Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

324 Lubricant Additives: Chemistry and Applications
The intermediate ring decomposes to alpha ole n and acid products [35]. Temperatures on the order
of 250°C are needed to initiate this reaction.
Ester pyrolysis produces volatile alpha ole ns as well as acids or anhydrides along the polymer
backbone or perhaps monomeric acids or anhydrides should the reaction occur after depolymerization.
The fate of acid or anhydride in the polymeric backbone would still be depolymerization under the
vigorous thermal conditions present. The ultimate products are volatile under the conditions needed
to initiate the reaction.
Consequences of either thermal reaction are loss of activity as a VII or PPD and the genera-
tion of volatile small molecules. The products distill from the high-temperature reaction zone and
thus offer no further opportunity for the chemical reaction. The data in Table 11.1 indicate the
very high volatility of a PMA VII that has been exposed to extreme temperatures in air. There is
no evidence that unzipping or ester pyrolysis is important in normal lubricant applications. Most
applications generate temperatures less than reaction onset temperatures; thus, these reactions do
not appear to be an issue. A limited potential might exist in a microenvironment, such as if VII
were trapped in a piston deposit where temperatures near the combustion chamber exceed the
onset temperature [6].
11.3.1.3 Oxidative Scissioning
Like any hydrocarbon when exposed to severe oxidative conditions, PMAs can be subject to classic
oxidation reactions resulting in polymer scissioning [33]. The scissioning reaction yields two
fragments of various lengths each of which is obviously of lower molecular weight than the parent
chain, and consequently, there is some loss of viscosity contribution. The reaction takes place at
random sites along the backbone since oxidative or free radical attack may occur anywhere along
the polymer chain. Allylic, benzylic, or tertiary hydrogen are most susceptible to oxidative or free
radical attack. Methacrylates do not normally contain those structural elements; thus, the reaction
is not normally an important consideration. The pyrolysis data in Table 11.1 would seem to support
this conclusion, as scission fragments would by and large not be volatile under the conditions of the
experiment.
Proof of PMA oxidative stability and continued effectiveness has been demonstrated by
comparing viscosities of used oils exposed to the very severe Sequence IIIG oxidative engine
procedure. One oil contained a PMA PPD. The other had all the same components but had no
PPD; after the engine-aging procedure, this oil was treated with exactly the same PPD in the same
concentration as the PPD-containing oil. The resulting viscosities were essentially the same, and of
particular note, the low temperature, low shear rate viscosities did not differ in any signi cant way,
indicating that the PMA PPD was not degraded in the severe environment of a Sequence IIIG [36].
TABLE 11.1
Pyrolysis of PMA
Pyrolysis
Temperature (°C)
Percentage Weight
Loss After 290 315
2 min — 18.7
3 min 93.2
5 min 93.1 94.6
10 min 94.9 96.1
15 min 96.3 97.7
CRC_59645_Ch011.indd 324CRC_59645_Ch011.indd 324 10/31/2008 2:18:00 PM10/31/2008 2:18:00 PM
Polymethacrylate Viscosity Modifi ers and Pour Point Depressants 325
On the whole, PMAs are not prone to thermal or oxidation reactions under normal conditions of
use, and there is little evidence that these reactions are important in the vast majority of PMA-based
lubricant applications.
11.3.1.4 Mechanical Shearing and Free Radical Generation
A well-known, very important degradation reaction of any VII including PMAs is mechanical
shearing. Although polymer shearing begins as a physical process, it does generate free radicals.
For each polymer chain rupture, two transitory carbon-centered free radicals are generated. In
lubricants, the free radicals are apparently quickly quenched, presumably by abstracting hydrogen
from the surrounding hydrocarbon solvent or perhaps by the antioxidants in formulated lubricants.
Overall, there appear to be few if any further chemical consequences. However, there are important
viscometric consequences since the rupture leads to two lower-molecular-weight fragments that
provide a reduced viscosity contribution. The shearing process is initiated through the concentra-
tion of suf cient energy within the polymer chain to induce homolytic cleavage of a carbon–carbon
bond in the backbone of the polymer. The susceptibility of the polymer to mechanical shearing is
not related to its chemical structure; rather it is very clearly a function of polymer molecular weight
or even more appropriately to the end-to-end distance of the polymer chain [37]. Overall, VII shear
stability, although an important physical process, does not appear to carry any appreciable chemical
consequences. Further discussion of shearing can be found in the section on the effect of structure
on physical properties.
11.3.2 PHYSICAL PROPERTIES
The paramount properties of PMAs are those associated with their use in solution as PPDs, VIIs,
or dispersants. The dispersants may also be utilized for their VI improving or thickening proper-
ties, but in some cases, thickening properties are not needed, and the molecules are used solely as
dispersants. The useful properties of PMAs are related to both their physical (primarily molecular
weight) and chemical nature (primarily side chain structure).
11.3.2.1 Pour Point Depressants
PPDs are used to modify and control wax crystallization phenomena in paraf nic mineral oils.
As temperature decreases, waxy components begin to form small, plate-like crystals. The plates
eventually grow together to form an interlocking network that effectively traps the remaining liquid.
Flow ceases unless a force strong enough to break the relatively weak wax gel matrix structure is
applied. Control of wax crystallization in lubricants is often described as pour point depressancy
since one of the quanti able effects is to reduce the ASTM D 97 pour point. The pour point test
is fairly archaic as it utilizes a very rapid cool down to measure only ow versus no- ow condi-
tions. PPDs also control wax crystallization during various slower, more realistic cooling conditions
that better favor crystal growth. PPDs are used to maintain uidity of lubricants under various
cooling conditions to expand the operating temperature window into colder regimes. How much
the operating window can be expanded is a complex function of wax chemistry, its concentration
in base oil, the presence or absence of other waxy additives, the cooling conditions, the nal cold
temperature, and, of course, PPD chemistry and concentration [38].
PPDs do not affect either the temperature at which wax crystallizes from solution or the amount
of wax precipitate. PPDs cocrystallize on the edges of the growing wax plates by virtue of their
longer alkyl side chains. Thus, the growing wax crystal is attached to the polymer, and then, because
of the presence of the molecularly large polymer backbone, crystal growth is sterically hindered
in-plane. Further growth is redirected in a perpendicular direction, resulting in the formation of
more needle-like crystals. Thus, the usual tendency to form a three-dimensional structure based on
CRC_59645_Ch011.indd 325CRC_59645_Ch011.indd 325 10/31/2008 2:18:00 PM10/31/2008 2:18:00 PM

326 Lubricant Additives: Chemistry and Applications
plate-like crystals is disrupted, and wax gel matrices are prevented at least temporarily. At
exceedingly low temperatures, oils may eventually become so viscous as to appear to cease ow-
ing, but this is irrespective of wax issues [39].
PMAs were the rst polymeric PPDs and were commercialized in the 1940s by Rohm
and Hass Company. Today, they are the predominant chemistry in this application enjoying a
majority of the worldwide market. The reason for this success is related to the molecular struc-
ture, as shown in Figure 11.8, and its inherent chemical exibility in terms of polymer chain
length but more importantly its ability to include various side chain lengths and at appropriate
concentrations.
In Figure 11.8, R
1
and R
2
represent two different lengths of alkyl side chains; one is wax-
interactive and the other is “neutral,” or noninteractive with wax. But, the side chains are actually
complex mixtures of alkyl groups, which may be anywhere from 1 to more than 20 carbons. The
longer carbon side chains are intended to interact with wax; to do so, they should be linear and
typically be at least 14 carbons atoms in length. The interaction of a waxy alkyl side chain with
wax intensi es as its length increases. However, shorter chains are added to serve as inert diluents,
thereby ensuring a controlled degree of wax interaction or to act as spacers between the longer side
chains so as to better t into crystal lattice structures.
Pour point depressancy is largely independent of molecular weight over a broad range and
degree of polymerization, which may vary from ∼200 to ∼3000. But it is important to achieve a
minimum degree of polymer backbone size to provide enough steric hindrance to crystal growth as
described earlier.
The optimum positive interaction with wax requires a careful balance of the waxy alkyl groups
in terms of both type and concentration. This thought is sometimes expressed as a function known
as the wax interaction factor (WIF) that takes into account the amount of each alkyl group that
interacts with wax and the strength of the interaction. Since mineral oils contain a distribution of
wax chain lengths, PPDs often contain a distribution of waxy side chains to best interact with wax
in a speci c situation. As the wax structure and content change because of different base stocks
and additives, a different PPD with a different WIF may be the optimum. This effect is shown in
Table 11.2 where different base stocks with different wax chemistries and concentrations respond
differently to PPDs with different WIF.
Finished lubricant formulations often respond differently to PPDs than do the base oils used to
make the fully formulated oils. For example, in the MRV TP-1 measurement, two different 150N
base stocks respond to PPD 3: base stock A with some yield stress and base stock B with no yield
stress; however, when these base stocks are additized each with the same DI and VII, they respond
in an opposite way. The SAE 10W-40 based on oil A has passing rheology, whereas the SAE 10W-
40 based on oil B is a clear failure. PPD 4 with different WIF does provide passing results with the
formulation based on oil B. These data are shown in Table 11.3.
Previously (Section 11.3.1.3), the oxidative and thermal stability of PMA had been discussed
and a conclusion was reached that PMA PPDs remain effective even after exposure to a severe oxi-
dative environment. The background for the experimentation is a new low-temperature pumpability
requirement for used gasoline engine oils appearing in the International Lubricants Standardization
and Acceptance Committee (ILSAC) GF-4 standard for passenger car engine oils [40]. Pumpability
is measured by ASTM D 4684 on an oil after undergoing a Sequence IIIG engine-aging procedure
H
2
CC
CH
3
CH
3
((
CO
2
R
1
)
x
H
2
CC
)
y
CO
2
R
2
FIGURE 11.8 PMA pour point depressant.
CRC_59645_Ch011.indd 326CRC_59645_Ch011.indd 326 10/31/2008 2:18:00 PM10/31/2008 2:18:00 PM
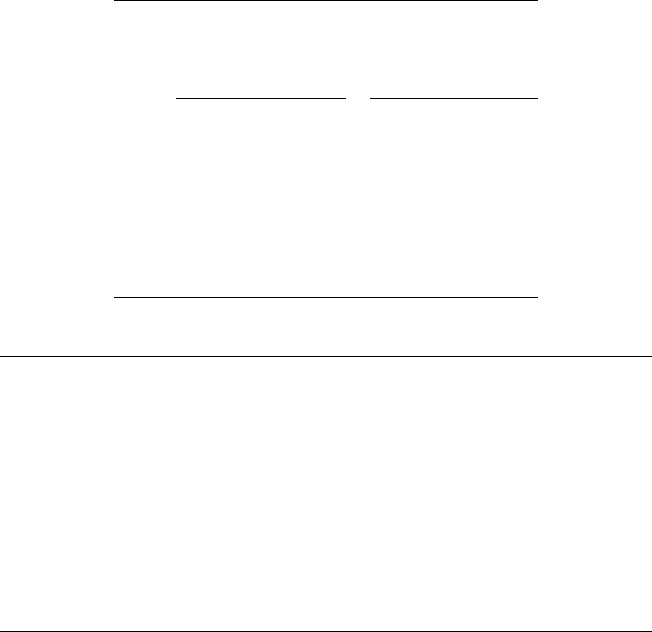
Polymethacrylate Viscosity Modifi ers and Pour Point Depressants 327
(ASTM D 7320), which is a severe oxidative and volatilization environment. One normally associ-
ates low-temperature pumpability with wax-related phenomena and ultimately with PPD activity;
however, the used oil pumpability requirement was added to address original equipment manufac-
turer (OEM) concerns of oil degradation in the eld, which could be identi ed by low-temperature
viscosity measurements and not necessarily by the usual higher-temperature kinematic viscosity
determinations [41]. The need for a low-temperature measurement is because of severe oxidation,
which may lead to the formation of polar molecular species that associate to form gel-like structures
at relatively cold temperatures but not necessarily at warmer temperatures.
11.3.2.2 Viscosity Index Improvers
VIIs are used to achieve the advantages of multigrade lubricating oils in numerous applications
including crankcase engine oils, automatic-transmission uids, high VI hydraulic uids, gear oils,
and other lubricants used primarily (but not necessarily) outdoors. VIIs, also known as viscosity
modi ers, are high-molecular-weight, oil-soluble polymers, which ideally provide increased viscos-
ity at higher temperatures and minimal viscosity contribution at lower temperatures [42]. Current
commercial chemistries are based on either of two chemical families: hydrocarbons such as ethyl-
ene–propylene copolymers or ester-containing materials such as PMAs. There are other examples
of each chemical family. PMA chemistry dominates applications where high viscosity index and
superior low-temperature properties are required [36]. These bene ts can usually be observed in
typical lubricant industry low-temperature, low-shear-rate rheology tests such as ASTM D 4684
mini rotary viscometer (MRV TP-1), Scanning Brook eld, and ASTM D 2983 Brook eld as well as
numerous others. Polymer molecular size in uences thickening at all temperatures, and the larger
TABLE 11.2
PPD Response
a
in Different Base Oils
Base Stock 1 Base Stock 2
Wt%
PPD 1
Low WIF
PPD 2
High WIF
PPD 1
Low WIF
PPD 2
High WIF
None −18 −12
0.05 −24 −21 −18 −18
0.10 −33 −30 −21 −27
0.20 −36 −30 −27 −33
a
ASTM D 97 pour point (°C).
TABLE 11.3
Comparison of PPD Response
a
in Base Stocks versus Fully Formulated
Fluids
PPD Treat Rate Base Stock A
Base Stock A +
DI and VII Base Stock B
Base Stock B +
DI and VII
0.1 wt% 150N SAE 10W-40 150N SAE 10W-40
PPD 3 31,100/35 39,700/<35 26,500/<35 62,000/70
PPD 4 — — 28,100/<35 35,800/<35
a
ASTM D 4684 TP-1 MRV at –30°C, viscosity (cP)/yield stress (Pa).
CRC_59645_Ch011.indd 327CRC_59645_Ch011.indd 327 10/31/2008 2:18:00 PM10/31/2008 2:18:00 PM
328 Lubricant Additives: Chemistry and Applications
the coil size, the higher the thickening power. Conversely, with smaller coil size, less thickening
occurs. PMA VIIs thicken oils well at higher temperatures but contribute relatively little low-tem-
perature viscosity. This desirable viscosity–temperature behavior stems from PMA ester function-
ality imparting polarity to the polymer in the nonpolar hydrocarbon solvent, mineral oil, leading to
a relatively small molecular coil size at low temperature. The ester polarity can be accentuated by
the use of short pendant side chain monomers.
Thickening properties for any chemical class of VIIs or viscosity modi er are related to their
immensely greater molecular size compared to that of the solvent in which they are dissolved. The
long polymeric strand, the backbone of the polymer, is con gured in a random coil shape. The size
of the coil, or more appropriately its hydrodynamic volume, is proportional to polymer molecular
weight as a rst approximation; but more precisely, it is proportional to the cube of the root mean
square end-to-end distance of the polymer. In the lattice theory of viscous ow, segments of poly-
mer molecules ll holes in the lattice (constructed of all surrounding molecules) and thereby limit
the ability of smaller molecules to participate in movement through the lattice [38]. The increase
in degree of viscosity depends on coil size; thus, higher-molecular-weight polymers provide more
thickening. The overall viscosity of a polymer-thickened solution is related to polymer concentra-
tion and molecular weight through the following equation developed by Stambaugh [6].
ln ln⫽⫹KM c k M c
v
a
v
a
⫺
′′
()
2
2
0
(11.1)
where
M
v
= VII viscosity average molecular weight
c = VII (or thickener) concentration
η
0
= solvent viscosity
exponent a relates to solubility of the speci c polymer chemistry, solvent, and temperature
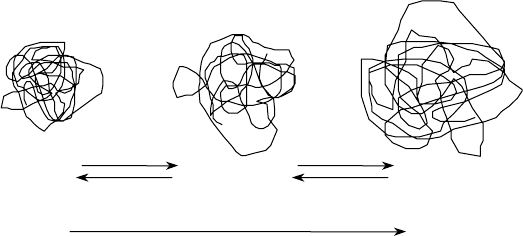
For a PMA VII in solution, the coil size expands and contracts with temperature [43]. At lower
temperatures, PMA is, on a relative basis, poorly soluble in oil. This is not meant to say that PMA
precipitates from solution, but the relatively poor solubility results in a contracted, smaller-volume
polymer coil, which has a relatively low viscosity contribution. As temperature increases, solubility
improves and polymer coils eventually expand to some maximum size and in doing so donate more
and more viscosity. The process of coil expansion is entirely reversible. The polymer coil will con-
tinue to expand or contract with temperature changes irrespective of the aging history of the solution
(see Figure 11.9). In contrast, nonpolar polymeric thickeners are well solvated by oils at all tempera-
tures and experience far less change to coil size with temperature [44]. For any VII or VM chemistry,
these solubility factors relate to the value of the exponent a in Equation 11.1 and, speci cally for
Temperature
Solvent power
FIGURE 11.9 PMA coil expansion.
CRC_59645_Ch011.indd 328CRC_59645_Ch011.indd 328 10/31/2008 2:18:01 PM10/31/2008 2:18:01 PM
Polymethacrylate Viscosity Modifi ers and Pour Point Depressants 329
PMA, are a function of the average length side chain chemistry: short, intermediate, and long alkyl
chains. This structural concept of three distinct chain lengths is represented in Figure 11.10.
A typical average side chain length of about eight carbons will provide PMA solubility in oil,
even down to extremely low temperatures. Thus, an intermediate-chain length monomer is used to
provide overall oil solubility and is normally selected from linear or branched chains composed of
8–14 carbons. The longer chains consisting of more than 14 carbons may be incorporated to provide
wax crystallization interactions as described earlier in the section on PPDs. Very short chains,
usually C1 or C4, are used to balance the composition and make the average chain length at least
eight carbons for paraf nic mineral oil solubility. To a very good rst approximation, the average
side chain length will determine viscosity–temperature properties rather than the detailed nature of
the side chain structures.
Building pour point depressancy into PMA VIIs by including longer-side-chain monomers
may involve compromises. An optimized wax interaction provided by the VII may not be possible
because of the many different base stocks (with different wax types and contents) in which the VII
might be used. However, VII treat rates are relatively high compared to PPDs, and the high polymer
concentration may simply overwhelm wax crystallization as it occurs and thereby prevent wax gel
matrix consequences (see Section 11.3.2.1).
Commercial PMA VIIs are available in various chemical compositions and molecular weights
ranging from ∼20,000 to 740,000 Da. The higher-molecular-weight materials are not only the most
ef cient thickeners and provide the greatest VI lift but also the most susceptible to shearing effects.
Selection of a suitable VII for a given application should focus on shear stability, thickening ef -
ciency, and VI lift.
Shearing effects, either temporary or permanent, are related to polymer backbone molecular
weight. High-molecular-weight polymers are subject to both temporary viscosity loss through shear
thinning and permanent viscosity loss when polymer chains are broken in mechanical mastication,
both of which result in loss of thickening.
Temporary viscosity loss occurs when polymer molecules become oriented along the axis of
ow at suf ciently high shear rates. This phenomenon, known as shear thinning, occurs at a mini-
mum, nominal value on the order of 10
4
s
–1
. Shapes of individual polymer coils change from a
spherical to an elongated con guration that occupies a smaller hydrodynamic volume and thus con-
tribute less viscosity. With further increases of shear rate, molecules increasingly deform leading to
a corresponding greater loss of viscosity contribution until maximum distortion is reached. Within
the lubricant community, non-Newtonian, shear-thinning behavior is better known as “temporary
loss of viscosity” since the process is reversible upon removal of high shear rates. Distorted polymer
molecules resume random coil shapes, reoccupy original hydrodynamic volumes, and contribute
viscosity just as before the application of a higher shear rate. The degree of temporary viscosity loss
depends on the level of shear stress and the molecular weight (size) of the polymer. Because of their
small coil sizes, low-molecular-weight polymers are less susceptible to shear thinning.
The temporary viscosity loss of PMAs is directly related to molecular weight, or even more
appropriately, to backbone molecular weight. PMAs are not associative thickeners and do not
experience viscosity losses through the loss of molecular associations in high shear stress elds.
Any loss of viscosity is related merely to at-rest molecular size and subsequent molecular shape
H
2
CC
CH
3
CO
2
R
1
)
(
x
CH
3
H
2
CC
CO
2
R
2
CH
3
((
)
y
H
2
CC
)
z
CO
2
R
3
FIGURE 11.10 PMA VII structure.
CRC_59645_Ch011.indd 329CRC_59645_Ch011.indd 329 10/31/2008 2:18:01 PM10/31/2008 2:18:01 PM
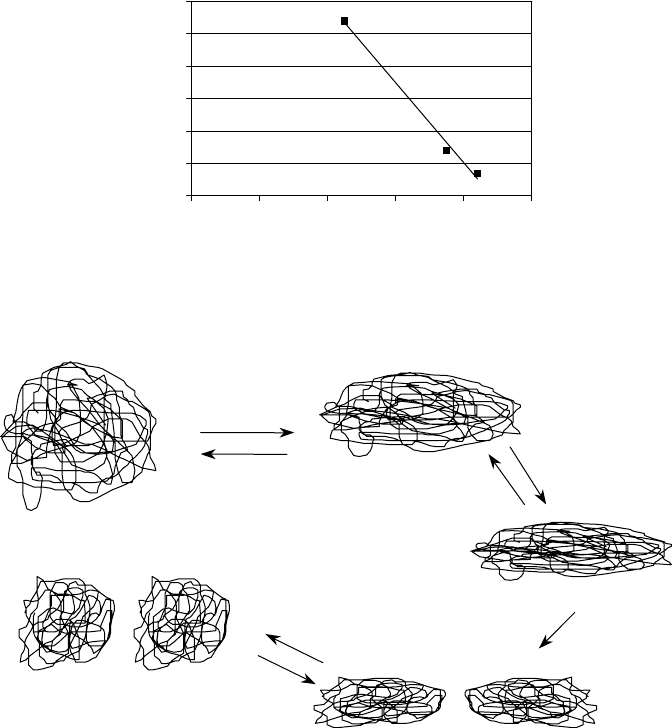
330 Lubricant Additives: Chemistry and Applications
distortion leading to lower hydrodynamic volume. Figure 11.11 is compiled with data taken from
a set of SAE 10W-40 oils blended to constant kinematic viscosity (14.5 mm
2
/s) and constant cold
cranking simulator (CCS) viscosity with the same compounding materials, except for the VII. Three
chemically equivalent d-PMA VIIs, differing only in molecular weight, were used in the formula-
tions. The resulting high-temperature, high-shear-rate (HTHS) viscosities are clearly a function of
polymer molecular weight, showing essentially an inverse linear relationship with polymer molecu-
lar weight.
Permanent viscosity loss through mechanical degradation occurs when very high shear stresses,
perhaps coupled with turbulent ow, lead to extreme polymer coil distortion and concentrate enough
vibrational energy to cause polymer chain rupture. Cavitation probably also plays an important role
by producing intense velocity gradients. Polymer chain rupture occurs through homolytic cleavage
of a carbon–carbon bond, statistically near the middle of the polymer chain. The cleavage pro-
duces two molecules each having approximately half the molecular weight of the original molecule;
Figure 11.12 represents molecular elongation and rupture concepts. The total hydrodynamic volume
of the two smaller molecules is less than that of the single parent molecule, resulting in lower
viscosity contribution. Since the bond scission is not reversible, the viscosity loss is permanent.
3.4
3.5
3.6
3.7
3.8
3.9
4
0 200 400 600 800 1000
MW
× 10
−3
HTHS viscosity, 150°C, (10
6
s
−1
)
FIGURE 11.11 Relationship of PMA VII molecular weight to temporary shear stability. (SAE 10W-40 oils
blended to 14.5 mm
2
/s at 100°C.)
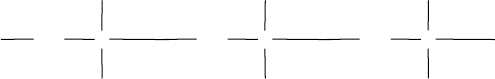
Shear
rate
Higher
shear rate
Removal of
shear rate
Bond
breakage
Shear
rates
FIGURE 11.12 Temporary and permanent shearing of polymer.
CRC_59645_Ch011.indd 330CRC_59645_Ch011.indd 330 10/31/2008 2:18:02 PM10/31/2008 2:18:02 PM
Polymethacrylate Viscosity Modifi ers and Pour Point Depressants 331
Higher-molecular-weight polymers are more susceptible to distortion and mechanical degradation,
whereas polymers of suf ciently low molecular weight may not even undergo permanent shearing.
The sheared polymer molecules are typically of suf ciently lower molecular weight that are not
normally susceptible to further degradation. Thus, the degradation process is self-limiting under
any given intensity of shearing.
As with shear-thinning phenomenon, PMAs of suf cient molecular weight are subject to
mechanically induced permanent loss of viscosity, again, the amount of viscosity loss for linear
PMA is a reasonably straightforward function of molecular weight. Molecular weight distribution
plays a secondary role. If the molecular weight distribution were skewed to larger fractions of high
molecular weight polymer, then the loss of viscosity would be greater than that of a polymer of simi-
lar average molecular weight but with a Gaussian distribution. It should also be noted that different
applications can create very different shear stresses, and because of this, viscosity losses of polymers
of a given molecular weight may vary by application. The net result is that viscosity loss to a rst
approximation is directly related to molecular weight and the amount of shearing force in a given
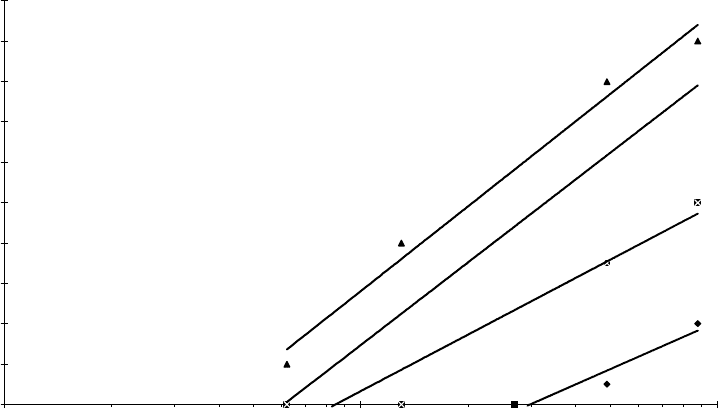
application or laboratory bench test. The relationship of shear stability index to polymer molecular
weight and shearing severity by application are shown in Figure 11.13 for a set of PMA VIIs [45].
Dispersant functionality may be incorporated into PMA chemistry by using a monomer that
contains a heteroatom, which will create polar zones along the otherwise oleophilic polymer chain.
Typically, the heteroatom is nitrogen incorporated in an amine, amide, or lactam, but oxygen-
containing monomers are sometimes used. As previously discussed, incorporation can be achieved
through monomer copolymerization, grafting a monomer onto the preformed polymer chain, or
incorporating a reactive chemical site on the polymer and then postreacting with an appropriate
chemical species, for instance, incorporating an anhydride, such as maleic anhydride, as the reac-
tive chemical site, then after the polymerization reacting with an amine, thus creating succinimide
sites along the polymer chain. The resulting polar region(s) serves to attract and peptize small polar
molecules or particles, that is, oxidized oil, oxidized fuel, or even soot. These undesirable materials
are often unintentional by-products of combustion or oxidation of the lubricant. Left undispersed,
0
10
20
30
40
50
60
70
80
90
100
10 100 1000
MW × 10
−3
SSI
Engine
oil
ATF
Hydraulic
fluid
Gear
oil
50 500
FIGURE 11.13 Relationship of shear stability index to PMA VII molecular weight and shear severity by
application.
CRC_59645_Ch011.indd 331CRC_59645_Ch011.indd 331 10/31/2008 2:18:02 PM10/31/2008 2:18:02 PM
332 Lubricant Additives: Chemistry and Applications
the small molecules may undergo further reactions, resulting in the formation of harmful deposits,
whereas particulate matter may become a source of abrasive wear.
d-PMAs are used as stand-alone dispersant products in a few applications but more typically as
dispersant VIIs, which augment the dispersancy provided by classic ashless dispersant molecules
found in DI packages. An example of a stand-alone d-PMA is its use in Society of Automotive Engi-
neer (SAE) speci cation J1899 piston aircraft engine oils. PMA dispersants can also be employed to
boost the overall dispersancy of a formulation. These materials have been used in engine oils either
to supplant some of the traditional ashless dispersants or simply to enhance dispersancy as well as
imparting the usual rheological properties. Table 11.4 contains data from Sequence VE engine test-
ing of a SAE 5W-30 API SG oil made from d-PMA VII (45 Bosch SSI) and another SAE 5W-30 oil
containing all the same compounding materials but with a nondispersant PMA VII (also 45 Bosch
SSI). The results in each case are the average of six engine tests. Clearly, the dispersant VII provides
enhanced dispersancy relative to its nondispersant analog.
11.4 MANUFACTURERS, MARKETERS, AND ECONOMICS
11.4.1 M
ANUFACTURERS AND MARKETERS
The following companies offer commercial quantities of PMA additives:
Afton Petroleum Additives, Inc.
330 South Fourth Street
P.O. Box 2189
Richmond, VA, 23219
Chevron Oronite
6001 Bollinger Canyon Road
San Ramon, CA 94583
CIBA Specialty Chemicals Corporation
540 White Plains Road
Tarrytown, NY 1059
In neum UK Limited
P.O. Box 1 Milton Hill
Abingdon Oxfordshire
OX 13 6BB, United Kingdom
Nippon NSC Ltd.
Specialty Synthetic Polymers Ginza Wall Bldg. 6-13-16, Ginza, Chuo-ku
Tokyo 104-0061, Japan
TABLE 11.4
Engine Performance
a
Dispersant PMA versus Nondispersant PMA
in SAE 5W-30 API SG Oils
PMA VIIs (45 SSI) Average Sludge Average Varnish Average Cam Wear
Dispersant 9.23 6.25 1.5
Nondispersant 4.55 4.56 5.8
a
Sequence VE test—summary data (average from six tests of each formulation).
CRC_59645_Ch011.indd 332CRC_59645_Ch011.indd 332 10/31/2008 2:18:02 PM10/31/2008 2:18:02 PM
Polymethacrylate Viscosity Modifi ers and Pour Point Depressants 333
The Lubrizol Corporation
29400 Lakeland Boulevard
Wickliffe, OH 44092-2298
RohMax Additives GmbH
Kirschenallee D-64293
Darmstadt, Germany
Sanyo Chemical Industries, Ltd.
11-1, lkkyo Nomoto-Cho, Higashiyama-Ku
Kyoto 605-0995, Japan
Toho Chemical Industry Co. Ltd.
6-4, Akashi-Cho, Chuo-Ku
Tokyo 104-0044, Japan
Within the realm of lubricant additives, NSC primarily offers PMA VIIs and PPDs. RohMax, Sanyo
Kasei, and Toho, although focused on PMAs, do offer other product types to the lubricant and oil-
re ning industries. CIBA offers additives other than methacrylates most notably antioxidants and
other ashless components. Afton, Chevron Oronite, In neum, and Lubrizol offer PMAs as well as
numerous other additive types and additive packages for lubricants.
11.4.2 ECONOMICS AND COST-EFFECTIVENESS
Current list prices for a few PMA VII products intended for different applications and PMA PPDs
are given in Table 11.5. The PPD price range covers products of varying complexity from the least
to the most expensive.
Of course, one must know an additive’s treat rate to do a meaningful economic study. Although
this is true for any additive, it is particularly true for VIIs as their treat rates may vary widely
depending on their chemical nature, base oil viscosities, as well as by the desired viscosity grade of
the treated oil. The cost-effectiveness of PMA VIIs varies widely by application. When enhanced
low-temperature performance, high viscosity index, and excellent shear stability are the require-
ments, then PMAs often enjoy excellent cost-competitiveness. Examples of these applications are
TABLE 11.5
List Price of PMA Products
Product Description Application
U.S. Dollars
per Pound
VII—45 SSI Engine oil 1.22
Dispersant VII—45 SSI Engine oil 1.60
Dispersant VII—25 SSI Engine oil 1.91
VII—15 SSI Hydraulic uid 2.29
VII—30 SSI Hydraulic uid 1.99
PPDs Various 2.03–2.72
Note: SSl values for engine oil application are for ASTM D 6278
(30 Pass Bosch Diesel injector). SSI values for hydraulic uid
applications are from ASTM D 5621 (40 min sonic shear).
Source: Courtesy of RohMax USA, LLC.
CRC_59645_Ch011.indd 333CRC_59645_Ch011.indd 333 10/31/2008 2:18:02 PM10/31/2008 2:18:02 PM
