Russell I. (ed.) Whisky. Technology, Production and Marketing
Подождите немного. Документ загружается.

[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 182 178-207
not necessarily the middle of the column as in Figure 6.2). Part of the vapour
condenses as it bubbles through the slightly cooler layer of liquid on the plate
immediately above, releasing its latent heat of condensation (or evaporation),
which is then available to vaporize a proportion of the liquid level at that level.
In this state of dynamic equilibrium the vapour phase, increasingly en riched
in the more volatile component, rises in the direction of decreasing tempera-
ture. The liquid stream flows down the column in the direction of hi gher
temperature, increasingly losing the more volatile component at each lower
level of the cascade.
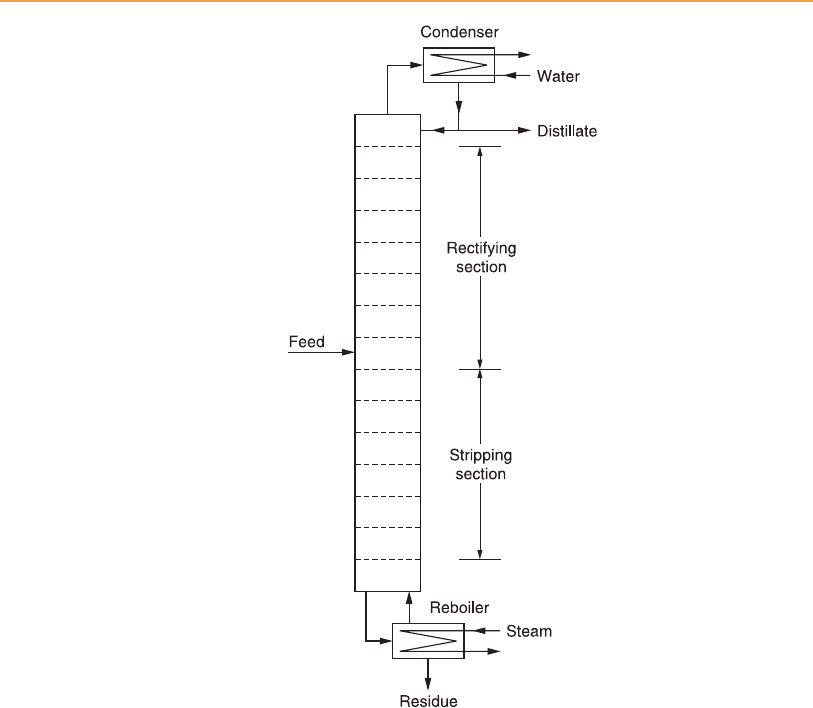
Liquid leaving the bottom section of the still is re-heated in an external
calandria, or reboiler, to generate the vapour for operation of the still, inciden-
tally evaporating any residual ethanol. Technically it is a partial reboiler, since
only part of the bottom product is evaporated and the residue is discharged,
182 Whisky: Technology, Production and Marketing
Figure 6.2
Simple distillation column.
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 183 178-207
either as a purified product (as in the petrochemical industry) or as effluent (as
in the Scotch grain whisky industry) . For an aqueous mixture like alcohol and
water the heat source could be steam injected into the base of the column, but
an important advantage of a reboiler is that the primary steam supply is a
closed loop returning to the main boiler. With direct injection, steam is lost
into the still system and has to be replaced continuously by expensively trea-
ted boiler-feed water. Also, the bottom product is diluted by the condensed
steam.
The vap our leaving the top of the column is condensed and a proportion of
the resulting liquid is returned to the top plate to maintain the liquid level
there, and to sustain the downflow of reflu x to lower sections. It is a total
condenser in the sense that all vapour is condensed, but only part of the
distillate can be drawn off as top product; the remainder mus t be returned
to the top plate as reflux.
Distillation in a continuous still can conveniently be represented as the
cascade shown in Figure 6.3.
Chapter 6 Grain whisky distillation 183
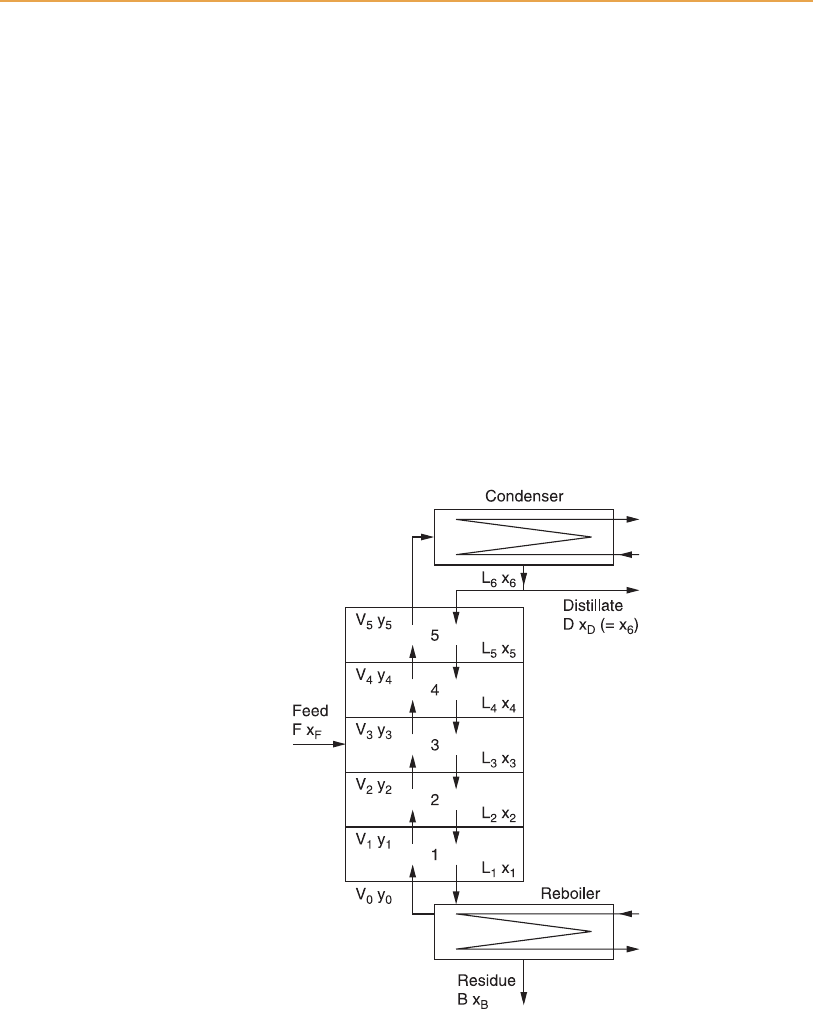
Figure 6.3
Equilibrium stages of a simple distillation column. B, flow rate of still bottom product
(residue from reboiler); D, distillate flow rate; F, feed flow rate; L, liquid flow rate; V,
vapour flow rate; x, mole fraction of more volatile component in liquid ; y, mole fraction
of more volatile component in vapour (Seader and Kurtyka, 1984).

[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 184 178-207
For simplicity only five equilibrium stages of the column are shown – the
feed plate itself and two plates above and two below – although the reboiler
and top condenser, conventionally not shown as part of the column, also
function as equilibrium stages 0 and 6 respectively. So in total Figure 6.3
shows seven equilibrium stages. Continuous distillation is a multi-stage
counter-current process in which liquid and vapour phases of the mixture
are in contact at each of the equilibrium stages, and are separated before
rising (vapour) or falling (liquid) to the adjacent stage. The composition x
and y of liquid and vapour respectively will be different at the individual
levels of the cascade. Since the system operates under steady-state conditions
there is no sudden change in co mposition around plate 3, the feed plate. The
continuous flow of saturated liquid feed to stage 3 will adjust to the tem-
perature (and pressure, if relevant) at that point to form an equilibrium
vapour–liquid mixture. The vapour , V
3
, of composition y
3
rises from stage
3 to bubble through, and partially conde nse in, the layer of liquid on plate 4,
which, having a higher concentrat ion of the more volatile component, is at a
lower temperature than plate 3. Liquid descending from plate 5 (L
5
,of
composition x
5
) to flood plate 4 and rising vapour V
3
mix on plate 4 and
reach a new liquid-vapour equilibrium, and vapour V
4
of composition y
4
rises to plate 5. At each higher level the process is repeated, at a lower
temperature, and y
3
,y
4
and y
5
represent increasing percentages of the
more volatile component. Referring to Figure 6.1, vapour rising from a
plate equivalent to the line MKL contains the equivalent of L per cent of
the more volatile component, which creates a new temperature and compo-
sition equilibrium on the plate abov e.
The liquid component flows down the column in the direction of increasing
temperature, becoming increasingly enriched in the less volatile comp onent.
Therefore y
0
to y
5
represent an increasing concentration of the volatile com-
ponent in the vapour, and x
6
to x
1
represent a decreasing proportion of the
volatile component in the liquid.
The number of stages to achieve the required separation can be calculated
from material balances at each plate, and determining the equilibrium com-
positions of vapour and liquid at each plate. For a discussion of these aspects
of distillation, refer to specialist chemical engineering sources (e.g. Seader
and Kurtyka, 1984; Coulson et al., 1991). However, such texts co ncentrate on
the mathematical treatment of two-compo nent systems and, perhaps with
good reason, do not consider the more complex composition of distillery
wash.
The theoretical model of Figures 6.2 and 6.3 is inappropriate to the distilla-
tion of grain whisky spirit for several reasons:
1. The still is required to separate several hundred flavour congeners from fer-
mented wash (Nykanen and Suomalainen, 1983; Korhola et al., 1989), not just
ethanol from water
2. The concept of theoretical plate is not entirely relevant to distillation in prac-
tice, since more than one actual plate is required for each theoretical plate of
the calculation
184 Whisky: Technology, Production and Marketing

[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 185 178-207
3. The top condenser cannot be a total condenser since a proportion of the most
volatile congeners must be vented off to prevent an unacceptable increase in
the spirit over the duration of the distillation run
4. Even so, the highest plates have an unacceptable concentration of these more
volatile congeners and the spirit is drawn from the column at a point several
plates down from the top.
5. Also, at the highest plates the ethanol concentration may reach the legal max-
imum of 94.7 per cent ABV **27 for Scotch whisky spirit, but a concentration no
more than 94.0 per cent in the collected spirit is preferred in practice, for
increased content of flavour congeners.
6. A reboiler cannot be used, since flavours pr oduced by boiling the spent wash
would be unacceptable and direct injection of steam to the column is neces-
sary; also, grain and yeast material in the wash would bake on to the steam
coil of a reboiler, creating heat transfer problems as well as off-flavours
7. Finally, distillation of wash, which is unlikely to excee d 0.03 mole per cent
ethanol (9.14 per cent by volume), require s so many plates that a single col-
umn would be too tall, and the strip ping and rectifying sections must be
constructed as two adjacent columns.
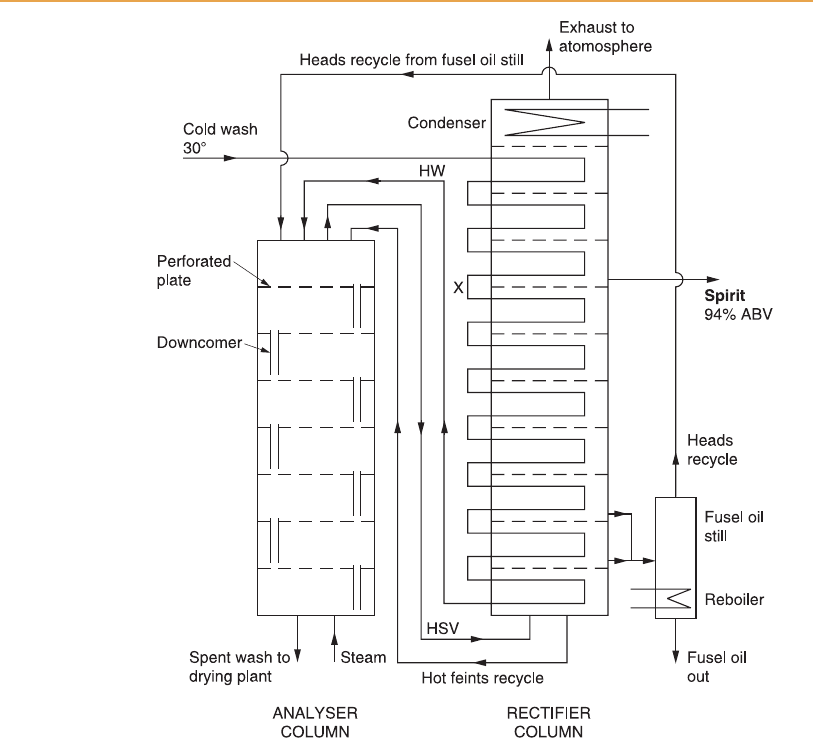
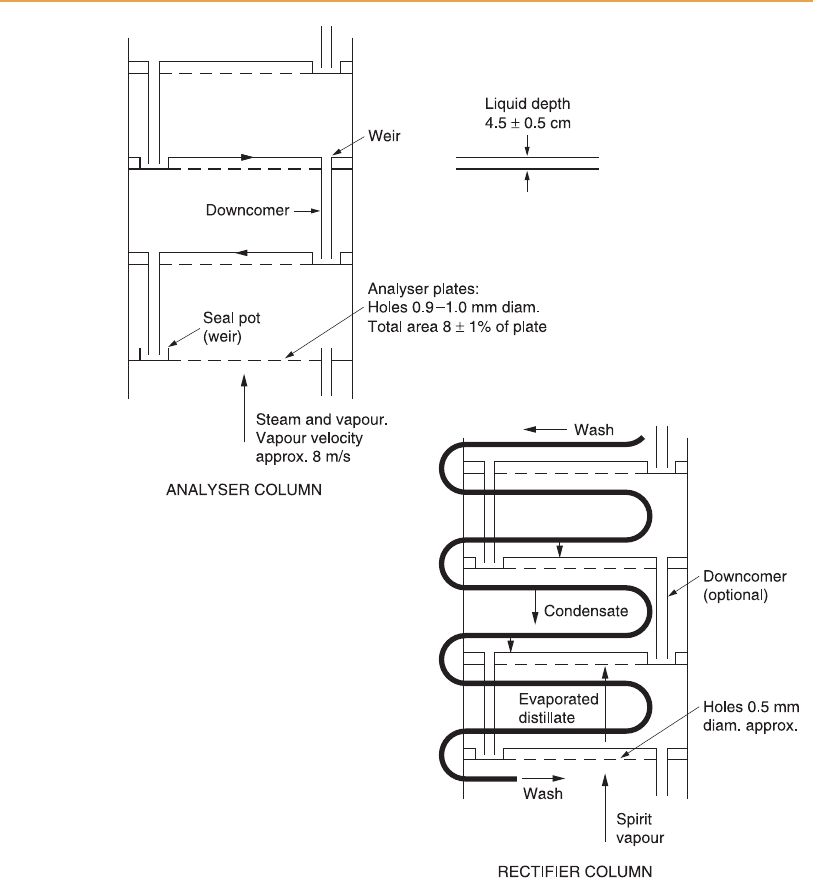
Therefore the stripping sec tion of the still in Figure 6.2 is represented by the
separate analyser column in Figure 6.4, with the heated feed entering at its top
plate. Since the rectifying section is a separate structure, vapour equivalent to
V
3
of Figure 6.3 is piped from the top of the analyser to the bottom of the
rectifier column. Although only a single pipe is shown for hot spirit vapour in
Figure 6.4, normally there are two, of sufficient diameter to permit a free flow
of vapour between the columns. The wash feed is heated as it flows within a
copper pipe (the wash coil) through the rectifier column, so it is at bubble
point temperature as it is discharged on to feed plate, the top plate of the
analyser. The wash coil itself also provides additional reflu x as the vapour
within the rectifier column condenses on its external surface.
Feedstock for distillation
The physical comp osition of wash varies between different grain whisky dis-
tilleries (see Chapter 3). In some, the complete mixture of ground malt and
cooked cereal is cooled to the initial temperature of fermentation, usually
about 208C, and inoculated for an ‘all grains in’ fermentation. In others,
some form of separation of solids is practised between mashing and fermenta-
tion: removing the coarser particles of cereal between mash tun and washback
minimizes damage to pipework, washback and still by abrasion. There is the
additional possibility of chemical and physical variation according whether
maize, wheat or other cereal is used – for example, the higher oil content of
maize has an valuable antifoam effect during fermentation and in the analyser
column.
Chapter 6 Grain whisky distillation 185
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 186 178-207
The temperature rises to 30–34 8C during fermentation, and as an energy-
saving procedure the wash should be distilled before a ny significant cooling
occurs. It is common practice to discharge the washbacks into a still charger
vessel of capacity approximately that of two washbacks, and equipped with a
mixer to ensure homogeneous composition (and incidentally to remove much
of the CO
2
). Wash is pumped continuously to the stills, and as the level of
wash in the charger falls below half full the contents of the next washback can
be added. However, there is a potential microbiological hazard in the contin-
ued use of the charger vessel over a period of time without regular cleaning
and sterilization.
186 Whisky: Technology, Production and Marketing
Figure 6.4
Essential features of the Coffey still. HSV, hot spirit vapour; HW, hot wash. X is the
wash coil bend at the spirit plate; the temperature here controls the flow rate of wash.

[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 187 178-207
To maintain stable operating conditions in the stills it is useful to maintain a
constant alcohol composition of the wash. Mixing in the still charger vessel
helps to balance slight variations in alcohol content between fermentations,
but for many years dilution with warm water (308C) to a constant percentage
of alcohol was considered to be necessary. This was normally the lowest con-
centration typically achieved in fermentation.
Although weaker wash may be preferable for more stable operation and
optimum separation of flavour congeners, the potential energy savings from
distillation of the strongest possible wash are now more important. However,
in continuous stills for grain whisky production it becomes increasingly diffi-
cult to maintain the necessary steady-state conditions with increasing alcohol
content of the wash, and above about 8.5 per cent alcohol by volume they need
particularly careful attention .
Only to a limited extent can the still adjust itself to a change in the alcohol
content of the wash during the run. A weaker wash produces a smaller
amount of spirit and therefore an increase in reflux, since the propor tion of
the less volatile compounds has increased. Conversely, introduction of stron-
ger wash generates more ethanol and a decrease in reflux. Therefore less
fractionation occurs of the various flavour congeners. Ultimately with increas-
ing alcohol content the system requires greater fractionation than the still is
capable of providing by its fixed number of plates, so, whatever the energy
implications, it would be necessary to dilute the wash if it exceeds the max-
imum strength the still is capable of distilling to the desired quality standard.
In theory, increased alcohol content could be comp ensated by reducing the
steam supply (as in pot stills, slower distillation means more reflux), but in
practice that would probably not be an option, since the wash would then tend
to fall through the holes in the plates. The still is designed for a specific flow
rate of steam, within narrow limits.
Design and operation of continuous grain whisky stills
Various designs of continuous still are used in the Scotch grain whisky indus-
try. Figure 6.4 shows a simplified drawing of a Coffey still, which until
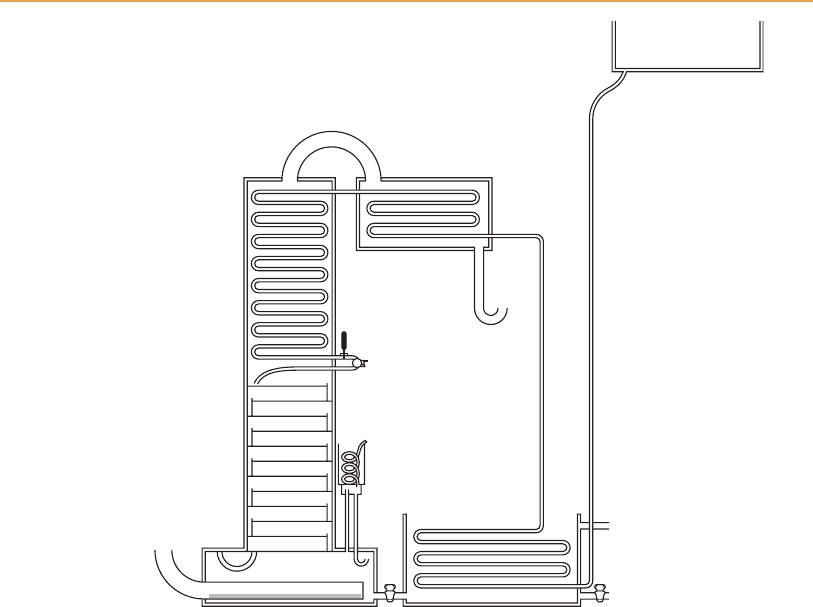
recently was the commonest type in Scotland. Coffey’s original design
(Figure 6.5) differs in several respects from the modern still bearing his
name, but the essential features are recognizable. In other designs for Scotch
grain whisky production (Walker, 1988; Panek and Bouch er, 1989) the ba sic
principle is the same except in one respect. In the Coffey still, the incoming
wash is heated to at least 908C by passage through the rectifier section in a
copper coil, before discharge onto the feed plate of the analyser. In other
designs of still, the wash is pre-heated to 90–938 in a separate heat exchanger
before discharge onto the top plate of the analyser.
The newer designs show the influence of North American whiskey distilla-
tion procedures. However, not only do contin uous distilleries in Canada and
Kentucky have different nomenclature of the equipment and distillates, and of
Chapter 6 Grain whisky distillation 187
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 188 178-207
course different raw materials; their maximum permitted spirit strength of 80
per cent ABV (1608 US proof), and normally in the range 60–70 per cent ABV,
also gives a higher concentration of flavour congeners in the distillate than
would be the case in Scotland. The production of American bourbon and
Canadian whiskies has been reviewed recently by Travis (1998) and Wright
(1998) respectively, as well as featuring prominently in the review by Panek
and Boucher (1989).
In Figure 6.4 only seven plates are shown in the analyser and nine in the
rectifier (ten, counting the top condenser), far short of the number actually
required. Usually 35–40 are fitted to each column of a Coffey still, but up to 60
are required in a rectifier lacking the reflux effect of the wash coil of a Coffey
still. Another simplification is the diagrammatic layout of the wash coils,
which are usually in one horizontal plane above each perforated plate as
shown in Figure 6.6, but in a very large still could be two superimposed
horizontal coils in the space above each plate. Also, in mos t distilleries the
bottom product from the rectifier does not return directly to the top of the
analyser but is collected in a hot feints tank from where it is drawn as required
188 Whisky: Technology, Production and Marketing
Figure 6.5
Coffey’s original design of still (Whitby, 1992).
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 189 178-207
to the to p of the analyser. This is useful to even out the flow of hot feints,
which must be recycled at constant temperature and flow rate. The actual
layout is explained in a later part of this chapter dealing with the recycling
of all feints streams of the still system. For a similar reaso n, the top product of
the feints still is collected as cold feints before recycling.
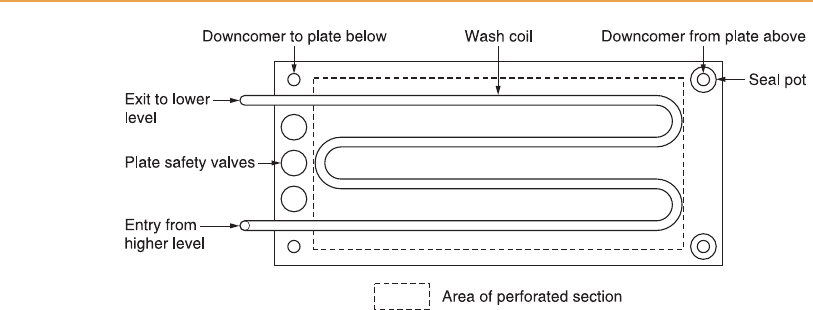
Different sized perforations are required in the plates of the two columns:
larger holes are necessary in the analyser to prevent blockage by grain solids
and yeast (Figure 6.7). For obvious reasons, in a distillery operating with
‘grains-in’ fermentations the holes in the analyser plates must be larger than
for distilleries using partially clarified wort, so a particular distillery is effec-
tively committed to one system. Even so, in addition to the pressure and
vacuum relief valves fitted to the tops of the two still columns, the individual
plates are fitted with a row of safety valves to protect against the effects of
blockage.
Although perforated copper plates have always been used in Co ffey stills,
originally the frame was constructed of baulks of wood and was therefore
rectangular. When it became the custom to construct entirely of copper the
rectangular shape was retained, but the more modern designs of stainless steel
stills are normally circular in cross-section. Stainless steel stills have a longer
working life, since corrosion of the structure is negligible in comparison with
copper, but sacrificial copper, usually as copper turnings, must be inserted to
react with and remove sulphur compounds at sensitive points. Copper is
certainly required in the vicinity of the spirit plate of the rectifier.
Also, it is convenient to fit copper mesh to the vapour pipes at the top of a
stainless steel analyser to remove volatile sulphur compounds from the spirit
vapour, and to remove entrained wash droplets, as well as acting as a flame
arrester. The distance between the still plates, usually 0.4–0.5 m, should be
sufficient to avoid problems from normal frothing of wash on the analyser
plates. The feed plate at the top of the analyser is the most likely place for
frothing, and a particularly frothy wash could contaminate the lower levels of
Chapter 6 Grain whisky distillation 189
Figure 6.6
Plan view of a rectifier plate and its wash coil in a Coffey still.
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 190 178-207
the rectifier with wash and cause off-odours in the spirit. Such transfer can be
prevented by a cyclone in the hot spirit vapour line to remove droplets of
wash. Although surface-active components of the malt, cereal or yeast are also
possible causes of frothing, entrained CO
2
is certainly important, but can be
largely eliminated by sufficiently vigorous agitation in the wash charger.
190 Whisky: Technology, Production and Marketing
Figure 6.7
Detail of analyser and rectifier plates of a Coffey still.
[15:36 13/3/03 n:/3991 RUSSELL.751/3991-006.3d] Ref: 3991 Whisky Chapter 006 Page: 191 178-207
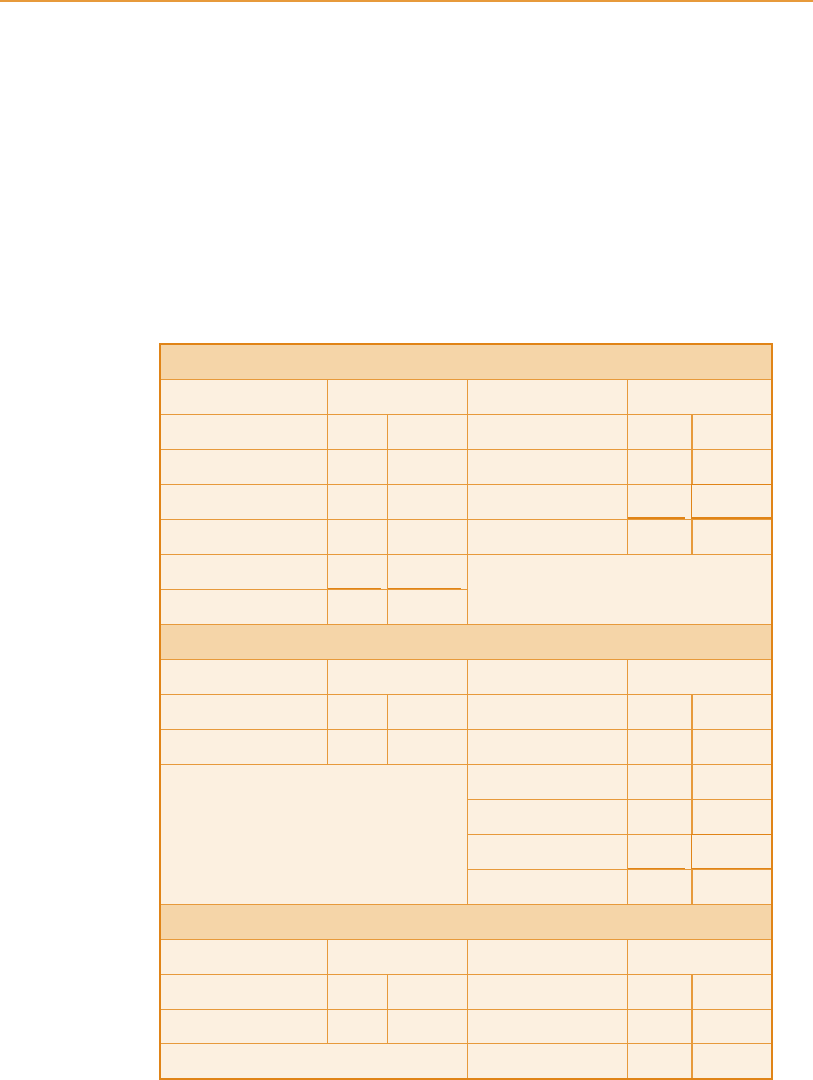
The amounts of ethanol at the various stages of the still system are shown in
Table 6.2. Wash, usually 7.5–8.5 per cent ABV and 30–348C, is pumped
through the coil at the designed rate, with fine adjustment to maintain con-
stant temperature in the wash coil at the level of the spirit plate. A temperature
sensor at this point (X in Figure 6.4) controls the rate of the wash pump
automatically. Constant temperature at this stage is believed to be more
important for spirit quality than constant flow rate, but ideally, with a stable
temperature of incoming wash the flow rate is also constant. Alternatively,
Chapter 6 Grain whisky distillation 191
Table 6.2
Mass balance of continuous grain whisky distillation
Analyser column
UNITS IN UNITS OUT
Mass Ethanol Mass Ethanol
Wash 100 7.5 Spent wash 103 0
Steam 12 0 Hot spirit vapour 20
9.4
Cold feints recycle 1 0.9 TOTAL 123 9.4
Hot feints recycle 10 1.0
TOTAL 123 9.4
Rectifier column
UNITS IN UNITS OUT
Mass Ethanol Mass Ethanol
Hot spirit vapour 20 9.4 Spirit 8 7.5
Feints 2 0.9
(Condenser vent) < 0.1 < 0.1
Hot feints recycle 10
1.0
TOTAL 20 9.4
Fusel oil still
UNITS IN UNITS OUT
Mass Ethanol Mass Ethanol
From rectifier 2 0.9 Cold feints 1 0.9
Fusel oil product 1 0
Strictly, this is not a mass balance since the units refer to alcohol by volume, the
measurements normally used in operating the process. However, all units of ethanol
are in the correct proportions.
