Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

4 Lubricant Additives: Chemistry and Applications
1.11.2 Bulk Oil Oxidation Test ............................................................................................. 33
1.11.2.1 Turbine Oil Stability Test (ASTM D 943, D 4310).....................................33
1.11.2.2 IP 48 Method ..............................................................................................34
1.11.2.3 IP 280/CIGRE ............................................................................................34
1.11.3 Oxygen Update Test ...................................................................................................34
1.11.3.1 Rotating Pressure Vessel Oxidation Test (ASTM D 2272) ........................34
1.12 Experimental Observations .....................................................................................................34
1.13 Antioxidant Performance with Base Stock Selection .............................................................37
1.14 Future Requirements ...............................................................................................................38
1.15 Commercial Antioxidants .......................................................................................................39
1.16 Commercial Metal Deactivators .............................................................................................41
References ........................................................................................................................................ 41
1.1 INTRODUCTION
Well before the mechanism of hydrocarbon oxidation was thoroughly investigated, researchers had
come to understand that some oils provided greater resistance to oxidation than others. The differ-
ence was eventually identi ed as naturally occurring antioxidants, which varied depending on crude
source or re ning techniques. Some of these natural antioxidants were found to contain sulfur- or
nitrogen-bearing functional groups. Therefore, it is not surprising that, certain additives that are
used to impart special properties to the oil, such as sulfur-bearing chemicals, were found to provide
additional antioxidant stability. The discovery of sulfurized additives providing oxidation stability
was followed by the identi cation of similar properties with phenols, which led to the development
of sulfurized phenols. Next, certain amines and metal salts of phosphorus- or sulfur-containing
acids were identi ed as imparting oxidation stability. By now numerous antioxidants for lubricating
oils have been patented and described in the literature. Today, nearly all lubricants contain at least
one antioxidant for stabilization and other performance-enhancing purposes. Since oxidation has
been identi ed as the primary cause of oil degradation, it is the most important aspect for lubricants
that the oxidation stability be maximized.
Oxidation produces harmful species, which eventually compromises the designated functiona-
lities of a lubricant, shortens its service life, and to a more extreme extent, damages the machinery it
lubricates. The oxidation is initiated upon exposure of hydrocarbons to oxygen and heat and can be
greatly accelerated by transitional metals such as copper, iron, nickel, and so on. when present. The
internal combustion engine is an excellent chemical reactor for catalyzing the process of oxidation
with heat and engine metal parts acting as effective oxidation catalysts. Thus, in-service engine oils
are probably more susceptible to oxidation than most other lubricant applications. For the preven-
tion of lubricant oxidation, antioxidants are the key additive that protects the lubricant from oxida-
tive degradation, allowing the uid to meet the demanding requirements for use in engines and
industrial applications.
Several effective antioxidant classes have been developed over the years and have seen use in
engine oils, automatic transmission uids, gear oils, turbine oils, compressor oils, greases, hydraulic
uids, and metal working uids. The main classes include oil-soluble organic and organometallic
antioxidants of the following types:
1. Sulfur compounds
2. Sulfur–nitrogen compounds
3. Phosphorus compounds
4. Sulfur–phosphorus compounds
5. Aromatic amine compounds
6. Hindered phenolic (HP) compounds
7. Organo–copper compounds
CRC_59645_Ch001.indd 4CRC_59645_Ch001.indd 4 12/4/2008 3:33:16 PM12/4/2008 3:33:16 PM
Antioxidants 5
8. Boron compounds
9. Other organometallic compounds
1.2 SULFUR COMPOUNDS
The initial concepts of using antioxidants to inhibit oil oxidation date back to the 1800s. One of the
earliest inventions described in the literature [1] is the heating of a mineral oil with elemental sulfur
to produce a nonoxidizing oil. However, the major drawback to this approach is the high corrosivity
of the sulfurized oil toward copper. Aliphatic sul de with a combined antioxidant and corrosion
inhibition characteristics was developed by sulfurizing sperm oil [2]. Additives with similar func-
tionalities could also be obtained from sulfurizing terpenes and polybutene [3–5]. Paraf n wax has
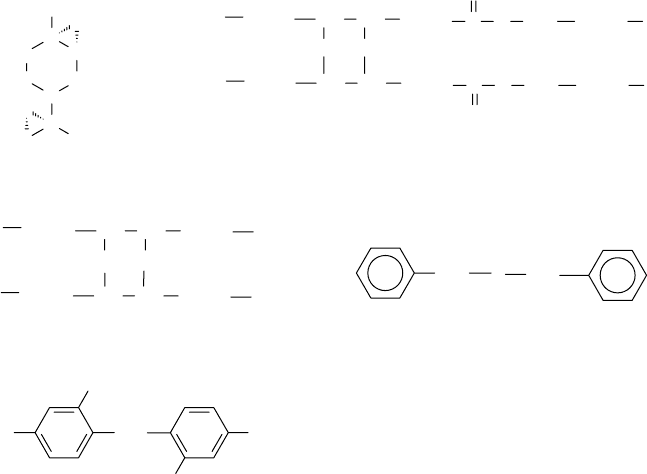
also been employed to prepare sulfur compounds [6–9]. Theoretical structures of several sulfur
compounds are illustrated in Figure 1.1. Actual compounds can be chemically complex in nature.
Aromatic sul des represent another class of sulfur additives used as oxidation and corrosion
inhibitors. Examples of simple sul des are dibenzyl sul de and dixylyl disul de. More complex
compounds of a similar type are the alkyl phenol sul des [10–15]. Alkyl phenols, such as mono- or
di-butyl, -amyl, or -octyl phenol, have been reacted with sulfur mono- or dichloride to form either
mono- or disul des. As shown in Figure 1.1, the aromatic sul des such as benzyl sul de have the
sulfur attached to carbon atoms in the alkyl side groups, whereas the alkyl phenol sul des have the
sulfur attached to carbon atoms in the aromatic rings. In general, the alkyl phenol sul de chemistry
appears to have superior antioxidant properties in many types of lubricants. Mono- and dialkyl-
diphenyl sul des obtained by reacting diphenyl sul de with C
10
–C
18
alpha-ole ns in the presence
of aluminum chloride have been demonstrated to be powerful antioxidants for high-temperature
lubricants especially those utilizing synthetic base stocks such as hydrogenated poly-alpha-ole ns,
diesters, and polyol esters [15]. The hydroxyl groups of the alkyl phenol sul des may also be treated
CH
2
CH
2
C
CH
2
CH
C
CH
3
S
C
CH
3
CH
2
S
Sulfurized dipentene
Sulfurized ester
CH
3
(CH
2
)
x
CH CH (CH
2
)
x
S
CH
3
CH
3
(CH
2
)
x
CH CH (CH
2
)
x
CH
3
Sulfurized olefin
CH
3
(CH
2
)
x
C
O
O (CH
2
)
x
CH
3
CH
3
(CH
2
)
x
CCH
2
(CH
2
)
x
CH
3
O
CH CH (CH
2
)
x
S
CH CH (CH
2
)
x
CH
2
S
CH
2
Dibenzyl sulfide
OH
R
HO
R
(S)
x
Dialkylphenol sulfide
S
O
S
CH
2
FIGURE 1.1 Examples of sulfur-bearing antioxidants.
CRC_59645_Ch001.indd 5CRC_59645_Ch001.indd 5 12/4/2008 3:33:17 PM12/4/2008 3:33:17 PM

6 Lubricant Additives: Chemistry and Applications
with metals to form oil-soluble metal phenates. These metal phenates play the dual role of detergent
and antioxidant.
Multifunctional antioxidant and extreme pressure (EP) additives with heterocyclic structures
were prepared by sulfurizing norbornene, 5-vinylnorbornene dicyclopentadiene, or methyl cyclo-
pentadiene dimer [16]. Heterocyclic compounds such as n-alkyl 2-thiazoline disul de in combi-
nation with zinc dialkyldithiophosphate (ZDDP) exhibited excellent antioxidant performance in
laboratory engine tests [17]. Heterocyclic sulfur- and oxygen-containing compositions derived from
mercaptobenzthiazole and beta-thiodialkanol have been found to be excellent antioxidants in auto-
matic transmission uids [18]. Novel antioxidant and antiwear additives based on dihydrobenzothio-
phenes have been prepared through condensation of low-cost arylthiols and carbonyl compounds in
a one-step high-yield process [19].
1.3 SULFUR–NITROGEN COMPOUNDS
The dithiocarbamates were rst introduced in the early 1940s as fungicides and pesticides [20]. Their
potential use as antioxidants for lubricants was not realized until the mid-1960s [21], and since then,
there have been continuous interests in this type of chemistry for lubricant applications [22]. Today,
dithiocarbamates represent a main class of sulfur–nitrogen-bearing compounds being used as antioxi-
dants, antiwear, and anticorrosion additives for lubricants.
Depending on the type of adduct to the dithiocarbamate core, ashless and metal-containing
dithiocarbamate derivatives can be formed. Typical examples of ashless materials are methylene
bis(dialkyldithiocarbamate) and dithiocarbamate esters with general structures being illustrated in
Figure 1.2. Both are synergistic with alkylated diphenylamine (ADPA) and organomolybdenum
compounds in high-temperature deposit control [23]. In particular, methylene bis(dialkyldithiocar
bamate) in combination with primary antioxidants such as arylamines or HPs and triazole deriva-
tives is known to provide synergistic action in stabilizing mineral oils and synthetic lubricating oils
[24–26]. This material has been used to improve antioxidation characteristics of internal combus-
tion engine oils containing low levels (<0.1 wt%) of phosphorus [27]. In another effort to reduce
phosphorus content in aviation gas turbine lubricants, methylene-bridged bis(dialkyl) or bis(alkylar
yldithiocarbamate) was used as high-temperature antioxidant and antiwear agent to replace tricresyl
phosphates that are of a concern to produce neurotoxic ortho-cresol isomers in trimethylolpropane
triester base oil under high-temperature service conditions [28].
It has been known that metal dithiocarbamates such as zinc, copper, lead, antimony, bismuth,
and molybdenum dithiocarbamates (MoDTCs) possess desirable lubricating characteristics includ-
ing antiwear and antioxidant properties. The associated metal ions affect the antioxidancy of the
additives. Within the group, MoDTCs are of greater interest particularly for engine crankcase lubri-
cants. Certain molybdenum additives posses good oxidation resistance and acceptable corrosion
characteristics, when prepared by reacting water, an acidic molybdenum compound, a basic nitro-
gen complex, and a sulfur source [29,30]. Oil-soluble trinuclear MoDTCs prepared by reacting
FIGURE 1.2 Ashless dithiocarbamates for lubricants.
N
C
S
S
R
R
S
C
S
N
R
R
N
C
S
S
R
R
C
C
C
R
C
O
O
C
O
O
Bis(disubstituted dithiocarbamate) Dithiocarbamate ester
R
R
R
CRC_59645_Ch001.indd 6CRC_59645_Ch001.indd 6 12/4/2008 3:33:17 PM12/4/2008 3:33:17 PM
Antioxidants 7
ammonium polythiomolybdate with appropriate tetralkylthiuram disul des were found to be supe-
rior to dinuclear molybdenum compounds in terms of providing lubricants antioxidant, antiwear,
and friction-reducing properties [31].
When combined with an appropriate aromatic amine, MoDTCs can exhibit synergistic antioxidant
effects in oxidation tests [32]. As a result, molybdenum dialkyldithiocarbamates (C
7–24
) and ADPAs
are claimed broadly for lubricating oils [33]. More restrictive are claims for molybdenum dialkyl-
dithiocarbamates (C
8–23
and C
3–18
) and ADPAs in lubricating oils that contain <3 wt% of aromatic
content and <50 ppm of sulfur and nitrogen [34]. Molybdenum dialkyldithiocarbamates and HP anti-
oxidants are jointly claimed for lubricating oils that contain 45 wt% or more one or two ring naph-
thenes and <50 ppm sulfur and nitrogen [35]. MoDTC was used to top-treat engine oils formulated
with group I base stocks (>300 ppm S) and an additive package designed for group II base stocks.
The oils passed the sequence IIIF oxidation test, in which the oils would otherwise fail without the
molybdenum top-treatment [36]. Further demonstrated is a combination of ADPAs, sulfurized ole n,
or HP and oil-soluble molybdenum compounds including MoDTC. The mixture is highly effective in
stabilizing lubricants, especially those formulated with highly saturated, low-sulfur base oils [37].
Thiadiazole derivatives, particularly the monomers and dimers, represent another class of
sulfur- and nitrogen-bearing multifunctional additives with antioxidant potency. For example, the
monomeric 2-alkylesterthio-5-mercapto-1,3,4-thiadiazole has been reported to increase oxidative
stability of engine oils under thin- lm oxidation conditions by using thin- lm oxygen uptake test
(TFOUT) [38]. Lithium 12-hydroxystearate grease containing 2,5-dithiobis(1,3,4-thiadiazole-
2-thiol), a dimer, exhibited superior oxidative stability in the American Society for Testing and
Materials (ASTM) D 942 pressure bomb oxidation test [39]. When used in conjunction with ADPA
and organomolybdenum compound, the thiadiazole derivative improved the thermal-oxidation
engine oil simulation test (TEOST) deposition (ASTM D 7097) characteristic of an engine oil from
the control oil containing sulfurized isobutylene instead [40]. In addition to providing antioxidant
bene t, the thiadiazole derivatives have been widely used as ashless antiwear and EP additives.
Some of them can also provide corrosion inhibition and metal deactivation properties to nonferrous
metals such as copper.
Phenothiazines are also well-known sulfur- and nitrogen-bearing antioxidants and have been
used to st abil ize av iation uids. Recent advances have lead to N-substituted thio alkyl phenothiazines,
having improved antioxidant activities and oil solubility [41]
as well as N-aminopropylpheno-
thiazine that can be used for further derivatization of the N-amino group [42]. For example, alkyl
phenothiazines together with aromatic amines can be attached to ole n copolymers to result in a
multifunctional antioxidant, antiwear agent, and Viscosity index (VI) improver for lubricants [43].
Diamine sul des, including diamine polysul des, can also provide effective oxidation control
when used in conjunction with oil-soluble copper. In demonstration, dimorpholine disul de and
di(dimethyl morpholine) disul de were compared to primary alkyl ZDDP and found to be superior
in controlling oil viscosity increase of engine crankcase lubricants at elevated temperatures [44].
1.4 PHOSPHORUS COMPOUNDS
The good performance of phosphorus as an oxidation inhibitor in oils was identi ed early on in
lubrication science. The use of elemental phosphorus to reduce sludge formation in oils has been
described [45]. However, elemental phosphorus, like elemental sulfur, may have corrosive side effects
to many nonferrous metals and alloys, so it is rarely incorporated in oils in this form, rather oil- soluble
organic compounds of phosphorus are preferred. Naturally occurring phosphorus compounds such
as lecithin have been utilized as antioxidants and many patents have been issued on these materials
for single use or in combination with other additives [46–49]. Lecithin is a phosphatide that has been
produced commercially as a by-product from the processing of crude soybean oil.
The antioxidant property of synthetic neutral and acid phosphite esters has been known for
sometime. Alkyl and aryl phosphites such as tributyl phosphite and triphenyl phosphite are ef cient
CRC_59645_Ch001.indd 7CRC_59645_Ch001.indd 7 12/4/2008 3:33:17 PM12/4/2008 3:33:17 PM

8 Lubricant Additives: Chemistry and Applications
antioxidants in some petroleum base oils, and many patents have been issued on such compositions
[50,51]. Table 1.1 summarizes the patenting activities of the past three decades on the stabilization
of various lubricants with organophosphites. For optimum antioxidant performance, phosphites are
customarily blended with aminic or HP antioxidants that can lead to synergistic effect. For better
hydrolytic stability, tri-substituted phosphites with sterically hindered structures such as tris-(2,4-
di-tert-butylphenyl) phosphite and those based on pentaerythritol as described in the U.S. Patent
5,124,057 [52] are preferred. The aluminum, calcium, or barium salts of alkyl phosphoric acids are
another type of phosphorus compound that displays antioxidant properties [53,54].
1.5 SULFUR–PHOSPHORUS COMPOUNDS
The identi cation of sulfur and phosphorus compounds as powerful antioxidants for protection
of hydrocarbons has led to the development of oil-soluble antioxidants, having both elements
in one molecule. Numerous patents have been issued on such compositions, and a considerable
number have been used commercially [60–67]. In fact, antioxidants containing both sulfur and
phosphorus are usually more effective and ef cient in a wider variety of base stocks than those
containing only phosphorus or sulfur. Many commercial oils have employed one kind or other of
these sulfur–phosphorus-type additives.
One widely used class of sulfur–phosphorus additive is the metal dialkyldithiophosphates,
which are typically prepared by the reaction of phosphorus pentasul de with alcohols to form
dithio-phosphoric acids, followed by neutralization of the acids with an appropriate metal com-
pound. Many types of alcohols such as the aliphatic, cyclic [62], and phenolic derivatives have
been used, and those of relatively high molecular weight (such as lauryl, octyl, cyclohexyl, methyl
cyclohexyl alcohols, and amyl [65] or butyl phenols) are preferred to give suf cient thermal stability
to the nal products while rendering suf cient solubility in oils. For the second-step reaction, zinc,
TABLE 1.1
Applications of Organophosphites as Antioxidants for Lubricants
Applications Phosphites Supplementary Antioxidants References
Compressor oils Trinonylphenyl phosphite, tributyl
phosphite, tridecylphosphite,
triphenylphosphite,
trioctylphosphite,
dilaurylphosphite
Secondary aminic and hindered
phenolic
55
Automotive and industrial
lubricants
Triaryl phosphites, trialkyl
phosphites, alkyl aryl phosphites,
acid dialkyl phosphites
Secondary aminic and hindered
phenolic
56
Automotive and industrial
lubricants
Triphenyl phosphite, diisodecyl
pentaerythritol diphosphite,
tri-isodecyl phosphite, dilauryl
phosphite
Secondary aminic and hindered
phenolic
57
Hydraulic uids, steam
turbine oils, compressor oils,
and heat-transfer oil
Steric hindered tributyl phosphite,
bis(butylphenyl pentaerythritol)
diphosphite
(3,5-Di-t-butyl)4-hydroxybenzyl
isocyanurate
52
Steam turbine oils, gas turbine
oils
Triphenyl phosphite, trialkyl-
substituted phenyl phosphite
Alkylated diphenylamine,
phenyl-naphthylamine
58
Hydraulic uids, Automatic
transmission uids
Trialkyl phosphites Secondary aminic and hindered
phenolic including bis-phenol
59
CRC_59645_Ch001.indd 8CRC_59645_Ch001.indd 8 12/4/2008 3:33:17 PM12/4/2008 3:33:17 PM
Antioxidants 9
barium, molybdenum, or calcium oxides are usually chosen. For more than 60 years, zinc salts
of dialkylthiophosphoric acids (ZDDP) have been one of the most cost-effective antioxidants and
therefore have been included as a key component in many oxidation inhibitor packages for engine
oils and transmission uids. In addition, ZDDPs show good antiwear properties, especially in the
valve train area owing to the formation of sul de and phosphate lms through corrosive reactions
on metal surfaces. These lms can also provide protection against corrosive attack from the organic
acids formed during the oxidation process. The salts of C
4
/C
5
dialkyldithiophosphoric acid are
the most common, but a broad range of other alkyl and aryl derivatives have been developed to
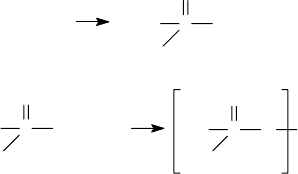
meet special needs, for instance, protection at higher temperatures. The reaction scheme of making
ZDDP is shown in Figure 1.3.
A number of patents describe modi cations to the rst step of the reactions shown in Figure 1.3; by
conducting preliminary condensation reaction of phosphorus pentasul de with unsaturated organic
compounds such as terpenes, polybutenes, wax ole ns, fatty acids, fatty esters, sperm oil, and so
on to form high-molecular-weight intermediate products [68–89]. During these reactions, hydrogen
sul de is liberated, and the intermediates are usually acidic. The mechanism of the P
2
S
5
reaction
with ole ns in these cases may be one of substitution (replacement of reactive hydrogen atoms) as
well as of addition. In preparing the nal additives, these acidic intermediates were neutralized by
the treatment with alkaline earth oxides or hydroxides to form metal salts. The calcium, barium, or
potassium salts are the most preferred products. Some additives may also display detergency char-
acteristics. The concept of conducting preliminary condensation reactions provides a facile route
to the synthesis of a wide variety of products from the reaction of phosphorus pentasul de and an
unsaturated organic moiety. Several of these, particularly the terpene and polybutene reaction prod-
ucts, have been used extensively in commercial applications.
To reduce the staining effect of ZDDP on metal parts (especially copper), addition of alkyl or
aryl phosphites during the synthesis has been attempted [90]. For example, triphenyl phosphite is
added to the dialkyldithiophosphoric acid and heated at 110ºC for an hour before the addition of
zinc oxide. In another patent, a novel dithiophosphate with improved oxidation stability is described
[91]. An acid is reacted with a glycol, to give a monoester having a hydroxyl group, which is then
reacted with P
2
S
5
to give the dialkyl dithiophosphoric acid. Zinc oxide is subsequently added to give
the novel dithiophosphates. To improve solubility, the salts can be made of lower dialkyl dithiophos-
phates by utilizing both primary and secondary alcohols, including butyl alcohols in the process
[92]. Mixed metal salts of dialkyl dithiophosphoric acids and carboxylic acids are claimed to have
higher thermal stability [93].
Many descriptions have recently appeared of organomolybdenum phosphorodithioate com-
plexes that impart excellent oxidation stability to lubricants. In certain circumstances, oil-soluble
molybdenum compounds are preferred additives owing to their multifunctional characteristics
such as antiwear, EP, antioxidant, antipitting, and antifriction properties. For instance, several
molybdenum dialkylphosphorodithioate complexes with varying alkyl chain length of amyl,
octyl, 2-ethylhexyl, and isodecyl were reported to exhibit appreciable antioxidation, antiwear,
FIGURE 1.3 Synthesis of ZDDP.
2 ROH + P
2
S
5
P
S
SH + H
2
S
RO
2 RO
P
S
SH + ZnO
RO
2 RO
RO P
RO
S
S
2
Zn + H
2
O
CRC_59645_Ch001.indd 9CRC_59645_Ch001.indd 9 12/4/2008 3:33:17 PM12/4/2008 3:33:17 PM
10 Lubricant Additives: Chemistry and Applications
and antifriction properties [94]. Novel trinuclear molybdenum dialkyldithiophosphates prepared
by reacting an ammonium polythiomolybdate and an appropriate bis(alkyldithiophosphoric) acid
possess excellent antioxidant as well as antiwear and friction-reducing properties [31]. Some
molybdenum compounds have been used commercially in engine oils and metal working uids
as well as in various industrial and automotive lubricating oils, greases, and specialties [95].
The combination of ZDDP with a molybdenum-containing adduct, prepared by reacting a phos-
phosulfurized polyisoalkylene or alpha ole n with a molybdenum salt, has been described [96].
In this case, the molybdenum adduct alone gave poor performance in oxidation tests, but the
mixture with ZDDP provided good oxidation stability. Novel organomolybdenum complexes pre-
pared with vegetable oil have been identi ed as synergist with ADPAs and ZDDPs in lubricating
oils [97].
Owing to increasing concerns on the use of metal dithiophosphates that are related to toxicity,
waste disposal, lter clogging, pollution, etc., there have been extensive research activities on the
use of ashless technologies for both industrial and automotive applications. A number of ashless
compounds based on derivatives of dialkylphorphorodithioic acids had been reported as multifunc-
tional additives. Upon reacting diisoamylphosphorodithioic acid with various primary and second-
ary amines, eight alkylamino phosphorodithioates with varying chain length from C
5
to C
18
were
obtained and found to possess excellent antiwear and antioxidant properties as compared to ZDDP
[98]. Alkylamino phosphorodithioates obtained from reacting heptylated or octylated or nonylated
phosphorodithioic acids with ethylene diamine, morpholine, or tert-alkyl (C
12
–C
14
) amines have
been demonstrated to impart similar antioxidant and antiwear ef cacy and superior hydrolytic sta-
bility over ZDDP [99]. Phosphorodithioate ester derivatives containing a HP moiety are also known
to have antioxidant potency. This type of chemistry can be obtained by reacting metal salts of
phosphorodithioic acids with HP halides [100] or with HP aldehydes [101]. Substituting the phenol
aldehydes with hindered cyclic aldehydes, in which the carbon atom attached to the carbonyl carbon
contains no hydrogen atoms, may also result in products having excellent antioxidant and thermal
stability characteristics [102].
1.6 AMINE AND PHENOL DERIVATIVES
Oil-soluble organic amines and phenol derivatives such as pyrogallol, gallic acid, dibutylresorcinol,
hydroquinone, diphenylamine, phenyl-alpha-naphthylamine, and beta-naphthol are early examples
of antioxidants used in turbine oils and lubricating greases [103,104]. In engine oils, these types of
compounds showed only limited effectiveness. Other amines and phenol derivatives such as tetra-
methyldiaminodiphenylmethane and alizarin were used to some degree, rarely alone, but more often
in combination with other types of antioxidants. For example, a mixture of a complex amine with
a phosphorus pentasul depolybutene reaction product has been reported [105]. Another reported
mixture is a complex phenol derivative such as alizarin in combination with an alkyl phenol sul de
and a detergent additive [106]. As technology advances, numerous amine and phenol antioxidants
have been invented, and many of them have become the most widely used antioxidants in the lubri-
cant industry.
1.6.1 AMINE DERIVATIVES
ADPAs are one of the most important classes of amine antioxidants being used today. Owing to their
higher reactivity over the unsubstituted diphenylamine, ADPAs have been workhorse antioxidants
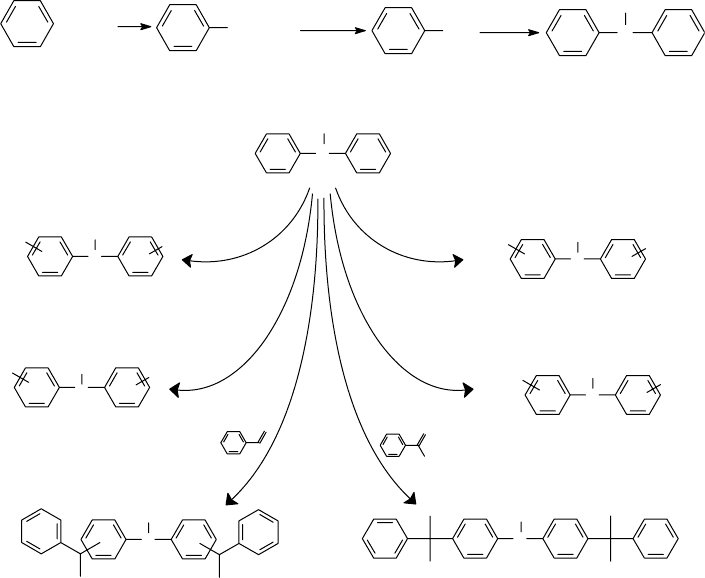
for engine oils and various industrial lubricants for more than two decades. Figures 1.3 and 1.4
illustrate the typical synthesis routes of some commonly used ADPAs. The reactions start with
benzene, which is rst converted into nitrobenzene [107], followed by a high-temperature reduc-
tion to aniline [108]. Under very high-temperature (400–500°C) and high-pressure (50–150 psi)
conditions, aniline can undergo a catalytic vapor-phase conversion to form diphenylamine [109].
CRC_59645_Ch001.indd 10CRC_59645_Ch001.indd 10 12/4/2008 3:33:18 PM12/4/2008 3:33:18 PM
Antioxidants 11
To make ADPAs, diphenylamine is reacted with an appropriate alkylating agent such as alcohol,
alkyl halide, aliphatic carbonyl compound, or an ole n. The ole ns are preferred for economic rea-
son. The most commonly used are isobutylene (C
4
), diisobutylene (C
8
), nonenes (C
9
), styrene, and
propylene tetramer (C
12
). Depending on the acidic catalyst, ole n, and other reaction conditions, for
instance, the temperature, the degree of alkylation will vary from mono- to di-alkylation.
Mono-ADPA is generally more effective than the corresponding disubstituted on a weight
basis because additional alkylation substantially reduces the number of moles of diphenylamine
per weight unit. However, in practice, obtaining monosubstituted diphenylamine in relatively
pure format is dif cult because as soon as the diphenylamine is monoalkylated, it quickly pro-
ceeds to dialkylation. Attempt in the preparation of high content of mono-ADPAs has led to
the use of novel clay catalyst with greater selectivity in alkylation reactions and C
6
–C
18
linear
ole ns to produce high levels (at least 50 wt%) of mono-ADPAs with lower levels of dialkyl
diphenylamines and undesirable unsubstituted diphenylamine [110]. Alkyl groups of six or more
carbon of mono-ADPA tend to render the material lower yellow color and higher resistance to
discoloration [111].
It was found that monosubstituted diphenylamines more readily oligomerize under various
conditions to produce higher-molecular weight, linear oligomers. Oligomers with 2–10 degrees of
polymerization are desirable antioxidants especially for high-temperature applications. Disubsti-
tuted and polysubstituted diphenylamines, however, are more restricted from forming oligomers
higher than dimers. Oligomeric versions of monosubstituted diphenylamine prepared from reacting
diphenylmine with C
4
–C
16
ole ns have been described for use in ester lubricants [112]. The prod-
ucts are claimed to be more effective than simple diphenylamines for extremely high-temperature
applications. Homo-oligomers of alkylated (C
4
–C
8
) diphenylamines, styryenated diphenylamines,
FIGURE 1.4 Synthesis routes of ADPA antioxidants.
N
H
N
H
C
8
, St
C
8
, St
N
H
C
4
, C
8
C
4
,
C
8
N
H
N
H
C
9
C
9
N
H
C
8
C
8
C
4
+ C
8
olefin
C
8
olefin + styrene
N
H
C
9
olefin
C
8
olefin
+ HNO
3
NO
2
+ H
2
NH
2
Catalyst
N
H
Benzene Nitrobenzene Aniline Diphenylamine
Catalyst
CRC_59645_Ch001.indd 11CRC_59645_Ch001.indd 11 12/4/2008 3:33:18 PM12/4/2008 3:33:18 PM
12 Lubricant Additives: Chemistry and Applications
or cross-oligomers of the ADPAs with substituted N-phenyl-α(β)-naphthylamine (PNA) are claimed
to possess superior antioxidant ef cacy in synthetic ester lubricants for high-temperature applica-
tions [113]. Oligomeric products derived from thermal and chemical condensation of ADPA and
alkylated PNA in the presence of aldehyde can provide high performance and nonsludging attri-
butes, as evident in the rotating pressure vessel oxidation test (RPVOT, ASTM D 2272) and the
ASTM D 4310 sludging tendency test designed for turbine oils [114].
There appears to be a great number of patenting activities on the process of using isobutylene
derivatives as alkylating agents. Under certain mole ratio range, diphenylamine can be reacted
with diisobutylene at a temperature of 160°C or higher to facilitate chain scission of diisobutyl-
ene [115]. In the presence of an acid clay catalyst, the resulting product has <25% of 4,4′-dioctyl
diphenylamine, which yields a liquid at room temperatures. In another process that involves two-
step reactions [116], a light-colored, liquid product is obtained by rst reacting diphenylamine with
diisobutene, followed by reaction with a second ole n, preferably isobutene. Speci c mole ratio,
reaction temperature, and reaction duration are critical to obtain the desired ADPAs. To obtain
higher levels (>50 wt%) of monosubstituted diphenylamine content in the nal product, diisobutyl-
ene is allowed to react at a lower temperature range of 105–157°C in the presence of a clay catalyst.
By carefully controlling mole ratio of the reactants together with reaction duration, the process,
as disclosed, selectively results in a higher proportion of mono-ADPA and a lower proportion of
unsubstituted diphenylamine and disubstituted or polysubstituted diphenylamines [90,117]. U.S.
Patent 6,355,839 [118] discloses a one-step process using highly reactive polyisobutylene oligomers
having an average molecular weight of ~160 to 280 and at least 25% of 2-methylvinylidene isomers
as the alkylating agents to make ADPAs and other types of alkylated diarylamine. The resulting
products are liquid at ambient temperatures.
Several antioxidant patents based on alkylation of benzotriazole compounds have been issued.
One particular bene t of using this class of antioxidant over the ADPAs is their additional activity in
the reduction of copper corrosion. Examples are N-t-alkylated benzotriazoles obtained by reacting a
benzotriazole with an ole n such as diisobutylene [119], and the reaction products of a benzotriazole
with an alkyl vinyl ether or a vinyl ester of a carboxylic acid such as vinyl acetate [120]. Antioxidant
and antiwear properties were reported for benzotriazole adducts of an amine phosphate [121] or an
organophosphorodithioate [122]. The former type also exhibited rust prevention characteristics in
the ASTM D 665 corrosion test.
Aromatic diamines are a broad group of aminic antioxidants suitable for lubricants. 3,5-
diethyltoluenediamines with the amino moieties being located on the 2,4 and 2,6 positions rela-
tive to the methyl group have been claimed to be effective in the prevention of oil viscosity
increase and acid buildup [123]. The additives are relatively noncorrosive to copper and lead
bearings and are compatible with seals at high temperatures and pressures. Substituted ben-
zylamines or substituted 1-amino-1,2,3,4-tetrahydronaphthalene is particularly useful for syn-
thetic lubricants such as polyalphaole ns (PAOs) or polyol esters. Oils bearing these additives
demonstrate very low metal corrosion, low viscosity increase, and low sludge buildup [124].
N,N′- diphenyl-p- phenylenediamines in which the phenyl groups may be substituted with methyl,
ethyl, or methoxy have been claimed as effective antioxidants [125].
A broader range of substi-
tuted p-phenylenediamines has been claimed for crankcase lubricating oils for use in environ-
ments where iron- catalyzed oxidation reactions can take place [126]. 2,3- Dihydroperimidines that
are prepared from the condensation of 1,8-diaminonaphthalenes with ketones or aldehydes show
good oxidation inhibition in the RPVOT (ASTM D 2272). Synergistic behavior of the amines was
also observed when an appropriate phenolic antioxidant is present [127]. Oils containing N,N′-
disubstituted-2,4-diaminodiphenyl ethers and imines of the same ethers have shown low viscosity
increase, low acid buildup, and reduced metal corrosion in bench tests [128,129]. The reaction
product of a hydrocarbyl succinic anhydride and 5-amino-triazole demonstrated antioxidant ef -
cacy in a railway diesel oil composition [130].
CRC_59645_Ch001.indd 12CRC_59645_Ch001.indd 12 12/4/2008 3:33:18 PM12/4/2008 3:33:18 PM
Antioxidants 13
1.6.2 PHENOL DERIVATIVES
Phenols, especially the sterically hindered phenols are another class of antioxidants being extensively
used in industrial and automotive lubricating oils and greases. Based on the chemical structure,
phenols may be customarily categorized into simple phenols such as 2,6-di-tert-4- methylphenol
(also known as BHT) and complex phenols that are typically in polymeric forms having molecular
weights of 1000 or higher. The structures, important physical properties, and typical applications of
some commonly used HPs are given in Table 1.2.
Similar to the alkyl phenol sul des discussed earlier, the combinations of HPs and sulfur chem-
istry have been widely reported. For example, the reaction products of simple phenols such as the
2,6-di-tert-butylphenol listed in Table 1.2 with selected thioalkenes have shown effectiveness in the
prevention of acid buildup and oil viscosity increase, without causing lead corrosion [131]. Another
patent describes a process for preparing hydrocarbylthio-HPs by reacting substituted phenols with
hydrocarbyl disul des using an aluminum phenoxide catalyst [132]. Using a 4,4′-methylene bis(2,6-
di-tert-butylphenol) as reference, the thiophenols were found to be superior in bulk oil oxidation
tests and bench corrosion test on bearings. High oligomeric phenolic antioxidants in the form of
hindered and sulfur bridged have been developed [133]. These compounds have lower volatility, bet-
ter thermal stability, and improved seal compatibility and corrosion properties. In general, sulfur-
bridged HPs are more effective than the conventional phenolics under high-temperature oxidation
conditions and are considered particularly suitable for the lubricants formulated with highly re ned
base stocks [134]. Figure 1.5 shows structures of some commercial sulfur-bridged HPs that have
found use in various lubricant formulations. Thioalkene-bridged hemi-HPs prepared from catalytic
reaction of HP with thioalkene have also been reported to be active in the stabilization of mineral
oils and synthetic oils [135].
1.6.3 AMINE AND PHENOL-BEARING COMPOUNDS
Given the high popularity and effectiveness of amine and phenol derivatives as lubricant anti-
oxidants, the combination of amine and phenolic moieties in one molecule represents a logic
approach to enhance performance. In a prior art [136], fusing amine with a long carbon chain 3,5-
di-tert-butyl-4-hydroxyphenalkyl group that separates the phenol group from the amino nitrogen
leads to novel products with lower volatility, better thermal stability, and higher solubility in oils.
Nelson and Rudnick [137] reacted an ethyoxylated alkyl phenol with an alkyl arylamine in the
presence of an aldehyde. The resulting product had improved antioxidant potency owing to a syn-
ergistic action between the phenolic moiety and the amine, and also showed enhanced solubility
in oils owing to the presence of alkylated aromatic moiety in the molecule. Phenolic imidazo-
lines have been prepared from polyaminophenols and carbonyl compounds [138]. In addition to
providing antioxidant activity, the products also have corrosion inhibition and metal deactivation
properties owing to the cyclic imidazoline moiety.
Multifunctional additives containing sulfur, nitrogen, and phenolic moieties in one molecule
have been reported. In this instance, mercaptobenzothiazoles or thiadiazoles are Mannich reacted
with HP antioxidants to yield oil-soluble compounds with antioxidant and antiwear properties [139].
More complex product having similar functionalities was obtained by reacting a sulfur-containing
HP ester with an ADPA [140].
1.6.4 MULTIFUNCTIONAL AMINE AND PHENOL DERIVATIVES
The industry-wide trend in the reduction of phosphorus and sulfur, in particular, ZDDP in nished
lubricants has led to increasing activities in the development of novel multifunctional additives
that have combined properties of antioxidancy, antiwear, and to some extent dispersancy, while
having low-to no-sulfur and phosphorus contents. It has been shown that products obtained from
CRC_59645_Ch001.indd 13CRC_59645_Ch001.indd 13 12/4/2008 3:33:18 PM12/4/2008 3:33:18 PM
