be supplemen-
ted by the hydrolysis of wood constituents during maturation, and interac-
tions between wood and distillate components that form new aroma
compounds. Subtractive reactions may involve the removal of constituents
by physical processes such as evaporation, adsorption/degradation by the
charred surface of the cask, and chemical degradation reactions such as oxida-
tion and the masking of distillate aromas either directly or through changes in
the whisky matrix.
Chapter 7 Maturation and blending 219

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 220 208-241
Additive activity
Cask charring generates high levels of colour and extractives in the cask
wood. A large proportion of these are extracted during the first use of the
casks, though the actual amount will depend on variables such as the
length of the matur ation period and the warehousing conditions. In sub-
sequent use further extraction of these thermal degradation products occurs,
and this is supplemented by the breakdown of lignin and hemicellulose
polymers in the wood by the combined actions of oxidation and hydrolysis.
Two mechanisms have been proposed for the degradation of lignin during
maturation (Reazin, 1981; Puech, 1984). The first involves extraction of an
ethanol–lignin complex by the spirit, which breaks down to form coniferyl
and sinapyl alcohols. These alcoho ls are then oxidized into conifer- and
sinapaldehydes, with further oxidation to vanillin and syringaldehyde
respectively. The second mechanism involves similar reactions, which take
place in the wood to produce aromatic aldehydes that are later extracted by
the spirit.
Degradation of hemicellulose under these condi tions has not been fully
investigated, but may give rise to sugars such as xylose and glucose rath er
than the furan compounds produced during toasting and charring. The level
of sugars obtained, however, does not reach the level required to impart
sweetness to the maturing spirit.
There are also a number of aroma co mpounds present in oak wood that
are not formed by the degradation of structural polymers. Of these, both oak
lactone (coconut aroma) and eugenol (spicy, clove aroma) have been identi-
fied in mature spirits. The sensory importance of oak lactone is complicated
by the fact that two diastereoisomers are present in oak, each with a different
sensory impact. The relative amounts of these depend on oak origin and its
pre-treatment. The highest concentrations are present in American oak, in
which the cis isomer predominates. Grain whisky matured for four years in
new, toasted American oak casks has been found to contain cis oak lactone at
a concentration twenty times greater than its odour threshold (Conner et al.,
2001). On its own, this level of cis oak lactone has a pronounced coconut
aroma, which was not detected in the grain whisky matured in the new
charred cask. Although it is undoubtedly an important aroma compound,
other aroma compounds present in the cask wood must mo dify its sensory
impact. With cask re-use the level of this compound extracted into the spirit
decreases, with a corresponding reduction in its sensory impact. Re-charring
casks produces only a small increase in oak lactone levels. The level of
eugenol in grain whisky matured in new, toasted American oak casks is
around its odour threshold level after four years. Consequently its sensory
impact is a lot lower, but cannot be discounted as it may play some role
in modifying the sensory impact of other aroma compounds such as oak
lactone.
Another grou p of constituents extracted from oak during maturation are the
hydrolysable tannins. These include gallic and ellagic acids and the various
complex combinations of these acids with sugars, which are known as gallo-
220 Whisky: Technology, Production and Marketing

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 221 208-241
and ellagitannins (e.g. vescalagin and castalagin). These compounds are
generally non- volatile and have no aroma, but may play a role in modifying
the mouth-feel and taste of mature whisky. They may also be important as
oxidative catalysts in the removal of sulphides from the spirit, and so contri-
bute indirectly to flavour changes duri ng maturation (See Chemical degrada-
tion, below). It should be noted that in many older studies Folin-Denis and
Folin Ciocalteau reagents were used to determine the level of tannins in
whisky. This method more accurately measures total phenolics, which
would also include any lignin breakdown products extracted from the cask
wood. Hydrolysable tannins gradually deplete with repeated cask use.
Although re-charring casks may give increased levels of ellagic acid, it has
little effect on the levels of gallic acid.
Another potential sour ce of aroma compounds in refill casks is from the
spirit or wine matured in a previous fill. In-drink by the cask dur ing matura-
tion results in the retention of some components of the distillate by the cask
wood, and these are then released during subsequent maturations. This effect
is most obvious in casks that are used to mature malt and then grain distillates,
with the grain spirit acquiring some of the characteristics (such as peatiness) of
the malt spirit. It is also possible to identify congeners from sherry and bour-
bon in ex-sherry and ex-bourbon casks, but it is not known if these constitu-
ents make a significant contribution to the distinctive flavo ur profile obtained
from such casks.
After a number of fills, heartwood constituents and thermal degradation
products have all but been depleted. The extraction of wood components
then relies on a combination of hydrolysis and oxidation reactions.
However, the proportion of lignin in the wood that is degraded by these
reactions is relatively small (Conner et al., 1993). With repeated use, suscep-
tible lignin in the inner surface of the wood becomes depleted and the locus of
degradation retreats further into the stave (Conner et al., 1995). This combina-
tion of depletion and migration from deeper in the stave wood slows the rate
of extraction and consequently maturation. Eventually a point is reached
where the cask fails to produce any sensory improvement, and it is then
termed ‘exhausted’ (Philp, 1989).
Subtractive activity
Changes in distillate character during maturation may be the result of the loss
or suppression of aroma compounds. This may involve:
. Evaporation of low boiling point compounds through the cask
. Adsorption/degradation by the charred surface of the cask
. Chemical reactions resulting in a less volatile product or one with different
sensory characteristics
. Masking of im mature characters either by sensory interaction or by physical
changes in the whisky matrix.
Chapter 7 Maturation and blending 221

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 222 208-241
Evaporation
Evaporation of volatile compounds through the cask surface occurs during the
course of maturation. For a model whisky, the rate of evaporation ranged from
32 per cent of the total present in the spirit for acetaldehyde to 5 per cent for
iso-amyl alcohols and 1 per cent for ethyl hexanoate and acetic acid (Hasuo
and Yoshizawa, 1986). Evaporation is thought to be the main route for the loss
of dimethylsulphide (Fujii et al., 1992) and dihydro-2-methyl-3(2H)-thiophene
(Nishimura and Matsuyama, 1989). Although evaporation occurs from all
casks, it is not known how factors such as porosity and stave thickness affect
its rate, and in turn spirit quality. Different warehouse conditions (tempera -
ture, humidity and the airflow round the cask) will also affect the evaporation
rate and again mature quality. As evaporation progresses the level of spirit in
the cask decreases, creating an air space. The increase d headspace may pro-
vide a larger pool of air to replenish the dissolved oxygen in the spirit that is
used up in oxidation reactions during maturation.
Adsorption/degradation by char
The ‘active’ carbon layer on the inner surface of the cask plays an important
role in the removal of immature character from the maturing spirit.
Experiments have shown that it promotes the oxidation of dimethyl sulphide
(Fujii et al., 1992) and may reduce the concentration of other sulphur com-
pounds by a combination of adsorption and oxidation (Philp, 1986). Two
mechanisms are possible for these reductions. It may be that char preferen-
tially adsorbs these compounds, or chemical degradation may play the major
role. Fujii et al. (1992) showed that approximately half the dimethyl sulphide in
model solutions was oxidized in the presence of char, but was unable to
identify the route by which the remainder was lost. Another potential role
of char is in retaining both cask extractives and wine/spirit congeners from
previous fills. Although fresh char contains few components, after maturation
it contains higher concentrations of wood and wine/spirit components than
the sub-surface layers. The char may therefore act as an important reservoir for
congeners that are released during the subsequent use of the cask.
Chemical degradation
Chemical reactions that alter distillate components include oxidation and
acetal formation. Examples of oxidation include the formation of acetaldehyde
and acetic acid from ethanol (Reazin, 1981), and the formation of dimethyl
sulfoxide from dimethylsulfide (Fujii et al., 1992). The breakdown of sulphur
compounds may also be enhanced by cask extractives, particularly hydroly-
sable tannins such as gallic and ellagic acids. Interaction between these tan-
nins, dissolved oxygen and copper ions produces active oxygen and peroxide,
which degrade sulphur compounds (Philp, 1986). The dynamics of this reac-
tion change during matur ation. Copper in the spirit is an important promoter
of this reaction, and is adsorbed by the cask during maturation (Muller and
McEwan, 1998). The availability of oxygen may also depend on cask factors
such as porosity, evaporation rate and the air space within the cask. The
222 Whisky: Technology, Production and Marketing

[15:37 13/3/03 n:/3991 RUSSELL.751/3991-007.3d] Ref: 3991 Whisky Chapter 7 Page: 223 208-241
situation is further complicated by a recent study (McPhail et al., 1999) that
identified these cask extractives as important antioxidants in mature spirits
with the ability to adsorb free radicals. Therefore, the role of these compo unds
and the mechanisms of oxidation in maturation need further clarification. If
oxidation reactions are initiated by active oxygen and peroxide, then hydro-
lysable tannins will act as promoters. If, however, it is initiated by free radi-
cals, then these compounds will act as inhibitors!
Acetal/aldehyde equilibria are established for most aldehydes, and are
important in whisky aroma. Aldehydes frequently have sour and pungent
odours, whereas acetals are pleasant and fruity (Perry, 1986). The equilibrium
between free aldehyde, hemi- acetal and acetal is affected by spirit pH (Perry,
1986), and hence is partly influenced by cask type. During maturation the
concentration of esters generally increases due to the esterification of free
acids by ethanol. A large part of this is due to the formation of ethyl acetate
from acetic acid, either extracted from the cask wood or the product of ethanol
oxidation (Reazin, 1981). Trans-esterification reactions are also thought to
occur, which in the presence of the large excess of ethanol favours the forma-
tion of ethyl esters.
Masking
The immature character of a spirit may be masked in a number of ways. The
first is a direct sensory interaction, where the presence of strong wood aromas
lessens the impact of sulphury or feinty characters. Less dominant wood aro-
mas may also interact by enhancing the perception of positive distillate char-
acters. However, the nature and extent of this type of interaction has not been
studied owing to the difficulties in creating realistic models of whisky aroma.
Masking may also occur through changes in the whisky matrix that reduce
the volatility of distillate components. The reduction in pH during maturation,
which may be cask-dependent, affects the ionization state of weak bases and,
consequently, their volatility (Delahunty et al., 1993). Decreases in pH had the
greatest effect on pyridines, due to their pKa values, greatly reducing their
perceived aroma in whisky.
It has also been known for some years that wood maturation of spirits
produces physicochemical changes in the liquid that are detectable by differ-
ential scanning calorimetry (Nishimura et al., 1983), small angle light scatter-
ing (Aishima et al., 1992) and mass spectrometric analysis of liquid clusters
(Furusawa et al., 1990). Recent research has shown that ethanol and water do
not form a homogeneous mixture over the whole compositional range
(D’Angelo et al., 1994). At spirit concen trations, ethanol exists as a micro-
emulsion in water. Whiskies consist mostly of ethanol and water, with
flavour-active components maintained in the aqueous emulsion by ethanol
(Conner et al., 1998). The aggregation of ethanol is affected by the presence of
wood extractives, which increases the solubility of aroma compounds and
consequently reduces their release into the headspace (Conner et al., 1999).
This effect, and the physicochemical changes, is consistent with an increase
in large ethanol polymer hydrates in wood-matured spirits, which have a
Chapter 7 Maturation and blending 223
