Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.


234 Lubricant Additives: Chemistry and Applications
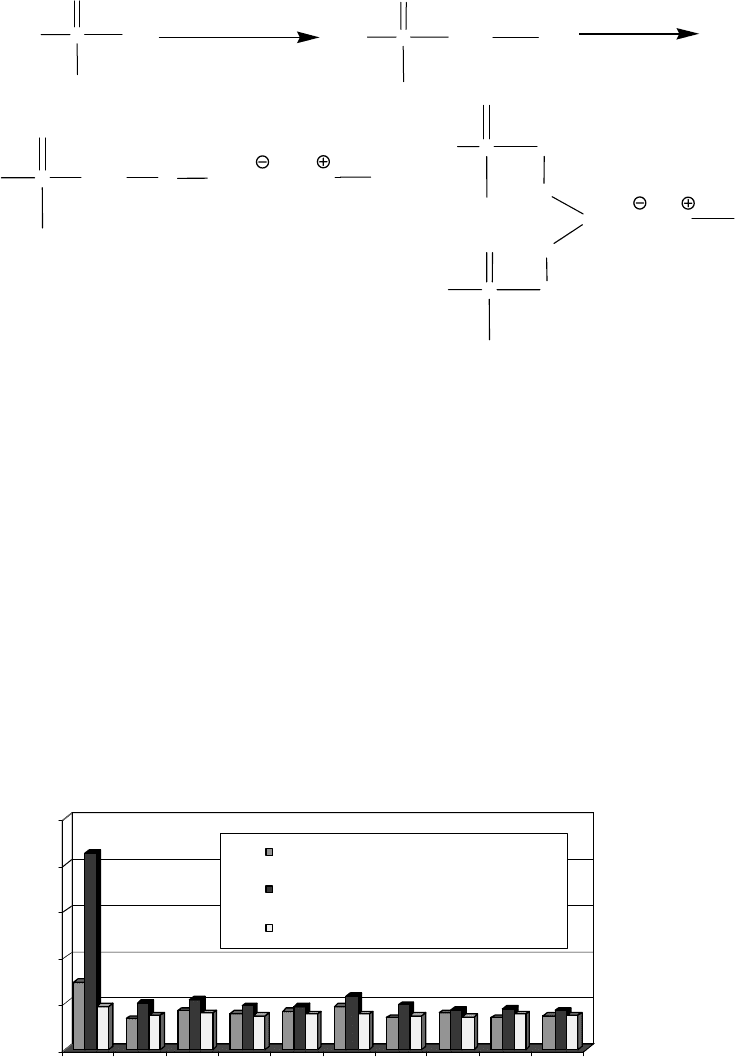
N
N
H
N
H
3
C
N
N
O
O
R
2
R
1
R
4
R
3
N
R
2
R
3
R
1
O
B
OR
5
OR
4
STRUCTURE Q (TTZ) STRUCTURE R (SHDZ) STRUCTURE S (BHPD)
Both BZT and TTZ derivatives are also effective copper deactivators at low concentrations.
Therefore, these types of additives indeed have dual functions. They nd applications in industrial
oils, greases, and fuels.
Table 8.2 lists a prototype engine oil formulation used for the evaluation of ODZ additives where
various ODZs can be blended at 1 wt% in place of the same amount of light base oil. Table 8.3 lists
the Four-Ball Wear performance data where a series of ODZs were evaluated against 0.5 wt% and
1 wt% ZDDP. As demonstrated, those ODZ additives exhibited fairly good antiwear properties in
this bench test [86].
8.2.7 ADDITIVES WITH MULTIPLE ELEMENTS
Complex additives with multiple elements can be derived from various S/P, sulfur/nitrogen,
phosphorus/nitrogen, and many other traditional additive building blocks. As a result, molecules
with more than four, ve, six, or even more elements are created (S/P/N/B in addition to C/H/O).
Derivatization frequently adds a degree of complexity, yet provides a chance of achieving better
synergisms among all critical elements that can not only satisfy the performance needs but also help
neutralize any potentially added costs associated with the new chemistry under development.
Many examples are available in the literature as well as in the marketplace, such as
amine salts of dithiophosphates and thiophosphates (Section 8.2.5.2); borated derivatives of
dithiophosphates [41], dithiocarbamates [50], and dimercapto-thiadiazole [93]; urethane deriva-
tives of dithiophosphates [94] (Structure T); and reaction adducts of dialkyl phosphites, sulfur,
and acylated amines [95].
S
P
RO
OR′
NH
C
S
NH
C
CH
3
O
O
R
O
O R
S
P
S
OR
OR′
STRUCTURE T
Several complex additives have different chemistries involved with the same element in a single
molecule to attain strong synergisms. As exempli ed in the following case, where both phosphite
chemistry and phosphate chemistry are incorporated into the same molecule, greater antiwear
performance can be achieved ([96]; reaction 8.38).
CRC_59645_Ch008.indd 234CRC_59645_Ch008.indd 234 10/31/2008 2:58:06 PM10/31/2008 2:58:06 PM
Ashless Antiwear and Extreme-Pressure Additives 235
PRO
OR′
O
H
R′′-CH=O
PRO
OR′
O
CHR′′ OH
P
2
O
5
Amines
PRO
OR′
O
CHR′′ O
P(O)(O)
2
(H
3
N R′′′)
2
O
O
P(O)-O
H
3
N
R′′
′
POR
OR′
O
CHR′′
PRO
OR′
O
CHR′′
+
(8.38)
The synergistic antiwear performance of the aforementioned complex phosphorus additives
are illustrated in Chart 8.1. Nine different analogues were synthesized and tested at 1 wt% in base
oils using three different conditions in the Four-Ball Wear test. As demonstrated, they all exhibit
exceptionally good antiwear properties.
8.2.8 HALOGEN ADDITIVES
Chlorine was one of the earliest antiwear and EP elements used in the lubricant industry.
Chlorine-containing additives are still used in cutting oils and related metalworking lubricants, in
combination with sulfur additives. Iodine was mentioned in aluminum-processing lubricants for
wear control. Fluorine, in per uorinated compounds, is well known to reduce wear and especially
friction.
WSD
0
0.5
1
1.5
2
2.5
BS BS +
1% (A)
BS +
1% (B)
BS +
1% (C)
BS +
1% (D)
BS +
1% (E)
BS +
1% (F)
BS +
1% (G)
BS +
1% (H)
BS +
1% (I)
Four-Ball Wear Test (WSD in millimeters)
Test 1−93C, 40 kg, 1800 rpm, 30 min
Test 2−93C, 60 kg, 1500 rpm, 30 min
Test 3−135C, 60 kg, 100 rpm, 30 min
All tested additives (A−J) were derived from dibutyl phosphite, butyraldehyde, and a selected amine at various molar ratios.
A and B: using Primene JMT at different ratios, C and D: using Adogen 183 at different ratios, E,G, and I: using Duomeen
O at different ratios, F and H: usin
g
bis 2-EH amine at different ratios.
CHART 8.1 Complex phosphorus additives. (BS, base stock; WSD, wear scar diameter.)
CRC_59645_Ch008.indd 235CRC_59645_Ch008.indd 235 10/31/2008 2:58:07 PM10/31/2008 2:58:07 PM
236 Lubricant Additives: Chemistry and Applications
The chlorine compounds act and function in that they coat the metal surface with a metal
chloride lm under the in uence of high pressure at point of lubrication and in the presence of
traces of moisture. FeCl
2
melts at 672°C and has low shear strength when compare with steel.
The effect of chlorine compounds depends on the reactivity of the chlorine atom, temperature,
and concentration. Hydrogen chloride formed in the presence of larger quantities of moisture can
cause severe corrosion of the metal surfaces. As the corrosion hazards increase along with the EP
properties with increasing reactivity of the chlorine atoms, a compromise must be found in the
development of chlorine-containing additives.
Chlorinated paraf ns such as trichlorocetane represent a group of important EP additives used
in the past. They can signi cantly increase the load stages in the FZG test with increasing con-
centration. The chain length has practically very little in uence on the EP effect; on the contrary,
the load-carrying capacity increases with increasing degree of chlorination. In practice, chlori-
nated paraf ns with ~40 to 70 wt% chlorine are used; however, they are sensitive to moisture and
light and can easily evolve hydrogen chloride [97]. Compounds such as phenoxy-propylene oxide,
amines, or basic sulfonates neutralize hydrogen chloride and thus act as stabilizers.
Good results are also obtained with chlorinated fatty acids and their derivatives; particularly
those with trichloromethyl groups in the end position, since the additives with CCl
3
groups are
particularly effective.
Owing to their high stability, chlorinated aromatics have less favorable EP properties than
the chlorinated aliphatics. Alkylaromatics with chlorinated side chains improve the load-carrying
capacity much more than those chlorinated in the ring; the ef ciency increases with the number of
carbon atoms in the side chain. Chlorinated fatty oils and esters as well as chlorinated terpenes and
amines have also been patented as EP additives.
Sulfur–chlorine additives were found to be satisfactory for gear lubrication in passenger cars
in the mid-1930s. Apparently, this type of additive could satisfy the high-speed and moderate-load
operation of passenger cars used in that time period. When sulfur and chlorine are combined in the
organic molecule, sulfur somewhat reduces the corrosive tendency of chlorine; on the contrary, the
EP properties of the combined moieties are improved in comparison with the individual compounds.
Chlorinated alkyl sul des, sulfurized chloronaphthalenes, chlorinated alkyl thiocarbonates, bis-(p-
chlorobenzyl) disul de, tetrachlorodiphenyl sul de, and trichloroacrolein mercaptals [Cl
2
C=CCl–
CH(SR′)–SR″, where R′ and R″ are alkyl or aryl] must be mentioned in this class. Reaction products
of ole ns and unsaturated fatty acid esters with sulfur chlorides contain highly reactive β-chlorosul-
des, which due to their reactive chlorine and sulfur atoms give very good EP agents, yet show more
or less strong corrosive tendencies. However, severe wear was frequently encountered in truck axles
where performance under high-torque, low-speed conditions is of greater importance. Later on, the
presence of chlorine, although a good EP agent, was found to be detrimental to lubricant thermal
stability. Hence, for the past 30 years, chlorine has not been used in gear oils.
Chlorinated trioleyl phosphate, condensation products of chlorinated fatty oils with alkali
salts of dithiophosphoric acid diesters, and reaction products of glycols with PCl
3
are examples of
chlorine–phosphorus additives used in earlier years.
The most serious drawback for chlorine antiwear and EP additives is in the environmental
area. Legislation around the industrial world limits the chlorine content of many lubricants to parts
per million. Therefore, except for the cutting oil industry, which is also under pressure to change,
chlorine additives are not considered a viable option for modern lubricants.
8.2.9 NONTRADITIONAL ANTIWEAR/EXTREME-PRESSURE ADDITIVES
Traditional sulfur, phosphorus, and halogen-related compounds are considered to be the dominant
antiwear/EP additives in the marketplace. However, as environmental concerns escalate, the future
trends will favor products that diminish potential hazard and disposal problems. Recent clean fuel
CRC_59645_Ch008.indd 236CRC_59645_Ch008.indd 236 10/31/2008 2:58:07 PM10/31/2008 2:58:07 PM
Ashless Antiwear and Extreme-Pressure Additives 237
activities are driving sulfur levels toward 10–50 wt ppm ranges. Subsequently, the petroleum indus-
try is favoring lower sulfur lubricants since sulfur is also known to poison the catalytic system
used for NO
X
reduction. Therefore, the use and development of nontraditional antiwear additives is
becoming more valuable.
A number of nonsulfur, nonphosphorus ashless antiwear additive technologies have been
reported in the literature [98–102]. Among these, high hydroxyl esters (HHE), dimer acids,
hydroxyamine esters, acid anhydrides, cyclic amides, and boron derivatives are recognized as
leading technologies. Graphite and polytetra uoroethylene (PTFE) possess excellent friction
reduction properties and indirectly contribute some antiwear/EP characteristics. However, both
materials need to be dispersed in the oil as they have very limited lubricant solubility, which ham-
pers their usefulness. Organic borates are considered as effective friction modi ers, antioxidants,
and cleanliness agents. Recent studies indicate that some borates can be good antiwear additives.
Potassium borates have been used in gear oils for years, but these types of metallic borates are
outside the scope of this chapter. Esters are known to possess good lubricity properties. The prop-
erties can be further improved to offer antiwear characteristics through proper functionalization.
Several companies have marketable products in this area.
8.3 MANUFACTURE, MARKETING, AND ECONOMICS
All major additive suppliers produce ashless antiwear and EP additives that are available as
components and packages. Following is a list of major producers (arranged in alphabetical order).
Afton Corporation
Akzo Nobel
Ato na Chemicals (former Elf Atochem NA and Pennwalt Corporation)
BASF
Chemtura (former Great Lakes Chemical’s Durad Division)
Ciba Specialty Chemicals
Chevron Corporation (Oronite Division)
Clariant
Dover Chemical (former Keil Chemical Division, Ferro)
Dow Chemical (former Angus Division)
Elco Corporation (Detrex)
FMC
Hampshire Chemical Corporation (former Evans Chemetics)
ICI America (Uniqema)
In neum International Limited
Lubrizol Corporation
Polartech
Rhein Chemie
Rhodia (former Albright & Wilson)
Zeneca
Ashless antiwear and EP additives are supplied in various chemistries, including single and
multiple blends formulated to maximize performance and minimize adverse effects (e.g., dropout
and corrosion). Product designations vary by chemical class and concentration. Many of them are
formulated into additive packages according to applications, such as passenger car engine oils,
heavy diesel engine oils, automotive transmission oils, automotive gear oils, hydraulic uids, and
others. Since the product offering information can be supplier-speci c, it is recommended to contact
the suppliers directly or go to their corresponding Web site for further information.
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
CRC_59645_Ch008.indd 237CRC_59645_Ch008.indd 237 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM

238 Lubricant Additives: Chemistry and Applications
There has been some consolidation in the additive business, but the market has not changed
much as a result. Following are the major changes by year.
1992 Ethyl acquired Amoco Petroleum Additives (U.S.) and Nippon Cooper (Japan)
1996 Ethyl acquired Texaco Additives Company
1997 Lubrizol bought Gateway Additives (Spartanburg, South Carolina)
1999 In neum, the new petroleum additives enterprise, a joint venture between
Exxon Chemical, Shell International Chemicals Ltd., and Shell Chemical
Company, unveiled its new corporate identity and became fully operational
on January 1, 1999 (the largest merge of additive companies in history)
1999 Crompton completed a merger with Witco
2001 Texaco Oil merged into Chevron Oil whereas Chevron Chemical Oronite
Division was kept intact
2003 Dover Chemical acquired the Keil Chemical petroleum additives business from
Ferro Corporation
2004 Ethyl Corporation transformed into NewMarket Corporation, the parent
company of Afton Chemical Corporation and Ethyl Corporation to maximize
the potential of its operating divisions—petroleum additives and tetraethyl
lead fuel additive business
2005 Chemtura was formed by the merger of Crompton and Great Lakes Chemical
Corporation
2007 Chemtura bulked up its specialty lubricants business with the assets of
Kaufman Holdings Corporation, parent company of Anderol and Hatco
Since most lubricant additives are produced through batch processes, consolidation can lead to
improved operations and reduced costs (e.g., reducing plant idle time with better chemical manu-
facturing management systems). There are still many manufacturing facilities using equipment and
procedures that are 30–40 years old. Hence, any investments in automation and continuous process-
ing for a plant will be a competitive advantage. However, the business is so cost-competitive that
most suppliers have dif culties in justifying major capital expenditures.
8.4 EVALUATION EQUIPMENT/SPECIFICATION
8.4.1 L
UBRICANT SPECIFICATIONS
Lubricant components and formulated products are manufactured as per the rigid speci cations
in petroleum re neries and lubricant blending plants, and must also meet detailed commercial,
industrial, and military speci cations. As an example, the U.S. Military has rigid speci cations
for automotive lubricants, although the automotive manufacturers have similarly rigid but not
necessarily the same speci cations to assure quality and consistency of lubricant manufacture.
In addition, there are performance speci cations that must be met from such original equipment
manufacturers (OEMs) as farm machinery and other off-highway automotive equipment. These
speci cations are designed to enable the user to select appropriate lubricants and to be assured of
adequate performance over a speci ed service life.
The industry is, for the most part, adequately self-regulating with minimal government input con-
cerning performance speci cations. The most elaborate system for developing and upgrading lubricant
and fuel speci cations is for automotive lubricants. The American Society of Testing and Materials
(ASTM), the Society of Automotive Engineers (SAE), and the American Petroleum Institute (API)
all have de ned roles in determining speci cations for products such as passenger car motor oils and
heavy-duty motor oils. These three organizations, working together in the United States, are known
as the Tripartite. Extending internationally, the International Lubricant Standardization and Approval
Committee (ILSAC) is also active in all phases of engine lubricant category development.
CRC_59645_Ch008.indd 238CRC_59645_Ch008.indd 238 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM
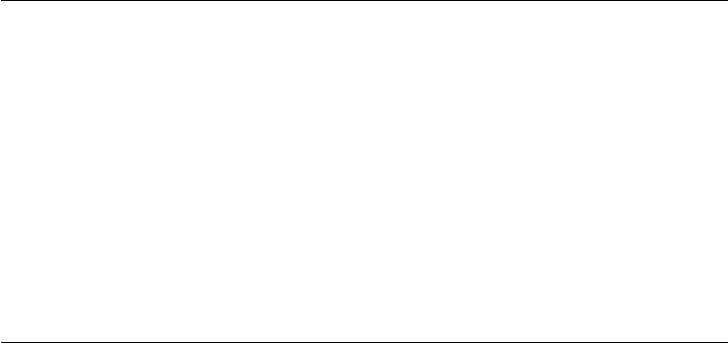
Ashless Antiwear and Extreme-Pressure Additives 239
In other product categories, lubricant and additive suppliers, OEMs, and industry trade associa-
tions work together to determine performance requirements and product speci cations. In addition
to the three industry organizations mentioned earlier, the National Lubricating Grease Institute
(NLGI), the National Marine Manufacturers Association (NMMA), the American Gear Manufac-
turers Association (AGMA), the Society of Tribologists and Lubrication Engineers (STLE), and
other groups, associations, and key equipment builders can in uence lubricant speci cations.
In addition to meeting all military and industrial speci cations, many leading lubricant mar-
keters and nished lubricant suppliers develop their own internal speci cations to be used for new
product launching, competitive product analysis, and future product development. Proprietary eld-
testing is an integral part of the overall new lubricant product development processes and is often
the most critical step to assure technical success and customer satisfaction for new products.
8.4.2 ADDITIVE SPECIFICATIONS
Speci cations for antiwear/EP additives focus primarily on application, base oil compatibility, and
quanti cation of elemental constituents. In addition, speci cations typically identify speci c and
critical performance standards for applications. Common speci cations for antiwear/EP additives
are shown in Table 8.4.
In addition to typical speci cations as reported in the Certi cate of Analysis (C of A) from
additive suppliers, individual lubricant marketers often prefer to conduct their own internal additive
speci cations, such as infrared analysis and key performance testing.
8.4.3 TEST METHODS AND EQUIPMENT
In the United States, a number of bench and advanced tests were developed and approved by ASTM,
and these tests have gained widespread reception throughout the industry. However, there are also a
few selected lab-bench and advanced tests that were developed and approved only by speci c OEMs,
but represent certain critical and desirable performance features (Figures 8.2 and 8.3). This chapter
is not intended to cover all evaluation tests in detail, but rather to illustrate a few representative tests
to highlight the key assessment criteria.
1. Four-Ball Wear and EP Test. This tester was developed to evaluate the antiwear, EP, and
antiweld properties of lubricants. It is a simple bench test machine designed to measure the
protection a lubricant provides under conditions of high unit pressures and various sliding
velocities. The Four-Ball Wear tester consists of four 1.5 in. diameter steel balls arranged
in the form of an equilateral tetrahedron. The three lower balls are held immovably in a
TABLE 8.4
Typical Specifi cations for Antiwear and EP Additives
Chemical Class Property Performance Test
Amine phosphates Percent of nitrogen,
phosphorus, and TAN/TBN
Four-Ball Wear, Four-Ball EP,
FZG, rust/oxidation test
Methylene bis-dialkyl dithiocarbamate Percent of sulfur, nitrogen,
and residual chlorine, amine
Four-Ball EP, FZG, Falex EP,
oxidation/corrosion test
Sulfurized lard, esters, fatty acids Percent of total sulfur Four-Ball Wear, Four-Ball EP,
stick-slip, Cu corrosionPercent of active sulfur
Triphenyl phosphorothioate Percent of sulfur, phosphorus,
and melting point
Four-Ball EP, FZG, Falex EP,
oxidation/corrosion test
Chlorinated paraf ns, fatty acids Percent of chlorine Four-Ball Wear, Falex EP,
Timken, Cu corrosionAcid value
CRC_59645_Ch008.indd 239CRC_59645_Ch008.indd 239 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM
240 Lubricant Additives: Chemistry and Applications
clamping pot, while the fourth ball is made to rotate against them. Test lubricant is added
in the test pot, covering the contact area of the test balls. During a test, wear scars are
formed on the surfaces of the three stationary balls. The diameter of the scars depends on
the load, speed, temperature, duration of run, and type of lubricant. The Four-Ball EP tes-
ter runs at a xed speed of 1770 ± 60 rpm and has no provision for lubricant temperature
control. A microscope is used to measure the wear scars. Two of the standard tests run
on the Four-Ball machine are Mean-Hertz Load and Load-Wear Index. ASTM D 2596
covers the detailed calculation procedure of Load-Wear Index for greases and D-2783
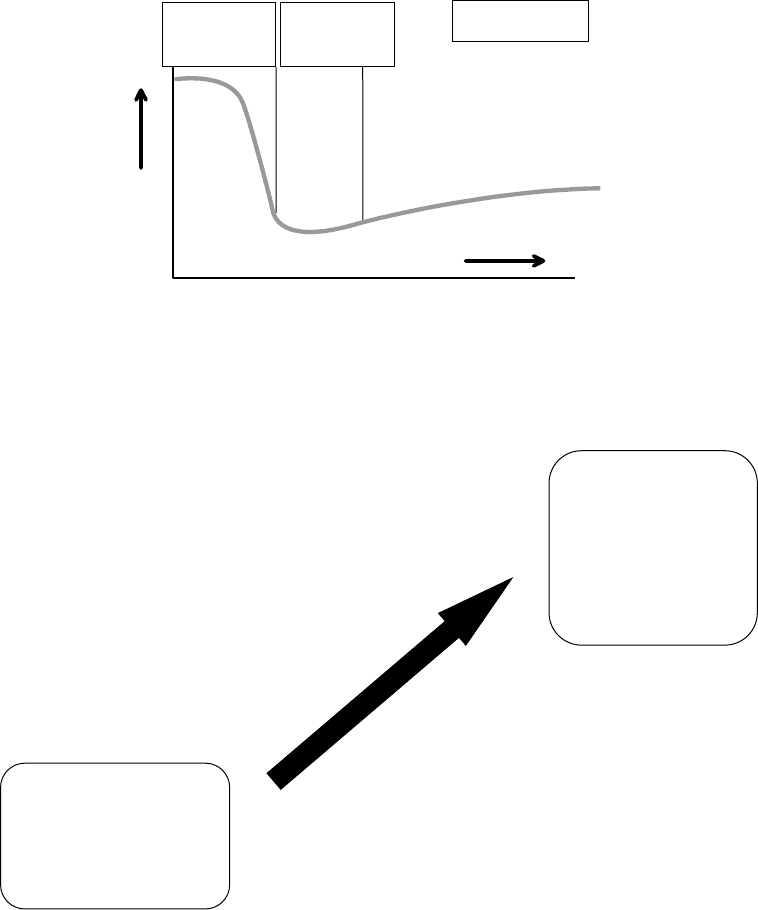
Elastohydrodynamic
Boundary Hydrodynamic
Machine ways
heavily loaded
gears
Gears rolling
element
bearings
Journal bearings
Friction
Viscosity × speed/load
1. Viscosity
Viscosity and viscosity index
Active antiwear and EP additives
Controlling lubricant factors
2. Pressure-viscosity coefficient
FIGURE 8.2 Lubrication regimes.
FIGURE 8.3 Ashless antiwear additives: availability, applicability, selection, and future needs.
• Availability
• Novel chemistries are available (S/P/N/B)
• Protecting film can be formed from
low-shear, nonmetallic species
• Process development, registration, and
commercialization plan pending on
market demand and timing
• Applicability
• Bench tests only serve as indicators
• Balance of performance is key
• Selection
• Identifying critical tests first
• Seeking combinations of additives
• Using QSAR analysis
Four-Ball Wear/EP
HFRR & Plint
Multispecimen
Optimol SRV
Pin-on-V-block
Seq IVA and IIIG
JAMA chain wear
FZG and FE8
M11 EGR
35VQ25 pump
OM 602/TU 3M
L37/L42
Engine and industrial oils
Reduced exhaust emissions
Improved stability
High temperature oxidation/nitration
Oxidation resistance/hydrolytic stability
Improved compatibility and cleanliness
Improved surface fatigue-wear protection
Pitting and micropitting
Antiscuffing
Low friction
Fuel economy and energy conservation
Improved durability
Extended drain
Fill for life
•
•
•
•
•
•
•
•
•
•
•
•
•
CRC_59645_Ch008.indd 240CRC_59645_Ch008.indd 240 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM
Ashless Antiwear and Extreme-Pressure Additives 241
for oils. These procedures involve the running of a series of 10 s tests over a range of
increasing loads until welding occurs. From the scar measurements, the mean load (load-
wear index) is calculated and it serves as an indicator of the load-carrying properties of the
oil being tested.
2. FZG Four-Square Gear Test Rig. The FZG test equipment consists of two gear sets,
arranged in a four-square con guration, driven by an electric motor. The test gear set is
run in the test uid, while increasing load stages (from 1 to 13) until failure. Each load
stage is run for a 15 min period at a xed speed. Two methods are used for determining
the damage load stage. The visual rating method de nes the damage load stage as the
stage at which more than 20% of the load-carrying ank area of the pinion is damaged by
scratches or scuf ng. The weight loss method de nes the damage load stage as the stage at
which the combined weight loss of the drive wheel and pinion exceeds the average of the
weight changes in the previous load stages by more than 10 mg. The test is used in develop-
ing industrial gear lubricants, ATFs, and hydraulic uids to meet various manufacturers’
speci cations.
3. Falex EP/Wear Tester. The Falex test machine provides a rapid method of measuring the
load-carrying capacity and the wear properties of lubricants. The test consists of rotating a
test pin between two loaded journals (V-blocks) immersed in the lubricant sample. There
are two common tests run in this machine: one is an EP test (subjecting a test lubricant to
increasing loads until a failure occurs) and the other is a wear test (subjecting a lubricant
to a constant load for a de nite period of time while measuring the wear pattern).
4. Timken EP Test. This test provides a rapid method of measuring abrasion resistance and
the load-carrying capacity of lubricants. A number of lubricant speci cations require
Timken “OK” loads above certain minimum values. The mode of operation consists of
rotating a Timken tapered roller bearing cup against a stationary, hardened steel block.
Fixed weights force the block into contact with the rotating cup through a lever system.
The OK load is the highest load the cup and block can carry without scoring during a
10 min run. Timken abrasion tests are run under xed loads for extended time periods,
and the weight loss of the cup and block are a measure of the abrasion resistance of the
lubricant.
5. L-37 High Torque Test. The CRC L-37 test operates under low-speed, high-torque condi-
tions. It evaluates the load-carrying ability, wear stability, and corrosion characteristics
of gear lubricants. The test differential is a Dana Model unit driven by a Chevrolet truck
engine and four-speed transmission. A complete, new axle assembly is used for each test
after a careful examination of gear tooth and bearing tolerance. After break-in at reduced
load and high speed, the test continues for 24 h under low-speed (80 axle rpm) and high-
torque conditions.
6. L-42 High Speed Shock Test. The CRC L-42 test is established to evaluate the antiscore
performance of EP additives in gear lubricants under high-speed, shock load conditions.
The test axle is a Dana Model unit driven by a Chevrolet engine through a four-speed truck
transmission. The procedure requires ve accelerations in fourth gear with inertia loading
and 10 accelerations in third gear with dynamometer loading. The lubricant evaluation is
based on the amount of scoring, and test results are expressed as percent tooth contact area
scored.
7. FAG FE-8 Test. FAG developed this test frame to be a exible tribological system to
conduct tests over a wide range of operating conditions with different test bearings. Short-
duration standardized tests have been developed for different applications. FAG also uses
longer-term testing (e.g., fatigue) for comprehensive evaluations. The FE-8 gear oil test
was developed speci cally to evaluate the effectiveness of antiwear additives. The test
runs under heavy load and low speed that forces the bearing to operate under boundary
lubrication conditions.
CRC_59645_Ch008.indd 241CRC_59645_Ch008.indd 241 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM
242 Lubricant Additives: Chemistry and Applications
Bearing Test Conditions
Bearings Cylindrical roller/thrust loaded
Speed 7.5 rpm
Load 114 kN
Bearing temperature Variable
Test duration 80 h
Other tests including Optimol SRV, Cameron-Plint, high-frequency reciprocating rig (HFRR), Falex
multi-specimen, Vickers vane pump, Vickers 35-VQ-25 pump, and Denison high- pressure pump
tests are also used widely in evaluation of various lubricants and greases. Appropriate eld tests are
also arranged in proprietary test sites to ensure good product quality and equipment compatibility/
friendliness before the introduction of a new product into the marketplace.
On the engine oil side, the ILSAC is active in all phases of passenger car category development,
and the SAE is the technical society for those with interest in transportation. Within the SAE is a
Fuels and Lubricants Division/Engine Oil Technical Committee (TC-1) that serves as a forum for
open discussion of technical issues related to current and future engine lubrication needs and stan-
dard development. With the introduction of GF-3 in 2001, the industry moved to a completely new
set of engine tests for validation of passenger car engine oil performance. Although some new tests
replaced previous tests, which were running out of parts, others provided a means to measure perfor-
mance in new areas. The current category is GF-4, which superseded GF-3 in the summer of 2004.
Among the GF-4 tests, the most critical engine tests related to antiwear/EP performance are
the Sequence IVA and the Sequence IIIG. The Sequence IVA is an ASTM designation of a test
previously referred to as the KA24E, originally developed by the Japan Automotive Manufacturers
Association. It is included to replace the wear component of the Sequence VE. The Sequence IVA
is designed to evaluate an oil’s ability to prevent cam lobe wear in slider valve train design engines
operated at low temperature, short trip, and “stop and go” conditions (low-speed/low-temperature
operation). Following is a list of the test conditions and speci cations:
Engine Nissan 2.4 L inline 4 cylinder
Engine speed 800 and 1500 rpm cycles
Engine torque 25 N m
Oil temperature 50–60°C
Cycle duration 50 min low speed/10 min high speed
Test length 100 h
7-Point cam lobe wear 120 µm maximum
The Sequence IIIG is a replacement for the Sequence IIIF and uses a current production version
of the GM 3800 Series II V-6 engine. Special camshaft and lifter metallurgy and surface nishing
are used to increase wear. The Sequence IIIG procedure is designed to evaluate the oil resistance to
oxidation and wear in high-speed and high-temperature vehicle operation. The test conditions and
speci cations are summarized as follows:
Engine GM 3800 Series II V-6 (231 CID)
Engine speed 3600 rpm
Engine load 250 N m
Valve spring load 205 lb
Oil temperature 150°C
Coolant temperature 115°C
Test length 100 h
Average cam and lifter wear 60 µm maximum
CRC_59645_Ch008.indd 242CRC_59645_Ch008.indd 242 10/31/2008 2:58:08 PM10/31/2008 2:58:08 PM
Ashless Antiwear and Extreme-Pressure Additives 243
The next ILSAC category is ILSAC GF-5, which is targeted to be introduced around mid-2010. The IL-
SAC/Oil Committee has decided that the Sequence IIIG and Sequence IVA tests will be retained to
ensure that acceptable wear protection is achieved in the upcoming ILSAC GF-5 category.
In the heavy-duty diesel engine oil area there are a number of industry standard engine tests that
measure the wear performance. These tests required to meet both industry and engine manufacturer
requirements such as API CJ-4 and various speci cations from Caterpillar, Cummins, Detroit Die-
sel, Mack, and Volvo. The key wear tests assess the ability of an oil to control valve train or ring and
liner wear under severe operating conditions, which include high-load duty cycles, use of exhaust
gas recirculation, and high levels of soot contamination.
The API CJ-4 category requires three tests that include valve train wear as a pass/fail parameter.
The Roller Follower Wear Test (ASTM D5966) is run in a 6.5 liter V-8 GM diesel engine; it was
initially developed for the older API CG-4 category, which was developed for the introduction
of low sulfur (500 ppm maximum) fuel. However, this test has remained as a requirement in all
subsequent speci cations. At the end of this 50 h test, the used oil soot level is typically 3.5 to 4.0%.
The level of wear on the stationary pin in the hydraulic cam followers is measured. The Cummins
ISB test (ASTM procedure in progress) was introduced as an industry requirement for API CJ-4.
This 350 h test runs in a 5.9 liter in-line 6 cylinder engine running of ultra low sulfur (15 ppm maxi-
mum) diesel fuel. The rst 100 h are run at steady-state conditions to generate 3.25% soot in the oil.
The nal 250 h are run under cyclic conditions to stress cam and tappet wear, which are the primary
pass/fail criteria. The third diesel engine test that measure valve train wear is the Cummins ISM
test (ASTM procedure in progress). The Cummins ISM is the third in a series of Cummins heavy-
duty wear tests developed for API and engine builder diesel oil speci cations. Similar to previous
Cummins M11 HST (ASTM D6838) and Cummins M11 EGR (ASTM D6975) tests, the Cummins
ISM alternates between 50 h soot generation and 50 h wear stages. This test runs for 200 h using
500 ppm sulfur diesel fuel. The used oil typically contains 6 to 7% soot, and the key pass/fail wear
parameters are focused on the crossheads (bridges for the inlet and exhaust valves) and the adjusting
screw for the fuel injectors.
The Mack T-12 test (ASTM D7422) measures ring and liner wear under severe operation using
15 ppm sulfur fuel. This 300 h test runs with a very high EGR rate for the rst 100 h to generate
4.3% soot. During the nal 200 h, the engine runs over-fueled at peak torque conditions to create
a very severe environment for top ring weight loss and liner wear at the point of top ring reversal,
which are the key wear parameters for this test.
8.5 OUTLOOK
The additives business has experienced an economic upturn in recent years, primarily due to the
imbalance between demand and supply as a result of tight feedstock availability and increased
demand in the Far East region. The basic chemicals used to produce additives are subject to short
supply as new and large capacity has not been effectively added to the manufacturing side for
several years. A number of natural disasters such as the hurricane Katrina certainly made the situa-
tion even worse. The additive suppliers have successfully passed the raw material costs to their cus-
tomers resulting in escalated unit pricing and improved pro tability. The increased volume demand
has been neutralized by several factors, such as longer drain lubricants and the reduction of ash
additives. Despite the push for new engine oils meeting more stringent requirements, a major ratio-
nalization is occurring because of the ability to use additives longer and the recycling of products in
the industry. Consequently, the total additive volume demand is growing slowly. Ashless antiwear/
EP additives are no different from other additives in terms of market demand.
Antiwear additives are a mature function class, and business opportunities in the next few years
will be modest. The dominant position of zinc dithiophosphates in engine oils is gradually diminishing,
but is not expected to be in jeopardy in the near term. Therefore, a total switch to ashless antiwear
additives in engine oils is not likely to occur very soon, but minor changes are in progress.
CRC_59645_Ch008.indd 243CRC_59645_Ch008.indd 243 10/31/2008 2:58:09 PM10/31/2008 2:58:09 PM
