Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

224 Lubricant Additives: Chemistry and Applications
8.2.2.2.3 Applications and Performance Characteristics
Dialkyl (or diaryl) hydrogen phosphites, besides being excellent antiwear agents, are considered the
most potent form of phosphorus, suited to high-torque, low-speed operations. This is the area where
antiwear processes are taken to the extreme and is one of the most important sections of the EP per-
formance spectrum. Sulfur can be quite incapable of giving protection under such conditions. Only
a phosphorus source, if active enough and in suf cient concentration, can help here. Conversely,
phosphorus components are of little use in high-speed and shock operations where sulfur compo-
nents can be excellent. Dialkyl or diaryl phosphites are also potent antioxidants.
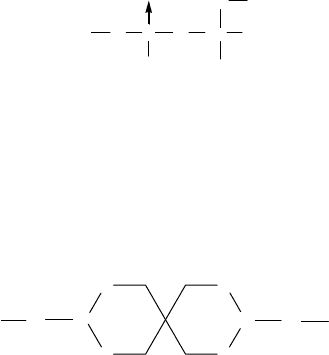
With dialkyl phosphites, it has been reported that oxidation produces a phosphate anion, which
tends to act as a bridging ligand to form an oligomeric iron (III) complex, that is, an iron oxide
complex resembling Structure C.
R
OPO
O
OH
Fe
O
O
OH
STRUCTURE C
However, there is also a weak, high-viscosity, nonsolid lm that increases the overall thickness
of the total lm at high speeds [24,31].
Dialkyl phosphites are widely used in gear oils, automatic transmission uids (ATF), and many
other applications. Spiro bicyclodiphosphites are also reported to be used in continuously variable
transmission uids [32] (Structure D).
O
O
P
O
P
O
O
OR
R
1
STRUCTURE D
8.2.2.3 Dialkyl Alkyl Phosphonates
Dialkyl alkyl phosphonates [R–P(=O)(OR)
2
] are stable organic phosphorus compounds that are
miscible with ether, alcohol, and most organic solvents. Besides being used as additives in sol-
vents and low-temperature hydraulic uids, they can also be used in heavy metal extraction, solvent
separation, and as preignition additives to gasoline, antifoam agents, plasticizers, and stabilizers.
Dialkyl alkyl phosphonates are prepared from either dialkyl hydrogen phosphites or trialkyl phos-
phites as described in reactions (Michaelis–Arbuzov reaction).
(RO)
3
P + R′X ⇒ (RO)
2
P(=O)R′ + RX (8.14)
(RO)
2
P(=O)H + R′OH + CCl
4
⇒ (RO)
2
P(=O)R′ + H
2
O (8.15)
(RO)
2
P(=O)H + NaOH ⇒ (RO)
2
P∙O∙Na + R′X ⇒ (RO)
2
P(=O)R′ + NaX (8.16)
In principle, the thermal isomerization of all phosphites to phosphonates can be carried out. The
stability of these compounds varies greatly; however, depending on the nature of the R group, other
products may be formed during heating. For R = methyl, complete conversion occurs at 200°C in
18 h, but for R = butyl, the compound is stable at 223°C. It is thought by some that isomerization of
phosphites may be possible only if traces of phosphonate are already present as an impurity [33].
(RO)
3
P + Heat ⇒ (RO)
2
P(=O)R (8.17)
CRC_59645_Ch008.indd 224CRC_59645_Ch008.indd 224 10/31/2008 2:58:03 PM10/31/2008 2:58:03 PM
Ashless Antiwear and Extreme-Pressure Additives 225
8.2.2.4 Acid Phosphates
Acid phosphates are also potent additives, useful in similar areas of antiwear and EP to the dialkyl
phosphites. Orthophosphoric (monophosphoric) acid (H
3
PO
4
), the simplest oxyacid of phosphorus,
can be made by reacting phosphorus pentoxide with water. It is widely used in fertilizer manufac-
ture. Orthophosphoric acid has only one strongly ionizing hydrogen atom and dissociates according
to the following reaction
H
3
PO
4
⇔ H
+
+ H
2
PO
4
–
⇔ H
+
+ HPO
4
2–
⇔ H
+
+ PO
4
3–
(8.18)
Since the rst dissociation constant, K
1
(7.1 × 10
−3
), is much larger than the second (K
2
= 6.3 ×
10
−8
), very little of the H
2
PO
4
produced in the rst equilibrium goes on to dissociate according to
the second equilibrium. Even less dissociates according to the third equilibrium since the third
constant K
3
is very small (K
3
= 4.4 × 10
−13
). The acid gives rise to three series of salts containing
these ions, for example, NaH
2
PO
4
, Na
2
HPO
4
, and Na
3
PO
4
.
8.2.2.4.1 Chemistry and Manufacture
Alkyl (aryl) acid phosphates are made from alcohol (phenol) and phosphorus pentoxide. Generally,
a mixture of monoalkyl (aryl) and dialkyl (aryl) phosphates is produced.
3ROH + P
2
O
5
⇒(RO)
2
P(=O)OH + (RO)P(=O)(OH)
2
(8.19)
Pure monoalkyl or dialkyl (aryl) phosphates can be synthesized through different reaction routes
as follows:
ROH + POCl
3
⇒ ROP(=O)Cl
2
⇒ (Hydrolysis) ⇒ (RO)P(=O)(OH)
2
(8.20)
(RO)
2
P(=O)H + Cl
2
⇒ (RO)
2
P(=O)Cl ⇒ (Hydrolysis) ⇒ (RO)
2
P(=O)(OH) (8.21)
8.2.2.4.2 Properties, Performance Characteristics, and Applications
Phosphoric acids tend to hydrolyze further when exposed to humidity. The extent of hydrolysis
depends on the moisture content of the ambient atmosphere and the duration of exposure. Wherever
possible, phosphoric acids should be handled in a dry nitrogen atmosphere to prevent hydrolysis.
Therefore, for applications where incidental moisture contact is inevitable, acid phosphates are not
recommended.
Acid phosphates are used as rust inhibitors and antiwear additives. However, they are not as
widely used as their amine-neutralized derivatives, for example, amine phosphates.
8.2.3 SULFUR–PHOSPHORUS ADDITIVES
Sulfur–phosphorus additives are used to provide protection against moderate to high pressure,
metal-to-metal contacts in boundary lubrication, and EHL. Metallic sulfur–phosphorus additives,
such as zinc dithiophosphates (ZnDTPs), are the most important antiwear/EP components used in
engine oils. Ashless sulfur–phosphorus additives are used less extensively, and the most commonly
available S/P additives in the marketplace are based on chemistries of dithiophosphates, thiophos-
phates, and phosphorothioates. Other important applications of S/P compounds are in matches,
insecticides, otation agents, and vulcanization accelerators.
8.2.3.1 Ashless Dithiophosphates
Numerous patents were issued on the use of phosphorodithioic acid esters in lubricating oils in the early
days. U.S. Patent 2,528,732 describes alkyl esters of phosphorodithioic acid. U.S. Patent 2,665,295
CRC_59645_Ch008.indd 225CRC_59645_Ch008.indd 225 10/31/2008 2:58:03 PM10/31/2008 2:58:03 PM

226 Lubricant Additives: Chemistry and Applications
describes the S-terpene ester, whereas U.S. Patent 2,976,308 describes an anti-Markovnikov addition
of phosphorodithioic acid ester to various ole ns, both aromatic and aliphatic. Amine dithiophosphates
and other novel dithiophosphate esters are reported in the literature [34–38]. Coupling with vinyl
pyrrolidinone, acrolein or alkylene oxides (to make hydroxyl derivatives) are also known [39–41].
8.2.3.1.1 Chemistry and Manufacture
Similar to metallic dithiophosphates, ashless dithiophosphates are also based on phosphorus
pentasul de (P
2
S
5
) chemistry. They can be prepared from the same precursor of ZnDTP, dithio-
phosphoric acid (reaction 8.22) through the reaction of alcohol (or alkylphenol) and P
2
S
5.
4ROH + P
2
S
5
⇒ 2(RO)
2
P(=S)SH + H
2
S (8.22)
The dithiophosphoric acids are further reacted with an organic substrate to generate ashless
derivatives. Typical organic substrates are compounds such as ole ns, dienes, unsaturated esters
(acrylates, methacrylates, vinyl esters, etc.), unsaturated acids, and ethers. The ef ciency and stability
of the ashless dithiophosphates very much depends on components used in their manufacture and
the reaction conditions.
The most common ashless dithiophosphate used in the marketplace is a dithiophosphate ester
made from ethyl acrylate and o,o-diisopropyl dithiophosphoric acid as described in the following:
[C
3
H
7
–O–]
2
–P(=S)S–CH
2
–CH
2
–C(=O)O–C
2
H
5
Treatment of terpenes, polyisobutylene (PIB), or polypropylene (PP) with phosphorus pentasul de
and hydrolysis give thiophosphonic acids [R–P(=S)(OH)
2
where R = PIB, terpenes, or PP).
They can be further reacted with propylene oxide or amines to reduce acidity. However, this type of
additive belongs to the same class of chemicals called ashless dispersants. Hence, they can be dual
functional dispersants with improved antiwear/EP properties.
8.2.3.1.2 Applications and Performance Characteristics
Unlike ZnDTP, ashless dithiophosphates are usually not as versatile, and therefore cannot be con-
sidered as multifunctional additives. Although ashless dithiophosphates have fairly good antiwear
and EP properties, their anticorrosion properties are not as good as ZnDTP. This is closely related
to the stability and decomposition mechanisms of ashless dithiophosphates. Relatively weak cor-
rosion protection also limits their application at high concentrations in engine oils as well as some
industrial oils.
Ashless dithiophosphates can be useful in metalworking uids, automotive transmission uids
(ATF), gear oils, greases, and non-zinc hydraulic uids [42,43].
8.2.3.2 Ashless Phosphorothioates and Thiophosphates
Numerous esters of the phosphorothioic acids are known. In salts and esters of these oxygen/sulfur
(O–S) acids, there may be a preferred location of the multiple bonds, but in general, this is not well
known. Thus in principle, there are two series of possible acids, each of which might give rise to
salts and esters as described in the following:
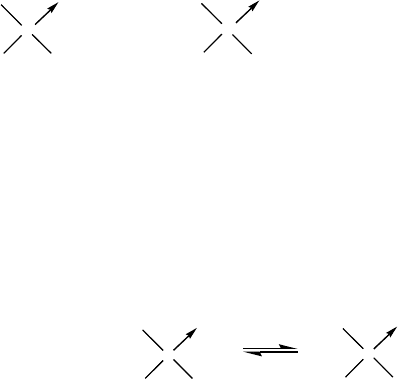
Phosphorothionic acid Phosphorothiolic acid Phosphorothioic acid
(Thionophosphoric) (Thiolophosphoric) (Thiophosphoric)
OHHO
HO
S
SHHO
HO
O
P
P
H
3
PO
3
S
CRC_59645_Ch008.indd 226CRC_59645_Ch008.indd 226 10/31/2008 2:58:03 PM10/31/2008 2:58:03 PM
Ashless Antiwear and Extreme-Pressure Additives 227
Phosphorothiolothionic acid Phosphorodithiolic acid Phosphorodithioic acid
(Thiolothionophosphoric) (Dithiolophosphoric) (Dithiophosphoric)
SHHO
HO
S
SH
HS
HO
O
P
P
H
3
PO
2
S
2
The “thionic” acids contain the group P=S, whereas the “thiolic” acids contain the group P–SH.
The term “thioic” is often used when the molecular form is unknown or when speci cation is not
desired. One form of these acids is usually more stable than the other, and it may not be possible to
prepare both esters as, for example, the isomers of phosphorothioic acid.
OHRO
RO
S
SHRO
RO
O
P
P
In the case of some esters, the thiolo form is the most stable, but the phenyl ester exists 80% in
thiono, (PhO)
2
P(=S)OH, and 20% in thiolo, (PhO)
2
P(=O)SH forms. The equilibrium of these com-
pounds is liable to be dependent on the nature of the R groups, the solvent used, and even the concen-
tration. Intermolecular hydrogen bonding may be expected to play a part in such equilibrium [33].
8.2.3.2.1 Chemistry and Manufacture
The creation of a compound with a phosphorus–sulfur linkage can often be carried out simply by
heating the appropriate phosphorus compound with sulfur [44]. Likewise, the replacement of oxygen
by sulfur in compounds containing P–O linkages can also be achieved simply by heating them
with P
2
S
5
. Inorganic phosphorothioates (thiophosphates) are usually prepared from sulfur-containing
phosphorus compounds. They are produced during the hydrolytic breakdown of phosphorus sul des
and are often themselves unstable in water. They hydrolyze to the corresponding oxy compounds
with the evolution of H
2
S. Phosphorus–sulfur compounds are often thermally less stable than their
oxy analogues. A few examples are listed as follows:
P
4
S
10
+ 12NaOH ⇒ 2Na
3
PO
2
S
2
+ 2Na
3
PS
3
O + 6H
2
O (8.23)
(BuO)
2
P(=O)SH + RI ⇒ (BuO)
2
P(=O)SR + HI (8.24)
(PhO)
3
P + S ⇒ (PhO)
3
P=S (8.25)
(PhO)
3
P + PSCl
3
⇒ (PhO)
3
P=S + PCl
3
(8.26)
Hydrolysis of phosphorothioate esters results in a progressive loss of sulfur as hydrogen sul de
(H
2
S) and its replacement by oxygen.
(RO)
3
P=S + H
2
O ⇒ (RO)
3
P=O + H
2
S (8.27)
8.2.3.2.2 Applications and Performance Characteristics
It has been known for many years that sulfur compounds form a lm of iron sul de, and phosphorus
compounds form iron phosphate, on the mating metal surfaces. Generally, the lms formed from
sulfur sources such as SIB are expected to contain FeS, FeSO
4
, as well as organic fragments from
CRC_59645_Ch008.indd 227CRC_59645_Ch008.indd 227 10/31/2008 2:58:03 PM10/31/2008 2:58:03 PM

228 Lubricant Additives: Chemistry and Applications
the additive decomposition. With phosphorus sources, such as dialkyl phosphites, lms containing
FePO
4
, FePO
3
, as well as organic fragments are expected. When both sulfur and phosphorus are
present, both elements contribute to the nature of the lm, and which one predominates depends on
the S/P ratio, the decomposition mechanisms, and the operating conditions, for example, high speed
and shock or high torque/low speed.
Ashless phosphorothioates are widely used as replacements for metallic dithiophosphates in
many lubricant applications where metal is less desirable [43,44]. Phosphorothioates are often
present (generated in situ) in lubricant formulations when both sulfur and phosphorus additives are
used. Aryl phosphorothioates provide good thermal stability and good antiwear/EP properties as
evidenced by their strong FZG performance.
8.2.4 SULFUR–NITROGEN ADDITIVES
Sulfur and nitrogen-containing additives are used to provide protection against moderate to high
pressure, metal-to-metal contacts in boundary lubrication, and EHL. Both open chain and hetero-
cyclic compounds have attracted a considerable amount of research effort to explore their potential
as antiwear and EP additives. Among open chain additives, dithiocarbamates are the most widely
used. Other additives, such as organic sulfonic acid ammonium salts [45], and alkyl amine salts
of thiocyanic acid [46] are reported in the literature, but are of relatively low commercial value.
Nitrogen and sulfur-containing heterocyclic compounds, such as 2,5-dimercapto-1,3,4-thiadiazole
(DMTD, Structure E), 2-mercapto-1,3-benzothiazole (MBT, Structure F), and their derivatives,
have been used for many years as antioxidants, corrosion inhibitors, and metal passivators; gener-
ally at relatively low concentrations.
NN
S
HS
SH
N
C
S
SH
STRUCTURE E STRUCTURE F
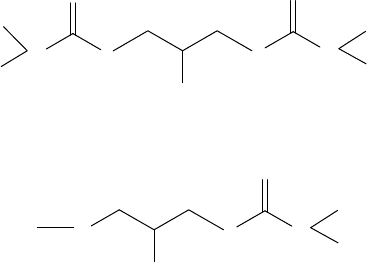
8.2.4.1 Dithiocarbamates
The dithiocarbamates, the half amides of dithiocarbonic acid, were discovered as a class of chemi-
cal compounds early in the history of organosulfur chemistry [47,48]. The strong metal-binding
properties of the dithiocarbamates were recognized early, by virtue of the insolubility of metal salts
and the capacity of molecules to form chelate complexes. Other than applications in lubricant areas,
dithiocarbamates have been used in the eld of rubber chemistry as vulcanization accelerators and
antiozonants.
8.2.4.1.1 Chemistry and Manufacture
Organic dithiocarbamates can be made by a one-step reaction of dialkylamine, carbon disul de, and
an organic substrate. The organic substrate is preferably an ole n, diene, epoxide, or any other unsat-
urated compounds as exempli ed in the literature [49,50]. Organic dithiocarbamates can also be
made through a two-step reaction involving ammonium or metal dithiocarbamate salts and organic
halides [51]. In the case of their ammonium salts, N-substituted dithiocarbamic acids, RNHC(=S)SH
or R
2
NC(=S)SH, are formed by reaction of carbon disul de with a primary or secondary amine in
alcoholic or aqueous solution before they are further reacted with ammonia. To conserve the more
valuable amine, it is a common practice to use an alkali metal hydroxide to form the salt.
RNH
2
+ CS
2
+ NaOH ⇒ RNHC(=S)S–Na + H
2
O (8.28)
The dithiocarbamic acid can be precipitated from an aqueous solution of dithiocarbamate by
adding strong mineral acid. The acids are quite unstable but can be held below 5°C for a short time.
CRC_59645_Ch008.indd 228CRC_59645_Ch008.indd 228 10/31/2008 2:58:04 PM10/31/2008 2:58:04 PM
Ashless Antiwear and Extreme-Pressure Additives 229
The most common additive, methylene bis-dibutyl dithiocarbamate, is prepared from sodium dibutyl
dithiocarbamate and methylene chloride.
2(C
4
H
9
)
2
NC(=S)S–Na + CH
2
Cl
2
⇒ [(C
4
H
9
)
2
NC(=S)S]
2
CH
2
+ 2NaCl (8.29)
8.2.4.1.2 Applications and Performance Characteristics
Unlike metallic dithiocarbamates that have been widely used in lubricants, ashless dithiocarbamates
have only been gaining more attention recently. Relatively high cost is certainly a major factor
in limiting wider use. The success of metallic dithiocarbamates also overshadows their ashless
counterpart. Certain metallic dithiocarbamates, such as molybdenum dithiocarbamates, offer
exceptionally good frictional properties that cannot be matched by their ashless analogues also.
However, ashless dithiocarbamates have been found to be versatile, multifunctional additives in
a few areas. They can be effective antiwear/EP additives as well as good antioxidants and metal
deactivators [52–55], (Structures Ga and Gb). They tend to generate less sludge or deposits than
mostly metallic additives and they are very compatible with various base oils.
N
S
S
R′
R
NS
S
R′
R
OH
STRUCTURE Ga
NS
S
R′
R
OH
S
R′′
STRUCTURE Gb
8.2.4.2 Dimercaptothiadiazole and Mercaptobenzothiazole Additives
Additives derived from DMTD and 2-mercaptobenzo-thiazole (MBT) are well documented in the
literature. Owing to strong ring stability (partial aromaticity and resonance delocalization), balanced
sulfur–nitrogen distributions, and reactive mercaptan groups, both heterocyclic compounds can be
versatile core molecules to make many useful additives with many bene cial characteristics, such as
improved thermal/oxidative stability and reduced corrosivity. Unfortunately, some potentially good
reactions are hampered by the limited solubility of DMTD and MBT in common petrochemical sol-
vents. Therefore, a suitable sample preparation procedure is very critical to help achieve desirable
antiwear/EP additives.
8.2.4.2.1 Chemistry and Manufacture
Many differing organic reactions can be applied to functionalize the mercaptan groups of DMTD
and MBT. Oxidative coupling reactions involving other alkyl mercaptans can bring in additional sul-
fur for EP performance and additional alkyl chains for improved solubility [56]. (Addition reactions
with organic compounds containing activated double bonds can link DMTD or MBT heterocyclic
core molecules with long chain esters, ketones, ethers, amides, and acids together [57–60]). Like-
wise, ring opening with epoxides to generate alcohol derivatives is also known [61]. Direct amine
salts formation and linking alkyl amines through Mannich base condensation are also extensively
studied [62–64]. A number of examples are listed in reactions 8.30 through 8.33, where TD is the
abbreviation for the thiadiazole moiety and BT is for the benzothiazole moiety.
CRC_59645_Ch008.indd 229CRC_59645_Ch008.indd 229 10/31/2008 2:58:04 PM10/31/2008 2:58:04 PM
230 Lubricant Additives: Chemistry and Applications
Oxidative coupling
DMTD + 2RSH + 2H
2
O
2
⇒ RS–S–(TD)–S–SR + 4H
2
O (8.30)
Mercapto alkylation and Mannich alkylation
DMTD + 2CH
2
=O + 2RSH ⇒ RS–CH
2
–S–(TD)–S–CH
2
–SR + 2H
2
O (8.31)
MBT + CH
2
=O + RNH
2
⇒ (BT)–S–CH
2
–NHR + H
2
O (8.32)
Amine salt formation
DMTD + 2RNH
2
⇒ RNH
3
–S–(TD)–S–NH
3
R (8.33)
8.2.4.2.2 Applications and Performance Characteristics
MBT is a light yellow powder with limited solubility in hydrocarbons. It is more soluble in aromatic
solvent (~1.5% in toluene), polar solvents, and highly aromatic oils. MBT is used as a copper
corrosion inhibitor in fuels as well as a corrosion inhibitor/deactivator in numerous industrial lubri-
cants such as heavy-duty cutting and metalworking uids, hydraulic oils, and lubricating greases.
DMTD is also a light yellow powder with very limited solubility in hydrocarbons. It is considered a
versatile chemical intermediate suitable for making various oil-soluble derivatives.
Both MBT and DMTD derivatives are widely used as copper passivators and nonferrous metal
corrosion inhibitors. Some proprietary load-carrying additives are substituted MBT and DMTD
compounds that are used in various applications either as a component or as a part of additive
packages with a speci c purpose [65,66]. In the absence of any phosphorus moiety in MBT and
DMTD, their oil-soluble derivatives are suitable for replacing zinc dithiophosphates in some lubri-
cant applications. For example, a commercial, high-density, powder-like MBT and DMTD deriva-
tives is used as a dual functional antioxidant/EP agent in greases.
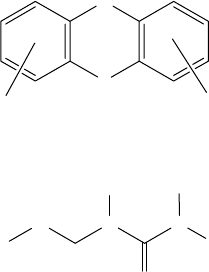
8.2.4.3 Other Sulfur–Nitrogen Additives
In addition to DMTD, MBT, and dithiocarbamate additives, there are other sulfur–nitrogen- containing
additives available in the marketplace or reported in the literature. Among these, phenothiazine
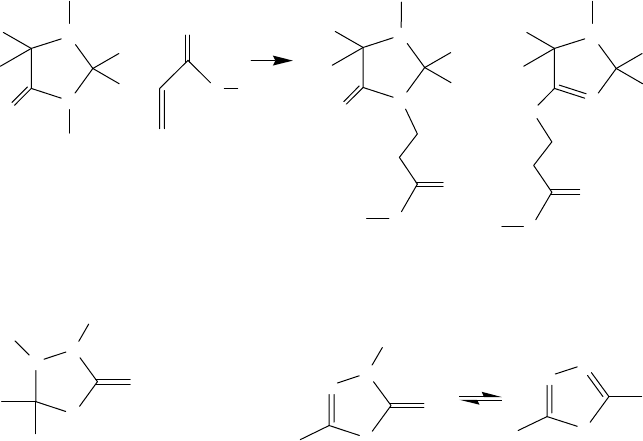
derivatives (Structure H, PTZ), substituted thiourea additives (Structure I, TU), thionoimidazolidine
derivatives (Structure J, TIDZ), thiadiazolidine and oxadiazole (ODZ) derivatives (Structures K
and L), thiuram monosul des, thiuram disul des, and benzoxazoles are of particular interest because
they are all sulfur- and nitrogen-rich molecules [67–73]. Thiuram disul des, chemically similar to
dithiocarbamates, can be used in the rubber industry as vulcanizers. 2-Alkyldithio-benzoxazoles
also offer good frictional properties in addition to strong antiwear/EP properties [74].
S
H
N
R
R′
STRUCTURE H, PTZ
R
3
X N N
R
2
S
R
1
R
4
STRUCTURE I, TU
CRC_59645_Ch008.indd 230CRC_59645_Ch008.indd 230 10/31/2008 2:58:05 PM10/31/2008 2:58:05 PM
Ashless Antiwear and Extreme-Pressure Additives 231
N
N
O
O R
5
S
R
3
R
4
R
1
R
2
N
N
S
H
H
R
3
R
4
R
1
R
2
N
N
S
H
R
3
R
4
R
1
R
2
H
+
O
O
O
O
R
5
R
5
STRUCTURE J, TIDZ
N
X
N
R
2
S
R
1
R
3
H
N
O
N
H
S
R
1
N
O
N
SH
R
1
STRUCTURE K, TDZL STRUCTURE L, ODZ
8.2.5 PHOSPHORUS–NITROGEN ADDITIVES
Phosphorus–nitrogen additives are used to provide protection against moderate to high pressure,
metal-to-metal contacts in boundary lubrication, and EHL. Ashless phosphorus–nitrogen additives
are used as dual functional antiwear/antirust additives extensively, and those that are most com-
monly available in the marketplace are based on chemistries of amine dithiophosphates, amine
thiophosphates, amine phosphates, and phosphoramides.
8.2.5.1 Amine Phosphates
Amine phosphates are by far the most important phosphorus–nitrogen-containing additives used
in lubricants. In fact, they are multifunctional additives possessing very good antirust properties as
well as antiwear/EP properties.
8.2.5.1.1 Chemistry and Manufacture
Amine phosphates are produced by treating acid phosphates with alkyl or aryl amines. Under various
conditions, neutral, overbased, and underbased amine phosphates can be synthesized. If mixed mono-
and dialkyl acid phosphates are used as starting materials, mixed mono and dialkyl amine phosphates
are produced. The nal additives usually possess high total acid number (TAN) and high total base
number (TBN), although reaction adducts are considered fairly neutral. It is known that a complete neu-
tralization of both phosphoric acid groups in monoalkyl acid phosphates with amines cannot be easily
achieved, and therefore, under normal conditions, a partially neutralized amine phosphate is formed.
(RO)
2
P(=O)(OH) + R′NH
2
⇒ (RO)
2
P(=O)O∙NH
3
R′ (8.34)
(RO)P(=O)(OH)
2
+ R′NH
2
⇒ (RO)P(=O)(OH)O∙NH
3
R′ (8.35)
8.2.5.1.2 Applications and Performance Characteristics
Amine phosphates are extensively used in industrial oils, greases, and automotive gear oils.
They offer very good rust protection as demonstrated in various bench rust tests (ASTM D665 and
CRC L-33). They also show very good antiwear/EP characteristics (Four-Ball Wear and Four-Ball
CRC_59645_Ch008.indd 231CRC_59645_Ch008.indd 231 10/31/2008 2:58:05 PM10/31/2008 2:58:05 PM
232 Lubricant Additives: Chemistry and Applications
EP, FZG, Timken, and CRC L-37). Since amine phosphates are very polar species, they interact
strongly with other additive components, making their performance very dependent on the formu-
lation. Hence, extra attention is needed when amine phosphates are used.
8.2.5.2 Amine Thiophosphates and Dithiophosphates
Amine thiophosphates and amine dithiophosphates can be found in engine oils and industrial oils
where zinc dithiophosphates and other nitrogen-containing additives are used, either as decomposi-
tion products or as in situ-produced products. They are critical to the lubricant performance because
of their high activity toward metal surfaces.
8.2.5.2.1 Chemistry and Manufacture
Amine thiophosphates are produced by reacting thiophosphoric acid with alkyl or aryl amines [75].
Likewise, amine dithiophosphates are synthesized from dithiophosphoric acid and amines.
(RO)
2
P(=S)SH + H
2
NR′ ⇒ (RO)
2
P(=S)S∙H
3
NR′ (8.36)
(RO)
2
P(=O)SH + H
2
NR′ ⇒ (RO)
2
P(=O)S∙H
3
NR′ + (RO)
2
P(=S)O∙H
3
NR′ (8.37)
8.2.5.2.2 Applications and Performance Characteristics
Amine thiophosphates and dithiophosphates are also multifunctional additives providing good rust
inhibition and antiwear properties. Owing to their high activity and low stability, amine thiophos-
phates and dithiophosphates are not as extensively used as either amine phosphates or metallic
dithiophosphates. A detailed study of their antiwear mechanisms suggested that a tribofragmenta-
tion process is involved [76,77]. Relatively poor corrosion control is one area of concern that needs
attention. With proper formulation adjustments, it is quite feasible to overcome certain intrinsic
weaknesses and apply both chemistries to various lubricant products.
8.2.5.3 Other Phosphorus–Nitrogen Additives
There are many other phosphorus–nitrogen-containing ashless antiwear additives reported in the
literature. Some are proprietary technologies, and their commercial status is unknown. Organophos-
phorus derivatives of benzotriazole (BZT) are a group of additives based on triazole and dialkyl
or dialkylphenyl phosphorochloridate chemistry [78]. Arylamines and dialkyl phosphites can be
coupled through a Mannich condensation reaction to form unique phosphonates that are used as
multifunctional antioxidant and antiwear additives [79]. Bisphosphoramides are also reported [80].
8.2.6 NITROGEN ADDITIVES
Nitrogen-containing additives are used to provide rust inhibition and cleanliness features in various
lubricant applications. For example, nitrogen-containing ashless dispersants are a key component
for engine oils, and alkoxylated amine compounds are used in lubricating greases to provide corro-
sion inhibition [81]. Furthermore, arylamines are widely used as antioxidants due to their ability to
terminate radical chain propagation and decompose peroxides. Very few nitrogen additives alone
are considered effective antiwear/EP additives, and their performance is either very speci c to
industrial applications or fairly dependent on product formulations. However, when used in combi-
nation with other sulfur, phosphorus, or boron additives, nitrogen-containing additives can be very
effective supplements to enhance antiwear/EP performance.
8.2.6.1 Chemistry, Manufacture, and Performance
Several novel chemistries are available in the literature for nitrogen-only antiwear additives. Among
these, dicyano compounds were tested and they exhibited very good Four-Ball Wear activities [82].
Polyimide-amine salts of styrene–maleic anhydride copolymers are also reported as antiwear
additives; however, high additive concentrations (5–10%) are needed [83]. Alkoxylated amines
(Structure M) and mixtures of fatty acid, fatty acid amide, imide or ester derived from substituted
CRC_59645_Ch008.indd 232CRC_59645_Ch008.indd 232 10/31/2008 2:58:06 PM10/31/2008 2:58:06 PM
Ashless Antiwear and Extreme-Pressure Additives 233
succinic acid or anhydride have been identi ed to be good fuel lubricity additives [84] (Structure M).
Alkyl hydrazide additives possessing two adjacent nitrogen atoms have also been claimed to exhibit
good antiwear properties [85] (Structure N). Products of nitrogen heterocycles, such as ODZ (Tables
8.2 and 8.3 for performance evaluations), BZT, tolyltriazole (TTZ), alkyl succinhydrazide (SHDZ),
and borated hydroxypyridine (BHPD) (Structures O, P, Q, R, S, respectively), with pendant alkyl-
ates, amines or carboxylic acids have been found to be effective antiwear additives in both lubricants
and fuels [86–92]. Although triazoles are costly chemicals, they have unique geometric structures
that contribute to high surface lm–forming ef ciency.
RO(C
4
H
8
O)
n
CH
2
CH
2
CH
2
NH
2
(M)
STRUCTURE M
R
1
N
N
R
3
H
O
R
2
N
O
N
R
2
R
3
R
4
R
1
N
N
H
N
STRUCTURE N (AHDZ) STRUCTURE O (ODZ) STRUCTURE P (BZT)
TABLE 8.2
SAE 5W-20 Prototype Motor Oil Formulation
Component Formulation A (wt%)
Solvent neutral 100 22.8
Solvent neutral 150 60
Succinimide dispersant 7.5
Overbased calcium phenate detergent 2
Neutral calcium sulfonate detergent 0.5
Rust inhibitor 0.1
Antioxidant 0.5
Pour point depressant 0.1
OCP VI improver 5.5
Antiwear additive
a
1
a
In the case of no antiwear additive present in the formulation,
solvent neutral 100 is put in its place at 1.0 wt%.
TABLE 8.3
Four-Ball Wear Results
Compound Formulation Wear Scar Diameter (mm)
No antiwear additive A 0.73 (0.74)
a
1.0 wt% ZDDP A 0.50 (0.51)
0.5 wt% ZDDP A 0.70 (0.67)
5-Heptadecenyl-1,3,4-oxadiazole A 0.38 (0.38)
5-Heptyl-1,3,4-oxadiazole A 0.54 (0.56)
5-Heptadecenyl-2,2-dimethyl-1,3,4-Oxadiazole A 0.7
5-Heptadecenyl-2-furfuryl-1,3,4-Oxadiazole A 0.38 (0.39)
a
Duplicated runs.
CRC_59645_Ch008.indd 233CRC_59645_Ch008.indd 233 10/31/2008 2:58:06 PM10/31/2008 2:58:06 PM
